1Department of Biotechnology, Prathyusha Engineering College Poonamalle Tiruvallur Road-602025
2Centre for Drug Discovery and Development, Sathyabama Institute for Science and Technology (Deemed to be University), Chennai 600119.
3California University of Science and Medicine, School of Medicine, CA, USA.
4Musculoskeletal Disease Research Laboratory US Department of Veteran Affairs, Loma Linda CA, USA.
Corresponding author email: jerrine.jj@gmail.com
Article Publishing History
Received: 13/01/2020
Accepted After Revision: 16/03/2020
The present study has been aimed to evaluate the anti-inflammatory property of P. guajava leaves by in-vitro using HRBC membrane stabilization method and anti-aging potential by in-silico method using AutoDock. The anti-inflammatory and anti-aging activity of leaf extracts of Psidium guajava collected from North Chennai region, India were evaluated in the present study. The in-vitro method showed significant anti-inflammatory property and anti-aging potential by binding with the target. The maximum membrane stabilization depicting the anti-inflammatory activity of P. guajava extracts was found to be 50% at a dose of 750 ug/ml. The effect of ascorbic acid from P. guajava leaves extract for preventing skin aging showing minimal binding energy for binding ligand (ascorbic acid) with the target protein (AP-1) was observed.
Psidium guajava, Anti-inflammatory, HRBC Membrane stabilization, Anti-aging, Docking.
Raghavi. R, Mohamoodha. N, Cholapandian. K, Rajasekar. T, Aruni W, Joseph J. In-vitro Anti-inflammatory and in-silico Anti-aging Properties of Psidium guajava Leaves. Biosc.Biotech.Res.Comm. 2020;13(1).
Raghavi. R, Mohamoodha. N, Cholapandian. K, Rajasekar. T, Aruni W, Joseph J. In-vitro Anti-inflammatory and in-silico Anti-aging Properties of Psidium guajava Leaves. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2QmXgxJ
Copyright © Raghavi et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Medicinal plants have a key role in combating human health issues since the Stone Age. They act as restorative, defensive and supportive agents for human body. The World Health Organization (WHO) reports revealed that 80% of populations in Asian and African countries rely on traditional medicines for primary health care necessities (Kim et al., 2012). A pivotal role of plants in the health scenario is attributed to bioactive compounds, which could delay or inhibit the inception of degenerative diseases and increase life expectancy (Jagadish et al., 200, Lakkadi et al 2018, Korkina et al., 2018 Aleksandra et al 2020).
Antioxidant medicinal plants, including phenolic and flavonoid are considered beneficial because of their protective actions in diseases as cancer. Phenol and flavonoids have been showed a wide range of biological activities (Bravo et al., 1998), including anticarcinogenic actions. Most of the beneficial health effects of flavonoids are attributed to their antioxidant and chelating abilities. Over product ion of reactive oxygen species (ROS) has shown to have detrimental effects on human health leading to cell/tissue damage and degenerative disorders such as inflammation, cardiovascular and neurogenic diseases, cancer, and aging related disorders. Many reports suggest that ROS are principal mediators of apoptosis (Simbula et al., 2007, Korkina, et al., 2018 Aleksandra et al 2020).
Antioxidants are added to food to slow the rate of oxidation and, if used properly, they can extend the length life of the food. ROS are produced by mitochondrial electron transfer processes and cytochrome P450 systems in hepatocytes (Robertaet al., 2005). Human hepatoma cell line (HepG2) is quite suitable for cytotoxicity evaluation due to the quality and stability of its enzymes and metabolic background (Ossenieet al., 2000). Many biological, chemical, and physical agents can generate inflammation with increased danger of human cancers (Nadia et al., 2016) many studies are currently going to develop inhibitors from medicinal plants to prevent or cure chronic inflammatory conditions for minimal side effects (Ashraf et al.,2016).Among the numerous traditional medicinal herbs, Psidiumguajava L. (Myrtaceae), commonly known as guava, has long been used in folk medicines as a therapeutic agent for the treatment of a number of diseases (Venkatachalam et al., 2012). The main constituents of Psidium guajava leaf extract are a variety of polyphenolics, flavonoids and triterpenoids, (Korkina et al., 2018 Aleksandra et al 2020).
Plants have long been used in the cosmetic industry as amongst others, skin lighteners and sun-screen agents. Dietary and topical ascorbic acid have beneficial effects on skin cells, and some studies have shown that vitamin C may help prevent and treat ultraviolet (UV)-induced photo damage (Gulluce et al.,2007). The present study aimed to evaluate the traditional anti-inflammatory, anti-oxidant and anti-aging potential of this species.
MATERIALS AND METHODS
Sample collection and extraction: The Psidiumguajava L. (Myrtaceae) plant leaves were collected from North Chennai region, India and were shade dried for 24 hours. The dried leaves were powdered and 25gm of the powdered leaves were subjected to soxhlet extraction using ethanol as the solvent.
Phytochemical screening: The ethanol extract and its fractions were tested by the LibermanBurchard, Lead acetate, Ferric chloride, Magnesium tracings, Vanillin sulphuric acid, Dragandroff’s reagent, Millon’s reagent and Liquid ammonia tests to determine the presence of steroids, phenolic compounds, tannin, flavonoids, saponins, alkaloids, proteins and anthraquinones respectively (Korkina et al., 2018).
Purification using thin layer chromatography: TLC plates were prepared by the application of a uniform layer of adsorbent (silica gel) on to 25mmX75mm glass slide. The plates were heated at 100 for 15 minutes to activate the silica gel. The sample is loaded on the plates leaving 1.5 cm from the bottom of the plates. The plates were inserted into the beaker containing the mobile phase (ethyl acetate and hexane). After the development of the chromatogram, the compounds were located and the retention factor for each compound was calculated using the following formula
Rf value = distance travelled by the sample/ distance travelled by the solvent.
Total Phenol Content: Total phenolic content was estimated by FolinCiocalteu’s method. One milliliter of aliquots and standard gallic acid (100, 200, 300 μg/ml) was positioned into the test tubes and 5 ml of distilled water and 0.5 ml of Folin Ciocalteu’s reagent was mixed and shaken. After 5 minutes, 1.5 ml of 20 % sodium carbonate was added and volume made up to 10 ml with distilled water. It was allowed to incubate for 2 hours at room temperature. Intense blue color was developed. After incubation, absorbance was measured at 750nm spectrophotometer. The blank was performed using reagent blank with solvent. Gallic acid was used as standard. The data for total phenolic contents of polyherbal formulation were expressed as mg of gallic acid equivalent weight (GAE) per 100gram of dry mass.
Antimicrobial Activity:Antibacterial activity was carried out by the disc diffusion method (Aliero et al., 2006). First, the different extracts of plant parts tested were dissolved in DMSO at a concentration of 100 mg/mL and filtered through 0.45 µm sterile filter membranes. Then, 100µL of bacterial inoculums containing 108 CFU/ml were spread over plates containing Mueller Hinton agar, and discs (6 mm in diameter) impregnated with 10 µL of the extracts (1 mg/disc) were placed on the surface of the media. Two control discs were used containing DMSO and Gentamicin (10 µg/ disc) as negative and positive controls, respectively. The plates were incubated for 24 h at 37 °C, and the experiments were performed in duplicate. The diameters of inhibition zones were measured and antibacterial activity was considered for diameters of inhibition zone greater than 9 mm.
Antibacterial and Antifungal activities were determined using agar diffusion methods against gram positive bacteria (Bacillus subtilis), gram negative bacteria (Escherichia coli) and a fungal species Aspergillus niger. Nutrient agar medium was prepared and the organisms were separately inoculated in the respective petri plates. Different concentration of the sample ranging from 20-80µl were added to the disc prepared from Whatman filter paper. It was incubated 24 hrs for bacterial pathogens and 48hrs for fungal pathogen and the results were observed. The diameter of the zone of inhibition was measured (Dharmanda et al., 2003).
Anti-inflammatory Activity :The blood was collected from healthy human volunteers and mixed with equal volume of Alsever’s solution (2% dextrose, 0.8% sodium citrate, 0.5% citric acid and 0.42% NaCl) and were centrifuged at 3,000 rpm. The packed cells were washed with iso saline and a 10% suspension was made. Various concentrations of extracts were prepared (250, 500 and 750 mcg/ml) using distilled water. To each concentration 1 ml of phosphate buffer, 2 ml hyposaline and 0.5 ml of HRBC suspension were added. It was incubated at 37°C for 30min and centrifuged at 3,000rpm for 20min. The haemoglobin content of the supernatant was estimated spectrophotometrically at 560 nm. Diclofenac (50 mcg/ml) was used as reference standard and a control was prepared by omitting the extracts. The percentage inhibition of lysis was calculated as follows:% hemolysis= (OD of test sample/ OD of control) X100
% protection= 100 – [(OD of test sample/ OD of control) X 100]
Antioxidant Activity: The antioxidant activity of ethanol leaves extract was measured in terms of hydrogen donation or radical scavenging activity using the stable radical DPPH (2,2-diphenyl-1- picrylhydrazyl) assay. DPPH radical scavenging activity of the samples was estimated according to the methods of (Venkatachalam et al., 2012). Different concentration of samples (100 and 200 μl) and DPPH solution (200 μM) were prepared using methanol. DPPH solution was mixed with sample, and the reaction mixture was left to stand for 30 min at room temperature in the dark. The scavenging activity of samples was estimated by measuring the absorption of the mixture at 515nm, which reflects the amount of DPPH radical remaining in the solution. The percentage of antioxidant activity was calculated using the following formula.
% of antioxidant activity = [Abs (control) – abs (sample)] x 100/ Abs (control)
Anti-aging activity :3D Structure of the target protein, AP-1 was retrieved from the protein data bank (PDB), with PDB ID of 1FOS. The DNA bound with the transcription factor was removed to prevent the interference during binding site prediction using Chimera software. The 3D structure of the active ingredient (Ascorbic Acid) are obtained from Pubchem in the SDF file format (*.sdf). A part from the active ingredient from natural source, 3D structure of SP100030, and a synthetic AP-1 inhibitor also retrieved which was used as a control. Auto Dock is a suite of automated docking tools. It is predicted to design how small molecules, such as substrates or drug candidates, bind to a receptor of known 3D structure.
RESULTS AND DISCUSSION
Phytochemical methods could provide the needed preliminary observations necessary to select among crude extracts, those with potentially useful properties for further chemical and pharmacological investigations (Joseph et al., 2010). A large variety of phytochemicals that have been reported from natural product research has been proven successful as anticancerous agents (Androutsopoulo et al., 2008). Elucidation of the chemical structures of these compounds can lead to the synthesis of more potent drugs with minimal toxicity. Plant parts that contain tannins are astringent in nature and have important roles as stable and potent antioxidants (Díaz-de-Cerio et al., 2016).The present results of the phytochemical screening of the leaves of Psidiumguajava L. revealed the presence of tannin, saponin, protein, steroids and phenol by positive reaction. Similarly, tannin, saponins, alkaloids, phenols, saponin, cardiac glycosides and carbohydrates found in the leaves of PsidiumguajavaL (Garode et al.,2014).
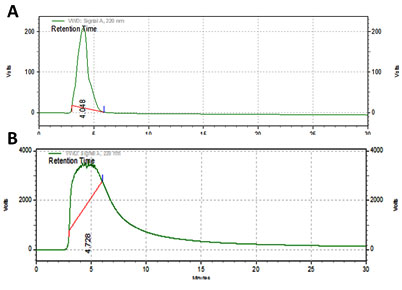
High performance liquid chromatography method has been validated to compare the ascorbic acid content in ethanolic extract of Psidiumguajavaleaves with the standard. The retention time is 4.728 min proved that ascorbic acid presence in the P.guajava leaves extracts.
(Figure 1). Similarly (Rahmanet al., 2018) reported that, HPLC analysis of P. guajava leaves exhibited the presence of gallic acid, in a high amount.The phenolic content was estimated as 49 mg of gallic acid equivalent/ g of dry material at 200ml concentration of the sample. Similarly, (Weni et al.,2011 ) reported that the ethanol extract of P. guajava leaves showed 201 mg/g of phenolic content.
Figure 1: HPLC Analysis of Sample (A) and Ascorbic acid (B)
The extracts of Psidiumguajava leaves showed potent antimicrobial activity against gram positive strains than gram negative strain and considerable activity against the fungal strain. As the concentration of the sample was increased, the radius of the zone of inhibition also increased (Table 1). Similarly,Harbone et al., (1984) showed the antibacterial activity of leaves extract of chloroform and ethanol of Psidium guajava L. against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi. It was found that ethanol extract showed maximum activity against S. typhi and lowest activity against S. aureus.
Table 1. Antimicrobial activity of Psidiumguajava leaves
| Concentration (µg/ml) | zone of inhibition (millimeter in diameter) | ||
| B. subtilis | A. niger | E. coli | |
| 20 | 7.5 | 7 | 5 |
| 40 | 12 | 15 | 8 |
| 60 | 15 | 30 | 12 |
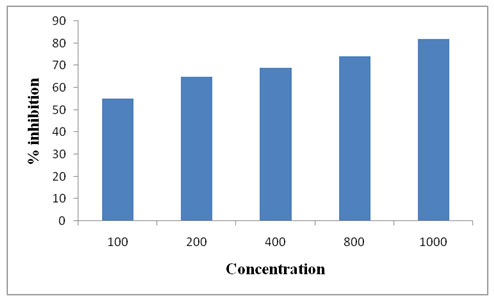
DPPH assay is considered as a valid method to evaluate scavenging activity of antioxidants, since the radical compound is very stable and do not have to generate as in other radical assays. DPPH radicals react with suitable reducing agents and then electrons become paired off and the solutions loses colourstoichiometrically with the number of electrons taken up such reactivity has been widely used to test the ability of plant extract to act as free radical scavengers. DPPH assay of ethanolic extract showed a dose dependent increase in the percentage of inhibition of free radicals (Lakkadi et al., 2013; Siwarungson et al., 2013). The ethanolic extract fraction was found to show a good antioxidant potential.The extract of Psidiumguajava leaves exhibited strong antioxidant activities and it was observed that as the concentration of the sample increases, the OD value decreases which indicates the increase in the antioxidant activity (Figure 2).
Figure 2: DPPH free radical scavenging activity of ethanolic extract of Psidiumguajava leaves
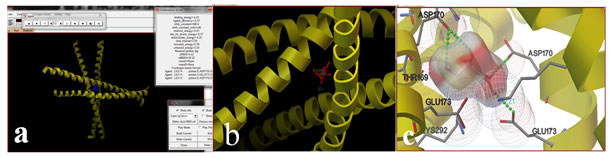
Figure 3: Binding of ascorbic acid with AP-1 (a) Closer view of binding (b) and Hydrogen bonding with amino acids (c)
Inflammation is a normal protective response to tissue injury caused by physical trauma, noxious chemicals or microbiologic agents. Inflammation is body’s response to inactivate or destroy the invading organisms, remove irritants and set stage for tissue repair. Inflammation is triggered by the release of chemical mediators from the injured tissues and migrating cells. The specific chemical mediators vary with the type of inflammatory process and include amines such as histamine, serotonin, and lipids such as prostaglandins and small peptides such as kinins (Jiménez-Escriget al., 2001). The anti-inflammatory activity of ethanolic extract of Psidiumguajava leaves carried out by HRBC stabilization method using diclofenac sodium as a standard revealed that the percentage of hemolysis decreases and the percentage of protection increases as the concentration of the sample increases (Table 2). Similarly, P. guajava extract reduced inhibitory percentage activities by 40.81, 55.45 and 43.61% (p< 0.05) respectively (SubbaRao et al., 2018).
Table 2. Anti-inflammatory activity
| Concentration (µg/ml) | Hemolysis (%) | Protection (%) |
| 250 | 76 | 24 |
| 500 | 56 | 44 |
| 750 | 50 | 50 |
| Diclofenac (100) | 8.8 | 91.2 |
Antioxidants and anti-melanoagents with redox properties can prevent or delay skin pigmentation by scavenging reactive oxygen species and reactive nitrogen species, known to induce melanin synthesis. They can also reduce o-quinones and other intermediates in the melanin biosynthesis thus delaying oxidative polymerization. Antioxidants in particular can prevent skin aging and degeneration of cells. It has often been noted that combinations of antioxidants and antimelanogents are more effective than one another acting independently. Thus high levels and quality of antioxidants and anti-melanogents, extracted from our suggested varieties of Thai Guava can not only be used as food supplements but also in cosmetic and pharmaceutical industries for developing products and drugs preventing skin aging and deterioration (Siwarungson et al., 2013).
The docking procedure was carried out with the ligand ascorbic acid and the target protein AP-1. 10 conformations were obtained out of which the binding energy was found to be the minimum for 10th conformation. The binding energy was found to be -4.43 (Fig 8, 9, 10). Similarly Lakkadi et al., (2018) reported that docking results reviled that molecules 12, 14 and 15 are the best active antioxidants for Poly (ADP- ribose) polymerase.
CONCLUSION
Psidiumguajava leaves are easily available and very cheap with abundant medicinal values. Their medicinal property such as antimicrobial and anti-inflammatory property was increases as the sample concentration increases. It exhibits strong antioxidant property by scavenging the free radicals generated by UV exposure, oxidative stress, environmental conditions etc., since it exhibits antioxidant property, it may invariable have antiaging property in which it maintains the healthy and normal integrity of the skin there by protecting the collagen network and reducing photo aging damages.
Authors Contributions: All authors have equal contribution in bringing out this research work.
Conflict of Interest: None.
REFERENCES
Aleksandra, Aleksandrova , Mariia Nesterkina , Svitlana Gvozdii , Iryna Kravchenko (2020)
Phytochemical analysis and anti-inflammatory activity of Cladophora aegagropila extract J Herbmed Pharmacol. 9(1): 81-85
Aliero, A.A. and Afolayan, A.J. (2006).Antimicrobial activity of Solanum tomentosum. African Journal of Biotechnology, 5(4), 369-372.
Androutsopoulos, V., Arroo, R.R., Hall, J.F., Surichan, S. and Potter, G.A. (2008).Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism.Breast Cancer Research, 10(3), R39.
Ashraf, A., Sarfraz, R.A., Rashid, M.A., Mahmood, A., Shahid, M. and Noor, N. (2016). Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidiumguajava leaf extracts. Pharmaceutical biology, 54(10), 1971-1981.
Bravo, L. (1998), Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews, 56 (11), 317-333.
Dharmanda, S., (2003).Gallnuts and the uses of tannins in Chinese Medicine-A paper presented at the Institute for Traditional Medicine.Portlant, Oregon, 3, pp.941-945.
Díaz-de-Cerio, E., Gómez-Caravaca, A.M., Verardo, V., Fernández-Gutiérrez, A. and Segura-Carretero, A. (2016). Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. Journal of functional foods, 22, pp.376-388.
Garode,AM and Waghode, S.M. (2014) Antibacterial activity of guava leaves extracts against S. mutans. Int. J. Bioassays, 3 (2), 1794‐1796.
Gulluce, M., Sahin, F., Sokmen, et al., (2007). Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food chemistry, 103(4), 3370 -3372
Harbone, JB., (1984) Phytochemical method-a guide to modern technique of plant analysis IInd ed. Champman and Hall, New York.:3-1
Jagadish, L.K., Krishnan, V.V., Shenbhagaraman, R. and Kaviyarasan, V. (2009).Comparitive study on the antioxidant, anticancer and antimicrobial property of Agaricus bisporus (JE Lange) Imbach before and after boiling.African Journal of Biotechnology, 8(4).654-661, 18.
Jimenez-Escrig, A., Rincon, M., Pulido, R. and Saura-Calixto, F. (2001).Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. Journal of Agricultural and food Chemistry, 49(11), 5489-5493.
Joseph, B. and Priya, R.M. (2010). Preliminary phytochemicals of Psidium guajava L. leaf of methanol extract and its cytotoxic study on HeLa cell lines. Inventi Rapid: Ethnopharmacology, 1(2),1121-15
Kim, J.H., Gupta, S.C., Park, B., Yadav, V.R. and Aggarwal, B.B. (2012). Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)‐κB and NF‐κB‐regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis. Molecular nutrition & food research, 56(3),454-465.
Korkina, L., Kostyuk, V., Potapovich, A., Mayer, W., Talib, N. and De Luca, C. (2018). Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics, 5(2), 32.
Lakkadi A, Vuppala S, Tigulla P. (2013) Novelin vitro antioxidant estimation of phenolic compounds and molecular modeling studies. International research journal of pharmacy 2. 148-152
Lakkadi, A., Vuppala, S. and Tigulla, P.(2018) Novel in vitro antioxidant estimation of phenolic compounds and molecular modeling studies.International Research Journal of Pharmacy. 4(9), 148-152.
Nadia L, Giovanna M. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells Immunobiology 2016; 221 (3). 486-493
Osseni, P. Rat, A. Bogdan, J.M. Warnet, Y. Touitou Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2 Life Sci., 68 (2000), pp. 387-399
Rahman, M.M., Zaman, S., Mamun, F., Gias, Z.T., Alam, M.N., Ulla, A., Hossain, M.H., Reza, H.M. and Alam, M.A. (2018). Phenolic content analysis in Psidium guajava leaves powder by HPLC‐DAD system and in vivo renoprotective and antioxidant activities in fludrocortisone acetate‐induced rats. Journal of food biochemistry, 42(6), 12687.
Roberta M, Roberta Di B, Rosaria V, Carmela F, Claudio G. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes The Journal of Nutritional Biochemistry.2005; 16(10) 577-586
Simbula G., Columbano A., Ledda-Columbano G.M., Sanna L., Deidda M., Diana A., Pibiri M. (2007).Increased ROS generation and p53 activation in α-lipoic acid-induced apoptosis of hepatoma cells. Apoptosis.12:113–123
Siwarungson, N., Ali, I. and Damsud, T. (2013).Comparative analysis of antioxidant and antimelanogenesis properties of three local guava (Psidium guajava L.) varieties of Thailand, via different extraction solvents. Journal of Food Measurement and Characterization, 7(4), 207-214.
SubbaRao Ch, Arun Kumar S, Javvad Ali, Priyadarshini P, Hinduja M, Keerthi R, Nivedita L, Priyanka D. In vitro antioxidant activity of Psidium guajava Linn.by using ethanolic extract fraction of leaves and bark. Adv Cell Sci Tissue Cult. 2018, 2 (1), 19-22.
Venkatachalam, R.N., Singh, K. and Marar, T. (2012).Phytochemical screening in vitro antioxidant activity of Psidium guajava.Free Radicals and Antioxidants, 2(1), pp.31-36.
Weni, L., Harliansyah, H. and Widayanti, W. (2011). Anti-inflammatory activity of the extract of guava leaves (Psidium guajava L) in the rat (Rattus norvegicus L). Indonesian Journal of Cancer Chemoprevention, 2(1), pp.169-172.