1Assistant Professor, Department of Zoology, DAV College, Kanpur, U.P, India
2Department of Zoology, Government PG College, Ratlam, M.P, India
Corresponding author email: reddysirr@gmail.com
Article Publishing History
Received: 11/07/2020
Accepted After Revision: 15/09/2020
In the present study we have investigated various enzymatic (SOD, CAT and GST) and non-enzymatic antioxidant parameters (GSH and MDA/LPO) and histopathological biomarkers in the liver and kidney of Mystus tengara collected from the upstream and downstream of Chambal River. Results revealed that the activities of antioxidant enzymes like SOD, Catalase (CAT) and glutathione S-transferase (GST) activities were significantly higher in both the tissues of fish from downstream than from reference site (p < 0.05), demonstrating initiation of antioxidant defense mechanisms. Similarly, LPO levels (MDA) were elevated in both the tissues of fish from the downstream site than reference site while reduced glutathione (GSH) concentrations in both the tissues were significantly decreased in the fish of downstream (P<0.05). The histopathology of the liver of fish from downstream exhibited marked differences like vacuolization, hemorrhage, presence of glycogen granules, necrosis, dilation of sinusoids and congestion of blood vessels while kidney showed a reduction in Bowman’s capsule space, degeneration of glomerulus, hemorrhage, necrosis vacuolation and reduction of tubular lumens.
The histopathological changes were evidently associated with contamination, being more severe in kidney than the liver. The activities of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT) and glutathione-S-transferase (GST) were significantly increased in renal tissue than liver tissues which were clearly reflected also in histopathology. These results point out that wastewater from urban and neighbouring industries discharged into downstream of the river provoked the most significant oxidative stress in the native fish which was also reflected in histopathology. On the whole, the current study recommends that the biomarkers of oxidative stress along with histopathological studies can serve as a valuable tool for examining the adverse effects of wastewater effluents on fish.
Biomarkers, Chambal River, Histopathology, In Situ Assessment, Oxidative Stress
Srivastava B, Reddy P. B. In Situ Stress Assessment of the Impact of River Pollution in a Catfish, Mystus tengara using Histopathological and Oxidative Biomarkers. Biosc.Biotech.Res.Comm. 2020;13(3).
Srivastava B, Reddy P. B. In Situ Stress Assessment of the Impact of River Pollution in a Catfish, Mystus tengara using Histopathological and Oxidative Biomarkers. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/31rIRGk
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Freshwater environs are frequently used as dump yards of the industrial and urban wastes. Such anthropogenic activities severely affect the aquatic ecosystem and its biota. Anthropogenic activities are the main cause of aquatic pollution. Several organic and inorganic chemicals like plastics, pharmaceuticals, insecticides, and heavy metals have frightening impacts on freshwater ecosystems (Reddy 2016, Srivastava and Reddy 2019). Yet, our knowledge to predict their adverse effects correctly is still inadequate. Periodically, the organisms undergo for local adaptation or maladaptation upon the exposure to chronic pollution which could cause a high intraspecific unevenness of sensitivity among wild populations (Jacquin et al, 2020).
Many pollutants, especially the heavy metals and xenobiotics present in wastewater are regarded as most hazardous due to their and persistent and non-degradable nature (Fatta-Kassinos et al, 2011, Kumar et al, 2019, Kumar et al, 2020). Many of these pollutants induce toxic effects through the production of reactive oxygen species (ROS) (Reddy 2017). The oxidative degradation of lipids (lipid peroxidation, LPO) is the widespread cause of reactive oxygen species (ROS) and therefore universally employed as a biomarker of fish health (Srivastava and Reddy 2017, Renuka et al, 2019, Brahma and Gupta 2020).
A number of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione S transferase (GST) and non-enzyme reduced glutathione (GSH) etc. are capable of protecting the tissues by stabilizing, neutralizing and or deactivating the effects of free radicals (oxidative damage). The role of these antioxidant enzymes against lots of toxicants was well studied in mammals (Kapoor et al 2010, Bhowmick et al 2015, Verma et al 2016, Singh et al 2018, Sutradhar et al, 2020) but still, such studies are comparatively scarce in fish species which can be effectively used as biomarkers for aquatic pollution (Javed et al, 2016, Srivastava and Reddy 2017, Louiz et al 2018, Corredor-Santamaría et al 2019, Tenji et al, 2020).
It is well-known fact that the results of oxidative damage is directly associated with histopathology of the tissues (Alchalabi et al 2016, Wei et al 2019). Therefore, the studies on histopathological examination of target tissues along with the examinations of oxidative stress markers would provide an inclusive risk assessment and toxic potential of River pollution in fish and other organisms (Ratn et al 2018, Awasthi et al, 2019, Kucukler et al 2020).Hepatic, branchial and renal tissues of the fish are predominantly used as target organs for biomarker studies (Camargo and Martinez 2007, Kroon et al 2017, Mohamed et al 2020).
The liver and kidney of the fish is the major metabolic and vital excretory organ respectively. The failure of kidney function leads bioaccumulation of pollutants which cause major deformities in the renal tissue. Periodic examination of these biomarkers in fish may offer an estimation of both ecosystem and fish health. However, as pollutants are usually present as complex mixtures in the natural environment, the correct assessment and prediction of probable toxicity is a big challenge (Dévier et al 2011, Altenburger et al 2019, Kumari and Kumar 2020). Because of this complicatedness, the use of biomarker responses of an organism to a stressor as a tool at the individual, tissue, cellular, molecular levels is well established in ecotoxicological studies (Reddy 2012 a, b, Reddy and Baghel 2012, Reddy 2016, Srivastava and Reddy 2017, 2019).
Fish may ingest a cocktail of pollutants through the food chain, gills and skin. Fractions of certain chemical pollutants can accumulate in tissues and cause weakening of fish health. A number of pollutants in freshwater ecosystem could lead to oxidative stress in exposed populations. Our earlier studies performed in the Chambal River at Nagda (M.P.india) confirmed municipal and industrial pollution in this region (Reddy 2012 a, b, and 2017). Therefore, the present study is aimed to explore the association of oxidative stress and histopathological injuries induced by aquatic pollution of Chambal River in the liver and kidney of Mystus tengara which is commonly prevalent in this zone.
MATERIAL AND METHODS
Fish collection: The live adult tengara catfish, (Mystus tengara, Hamilton, 1822) irrespective of the sex and of similar size and weight (n = 10), (8.3 ± 0.6cm; 7.2 ± 0.42g) were caught at two different zones (upstream and downstream) by means of a cast net with the help of skilled local fisherman, of the Chambal River at Nagda, Ujjain (23’27N and 75’25), (M.P.India) during winter months of 2018. The fish were placed in two separate containers (upstream and downstream) with river water and immediately transported to the laboratory for histopathological and oxidative stress examinations. Fish were washed and anaesthetized by 0.1 g/L of benzocaine and liver and kidney tissues were dissected out for the study of enzymatic and non-enzymatic antioxidants and histopathological studies.
Homogenate preparation: Both hepatic and renal tissues were taken out of the fish body. They were washed carefully with phosphate buffer and soaked. Afterwards, 10% of homogenate was prepared using homogenizing buffer (50 mM Tris -HCl mixed with 1.15% KCl at pH 7.4) by using a Teflon tissue homogenizer (Remi, India). The tissue homogenate was centrifuged (Refrigerated centrifuge Remi, India) at the 10,000 rpm for 20 min at -40C and the collected supernatant was directly stored in aliquots at -200C in glass vials for further analysis.
Oxidative stress markers: Antioxidant enzymes: Superoxide dismutase (SOD): The activity of Superoxide dismutase (SOD) in both hepatic and renal tissue was determined by an indirect method given by Marklund and Marklund (1974) with slight modifications. The technique is based on the ability of superoxide dismutase (SOD) to inhibit the autoxidation of pyrogallol into a yellow solution. The absorbance can be measured at 420 nm and expressed as µ/mg protein.
Catalase: Catalase (CAT) is a universally known antioxidant enzyme that degrades the hydrogen peroxide (H2O2) into water and oxygen. The activity of CAT was calculated by monitoring the decline in absorbance of H2O2 at 240 nm and expressed as μmol/mg protein/min (Aebi, 1984). Glutathione S-Transferase (GST): GST activity was measured by the procedure given by Pabst et al (1974) with few slight modifications. This method is based upon the ability of GST to conjugate 1-chloro-2, 4-dinitrobenzene (CDNB) to reduced glutathione. The absorbance of conjugation is determined at 340 nm and expressed as units /mg protein.
Non-enzymatic Antioxidants: Reduced glutathione (GSH): GSH is the common intracellular low-molecular-weight thiol. It involves metabolic defensive roles, including reduction of hydroperoxide, detoxification, and free radical scavenging (Reddy 2016). With few modifications, the levels of GSH were determined as per the protocol of Jollow et al. (1974) and the absorbance was measured at 412 nm. Lipid Peroxidation (LPO): Lipid Peroxidation in both hepatic and renal tissue homogenates was estimated by the method given by Buege and Aust (1978). It was determined by quantifying the formation of thiobarbituric acid reactive substances (TBARS) which enumerated as malondialdehyde (MDA) equivalents and the absorbance was measured spectrophotometrically at 530 nm.
Histopathology: Hepatic and renal tissues of downstream and reference site fishes were taken out and fixed in Bouin’s fluid for 48h. Tissues were cleaned under running tap water and dehydrated in ascending grades of alcohol, cleared twice in xylene and finally embedded in paraffin wax and 7µ thick sections were made by using a rotatory microtome. Sections were cleared in xylene, hydrated in serial dilutions of alcohol, stained with haematoxylene and eosin and finally mounted with DPX. The stained micro sections were evaluated under a compound microscope (Olympus BX46) and photographed by using the Omax 8.0MP Digital USB Microscope Camera.
Statistical analysis: The results of the current investigation were expressed as mean and standard error (mean ± standard error mean) for all the parameters. The data were tested for the significance by employing the software of student ‘t’test in Microsoft Excel Windows 10 version.
Results AND DISCUSSION
Water quality is vital as it plays a central role in regulating various metabolic and physiological processes. The results of our earlier publication evidently revealed that physicochemical properties of surface water at downstream of Chambal River at Nagda, Ujjain (M.P.India) exceed the standard limits of CPCB.
Enzymatic and Non- enzymatic Antioxidant parameters: The activities of enzymatic and non-antioxidant parameters in the liver and kidney of Mystus tengara from the Chambal River at Nagda are presented in Table 1. Results clearly reveal that the SOD, CAT and GST activities were significantly elevated in both hepatic and renal tissues of fish from the downstream ((P>0.05) but the percentage of increase was much higher in kidney than in the liver. The percentage increase of SOD, CAT and GST activities were 115.1% and 103.4%, 78.99% and 277.2% and 80.77% and 94.96% for liver and kidney respectively.
Table 1. Enzymatic and non-enzymatic activities in liver and kidney of Mystus tengara, Values are mean ±SE of six individual observations. Comparisons of means (upstream and downstream fish) were done by Student’s t-test. * Significant at 5% level
(p <0.05).
| Parameter | LIVER | KIDNEY | ||||
| Upstream | Downstream | % | Upstream | Downstream | % | |
| Superoxide dismutase (SOD)
U/mg protein |
11.2 ±0.6 | 24.1± 3.3* | 115.1 | 26.2 ± 3.1 | 53.3± 5.2* | 103.45 |
| Catalase (CAT) n mole/
min/mg protein |
11.9 ±0.7 | 21.3 ±4.1* | 78.99 | 2.2 ± 0.4 | 8.3± 0.8* | 277.2 |
| Glutathione-S-transferase (GST) µmole/ min/mg protein | 82.4 ±5.3 | 148.6±12.6* | 80.77 | 53.6 ± 4.1 | 104.5 ± 9.7* | 94.96 |
| lipid peroxidation (MDA)
n mole/ hr/mg protein |
7.1± 0.31 | 29.3± 3.9* | 312.6 | 4.1 ± 0.3 | 24.2 ± 2.2* | 490.2 |
| Reduced glutathione (GSH)
n mole/mg protein |
158.6 ±9.8 | 97.2 ± 7.2* | -38.7 | 133.8 ± 9.7 | 53.3± 5.4* | -60.1 |
The lipid peroxidation levels (MDA) formation in both liver and kidney of Mystus tengara from upstream and downstream were displayed in Table 1. Results from Table 1 clearly reveal that MDA levels were significantly elevated in both the tissues of the fish from downstream of the River compared to that of control fishes from the reference site (upstream). The percentage of increase in MDA levels was significant (P<0.001) and were found as 312.6% and 490.2% in liver and kidney respectively. However, the levels of GSH in both the tissues (liver and kidney) significantly reduced compared to that of control (upstream). The percentage increase in GSH levels were 81%, and 83% respectively for liver and kidney.
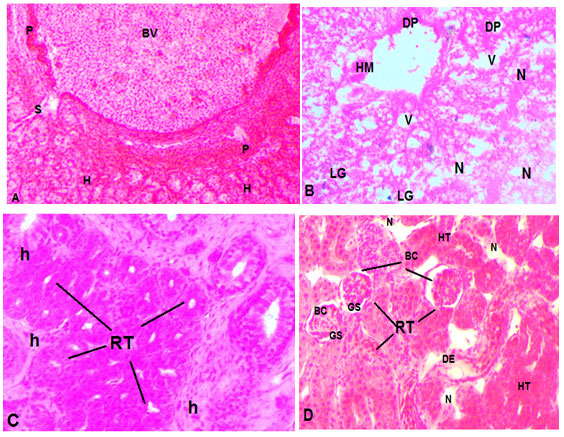
Histopathological studies: The specifications of histopathological examinations in the liver and kidney of M.tengara from the reference site (upstream) and downstream site are shown in Figs. 1&2 respectively. Fish liver from reference site showed normal architecture (Fig1) with central veins, sinusoids, normal hepatocytes and glycogen granules. However, the micro sections of liver from downstream fish had shown several histopathological anomalies like necrosis, vacuolation, ruptured and congested central vein and few broken hepatocytes. Aggregation of Melanomacrophages (MMC) and a higher amount of glycogen granules was also seen (Fig1.B).
The micro sections of the kidney of fish from upstream (control) exhibited normal structure with Bowman’s capsule (BC), renal tubules (RT), epithelial cells (EC) glomeruli (G), proximal convoluted tubules (PCT), and distal convoluted tubules (DCT) with brush borders (BB) (Fig 3). However, the photomicrograph of renal tissue from downstream showed several pathological lesions including a reduction in Bowman’s capsule space, degeneration of glomerulus, necrosis vacuolation and reduction of lumens (Fig 2.C&D).
Figure 1 A & B: Photomicrographs of the liver of Mystus tengara inhabiting in reference and polluted water. (A): Reference fish liver (B) Exposed fish liver; BV (blood vessel), H (hepatocyte), HM (hemorrhage) LG (lipid granule), P (pancreatic tissue), S (sinusoid), V (vacuolization). C &D. Photomicrographs of the kidney of Mystus tengara inhabiting in reference and polluted water. (C): Reference fish kidney (D) Exposed fish kidney; BC (Bowman’s capsule), DE (degeneration of epithelium) GS (Glomerular shrinkage), N (necrotic cell), RT (renal tubule), H (haemopoeitic tissue), HT (hypertrophy) LG (lipid granule), P (pancreatic tissue), S (sinusoid), V (vacuolization). Sections were prepared from multiple fish liver and kidney tissues (four animals). All the sections were stained with haematoxylene and eosin and photomicrographs were taken using a light microscope with 400×magnification.
Earlier studies performed in the Chambal River at Nagda (M.P.india) confirmed municipal and industrial pollution in this region (Reddy and Renu Singh 2011, Reddy 2012 a, b, and 2017). The River Chambal at Nagda (M.P.India) is receiving approximately 18,500 to 19000 kl./day treated effluent from various industrial complexes and about 8000 Kl/day urban untreated wastewater at Juna Nagda area (downstream) which is found to be a major source of river pollution (Reddy and Baghel 2012, MPCB 2019). Fish species are so sensitive to changing the water quality and are subject to encounter with several types of chemical pollutants. Several researchers have conducted experiments to study the harmful effects of pollutants and many of those are linked it with the induction of oxidative and such studies will be useful to prevent or minimize the impacts of oxidative stress in animals stress (Reddy 2016, Srivastava and Reddy 2019).
In the present study, we estimated the impact of pollution on certain biomarkers of oxidative stress and histopathological biomarkers of aquatic pollution in a native catfish Mystus tengara from the Chambal River at Nagda (M.P.india). Several researchers reported that environmental pollutants could lead to the formation of excessive free radicals which cause oxidative stress and disturb cellular homeostasis in fishes (Lackner 1998, dos Santos Carvalho et al 2012, Yadav et al 2015). Oxidative stress markers like SOD, CAT, GST and GSH can serve as perceptive bioindicators of aquatic pollution in fishes (Javed et al 2016, 2017, Reddy 2016, Srivastava and Reddy 2017).
SOD converts the superoxide radical anion (O.−2·) to H2O2. CAT counteracts or decomposes the toxic effects of H2O2. GST is the phase II type of detoxifying enzyme that protects the cellular macromolecules from ROS. Activities of enzymatic (SOD, CAT and GST) and non-enzymatic antioxidant parameters (LPO/MDA and GSH) were analyzed in hepatic and renal tissues to determine the impact of pollution on oxidative stress of liver and kidney of the fish and summarized in Table 1.
Results (Table 1.) clearly revealed a significant (p<0.05) and elevated levels of SOD, CAT, GST and reduced levels of GSH in the liver and kidney of the exposed fish compared to fish from upstream (reference site). The arrangement of SOD/CAT may be the earliest defense mechanism against ROS which are produced by the induction of pollutants. (Sharma et al 2012). The observed increase in SOD and CAT levels in liver and kidney of the fish exposed to wastewater specifies a strong detoxifying mechanism against the pollution-induced toxicity. We found similar interpretation in the hepatic tissue of the same fish, Mystus tengara exposed to wastewater in downstream of the river (Reddy 2016).
Similar results have also been documented by many workers in gill, liver and kidney of other fish species ( Javed et al 2016, 2017, Reddy 2016, Srivastava and Reddy 2017, Tyor and Pahwa 2017, Ratn et al 2018, Kumar et al 2019, Chowdhury and Saikia 2020). Enhanced levels of CAT is frequently observed in various fish species frequently in the presence of ecological pollutants (Yadav et al 2015, Reddy 2016) as CAT in combination with superoxide dismutase SOD) stands for the first line of defence against oxidative stress (Ighodaro and Akinloye 2018). For that reason, increased levels of CAT in the current investigation reflect a tough and resistant antioxidant response generated by wastewater.
The elevated GST activities in liver and kidney of the Mystus tengara from the downstream of the Chambal River could be provoked to defend against the toxicity of pollutants. Similarly, the study of Samanta et al (2016) showed elevated levels of antioxidant enzymes like SOD, CAT and LPO levels in association with histopathological alterations in liver and kidney of a crucian carp Carassius auratus exposed to sewage water. In the same way, Samanta et al (2018) again confirmed increased activities of antioxidant enzymes along with histopathological anomalies in three fish species collected from different water streams of Korea. In another study, Chang et al (2019) showed that urban effluent can cause oxidative damage by increasing MDA content and antioxidant enzymes in the liver of C. auratus.
The recent study of Huang et al (2020) confirmed that pollutants of UV filters used in personal care products can cause oxidative damage by inducing ROS and alterations in SOD, GST, GSH, and MDA in the hepatic tissue of zebrafish. The study had shown the enhancement of SOD, CAT, and GPx activities, as well as the reduction in GSH content. But, in contrast, dos Santos Carvalho et al (2012) observed decreased SOD and increased GST in the liver of fish Tilapia (Oreochromis niloticus) from downstream of Monjolinho River (Brazil). The in situ assessment of Kim and Jung (2016) has shown a significant decrease in the enzyme activities of CAT, SOD, and GST in fish (Z. platypus) liver from the downstream. Apart from the antioxidant defence mechanisms, the reduced glutathione (GSH) (non-enzymatic) can also aid in the protection of the cell by scavenging of ROS/free radicals. Glutathione (GSH) is a tripeptide and serves as an important cofactor for antioxidant enzymes like GST and GPx. In the present experiment, GSH levels were significantly (P< 0.05) reduced in liver and kidney of exposed fish (Table 1). The reduction in GSH in hepatic and renal tissues of the exposed fish could be due to its oxidation to GSSG which occur during higher oxidative stress (Reddy 2016).
The induction of antioxidant enzymes takes place as a protection mechanism against the increased production of ROS. However, the responses of antioxidant parameters to pollution are not identical but differ for different species tissues and the amount of single or mixed pollutants. Great, variation can be found in wild situations (Livingstone 2001, Aljahdali and Alhassan 2020). The outcomes of this study suggest that the fish exploits both enzymatic and non-enzymatic mechanisms to abide by the effects ROS induced oxidative stress. For that reason, quantification of enzymatic and non-enzymatic (SOD, CAT, GST and GSH) constraints has been established as useful biomarkers of environmental pollution.Lipid peroxidation (LPO) is one of the major actions linked with cellular damage which expresses itself in the form of tissue injuries due to oxidative stress. Lipid peroxidation (LPO) changes the organization of cell membranes and affects the physiological functions of cell membranes (Reddy 2016, Srivastava and Reddy 2017).
In the present study, the MDA levels (lipid peroxidation product) were significantly (P<0.05) higher in both liver and kidney of Mystus tengara exposed to polluted water. The higher MDA levels potentially denote the damage of cell membranes. We found similar rationalization in the hepatic tissue of the same fish, Mystus tengara exposed to wastewater in downstream of the river (Reddy 2016). The elevated levels of LPO serve as a compensatory mechanism against surplus production of ROS/free radicals due to less effective antioxidant defence mechanism. The increased enzyme activities are indicative of the beginning of self-defence actions to alleviate impacts of ROS and free radicals to reinstate the redox balance and homeostasis in cells. The enhanced LPO and CAT, SOD, and GST in fish from downstream signify as combat mechanism against overproduction of ROS to minimize harm to the fish. The outcomes of current investigation evidently imply that the fish inhabiting downstream water were subjected to oxidative stress because of the high amount of pollutants and inadequate levels of antioxidants. Therefore, liver and kidney tissues were further processed for histopathological examinations.
A number of researchers applied histopathological signs as direct indicators of chemical exposures (Reddy and Rawat 2013, Javed et al 2016, Kumar et al 2017, Samanta et al 2016, 2018, Nofal 2019, Weber et al 2020). The liver is the chief metabolic organ and is the site for xenobiotics metabolism, detoxification and elimination of the toxicants. Exposure to xenobiotics induced histopathological changes in the hepatic tissue such as necrosis and hypertrophy. The apparent tissue damage in the liver and kidney might also be linked to the impact of oxidative stress. It is quite oblivious that the interactions of both ROS and toxicants interact with cellular apparatus cause tissue lesions and other forms of damage. The extensive accumulation of fats and portal swelling observed in the hepatic tissue reveals the detrimental effects of wastewater on native catfish. Accumulation of fat is a general cellular reaction to toxic pollutants that affects the lipid metabolism and weakens liver function. In such conditions, fish may be susceptible to parasitic infections due to reduced immunity (George et al 2017, Tan et al 2018).
Reduction in the immunity, enhanced secondary infections, impaired health in fish could ultimately result in a reduction in the reproduction process and an overall decline in the fish catch. The kidney is an important haemopoeitic and osmoregulatory organ in fish. It is also the site of excretion and transformation of xenobiotics. For this reason, entry of pollutants through the branchial artery may potentially induce histopathological alterations and alter the biochemical composition. Therefore, the expected pathological alterations in renal tissue can be used as biomarkers of environmental pollution. The histopathological assessment of kidney demonstrated that fish exposed to pollution-induced the manifestations of degeneration of tubular epithelium, necrosis, hemorrhages, and shrinkage of the glomerulus and decreased the space between glomerulus and Bowman’s capsule. Similar results were found in Channa punctatus from sugar mill effluent at Aligarh, India (Javed et al, 2016), Oreochromis mossambicus from Bhima River of Maharashtra, India (Kumar N et al, 2017), Carassius auratus from Sincheon stream of Korea (Samanta, P et al, 2018), Oreochromis niloticus from Manzala fish farm of Egypt (Nofal, M.I 2019) and Hoplias intermedius and Hypostomus affinis from Doce River basin (Weber, A.A et al 2020).
CONCLUSION
This study has evidently shown that fish in downstream of the river live in the polluted environment was experienced from oxidative stress and tissue damage which consequently affects the fish growth, vulnerability to diseases and reproductive survival. The results in the current investigation clearly exhibit the threat posed by continuous discharge of treated effluents in the River may impair the fish health by inducing oxidative stress. The adverse effects of wastewater on the native and non-target catfish species is of concern as it may impair not only the fish health but also to the residents of the Nagda and neighbouring villagers who depend on the river for irrigation and for fish. The study recommends conducting more inclusive in situ measurements are required in order to resolve the current health status of fish populations in the Chambal River at Nagda (M. W.India).
Conflict of Interest: Authors declares no conflicts of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of DAV College, Kanpur, U.P, India.
REFERENCES
Aebi H (1984) Catalase in vitro. Methods Enzymology 105 Pages 121–126.
Alchalabi AS Rahim H Aklilu E Al-Sultan II Malek MF Ronald SH Khan MA (2016) Histopathological changes associated with oxidative stress induced by electromagnetic waves in rats’ ovarian and uterine tissues. Asian Pacific Journal of Reproduction1; 5(4) Pages 301-10.
Aljahdali MO and Alhassan AB (2020) Ecological risk assessment of heavy metal contamination in mangrove habitats, using biochemical markers and pollution indices: A case study of Avicennia marina L. in the Rabigh lagoon, Red Sea. Saudi Journal of Biological Sciences 4 Pages 1174-1184.
Altenburger R Brack W Burgess RM Busch W Escher BI Focks A Hewitt LM Jacobsen BN de Alda ML Ait-Aissa S Backhaus T (2019) Future water quality monitoring: improving the balance between exposure and toxicity assessments of real-world pollutant mixtures. Environmental Sciences Europe 31(1) Pages 1-7.
Awasthi Y Ratn A Prasad R Kumar M Trivedi A Shukla JP Trivedi SP (2019) A protective study of curcumin associated with Cr6+ induced oxidative stress, genetic damage, transcription of genes related to apoptosis and histopathology of fish, Channa punctatus (Bloch, 1793). Environmental toxicology and pharmacology 1; 71 Pages 103209.
Bhowmick D Srivastava S D’Silva P Mugesh G (2015) Highly efficient glutathione peroxidase and peroxiredoxin mimetics protect mammalian cells against oxidative damage. Angewandte Chemie. 13; 127(29) Pages 8569-73.
Brahma N and Gupta A (2020) Acute toxicity of lead in fresh water bivalves Lamellidens jenkinsianus obesa and Parreysia (Parreysia) corrugata with evaluation of sublethal effects on acetylcholinesterase and catalase activity, lipid peroxidation, and behavior. Ecotoxicology and Environmental Safety 189Page.109939.
Buege JA and Aust SD (1978) Microsomal lipid peroxidation. In Methods in Enzymology Academic Press. 52 Pages 302-310.
Camargo MM and Martinez CB (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotropical Ichthyology, 5(3) Pages 327-336.
Chang T Wei B Wang Q He Y Wang C (2019) Toxicity assessment of municipal sewage treatment plant effluent by an integrated biomarker response in the liver of crucian carp (Carassius auratus). Environmental Science and Pollution Research 27 Pages 1-9.
Chowdhury S and Saikia SK (2020) Oxidative Stress in Fish: A Review. Journal of Scientific Research, 12(1) Pages145-160.
Corredor-Santamaría W Torres-Tabares A Velasco-Santamaría YM (2019) Biochemical and histological alterations in Aequidens metae (Pisces, Cichlidae) and Astyanax gr. bimaculatus (Pisces, Characidae) as indicators of river pollution. Science of The Total Environment 20; 692 Pages 1234-41.
Dévier MH Mazellier P Ait-Aissa S Budzinski H (2011) New challenges in environmental analytical chemistry: identification of toxic compounds in complex mixtures. Comptes Rendus Chimie 1; 14 (7-8) Pages766-79.
dos Santos Carvalho C Bernusso VA de Araújo HS Espíndola EL Fernandes MN (2012) Biomarker responses as indication of contaminant effects in Oreochromis niloticus Chemosphere 1; 89(1) Pages 60-9.
Fatta-Kassinos D Kalavrouziotis IK Koukoulakis PH and Vasquez MI (2011) The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Science of the Total Environment, 409 (19) Pages 3555-3563.
George OO Amaeze NH Babatunde E Otitoloju AA (2017) Genotoxic, Histopathological and Oxidative Stress Responses in Catfish, Clarias gariepinus, Exposed to Two Antifouling Paints. Journal of Health and Pollution 7 (16) Pages 71-82.
Huang X Li Y Wang T Liu H Shi J Zhang X (2020) Evaluation of the Oxidative Stress Status in Zebrafish (Danio rerio) Liver Induced by Three Typical Organic UV Filters (BP-4, PABA and PBSA). International Journal of Environmental Research and Public Health 17 (2) Pages 651.
Ighodaro OM and Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria journal of medicine, 54(4) Pages 287-293.
Jacquin L Petitjean Q Côte J Laffaille P Jean S (2020) Effects of Pollution on Fish Behavior, Personality, and Cognition: Some Research Perspectives. Frontiers in Ecology and Evolution 7; 8 Pages 86.
Javed M Ahmad I Usmani N Ahmad M (2016) Studies on biomarkers of oxidative stress and associated genotoxicity and histopathology in Channa punctatus from heavy metal polluted canal. Chemosphere 1; 151 Pages 210-9.
Javed M Ahmad MI Usmani N Ahmad M (2017) Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Scientific reports 7(1) Pages 1-1.
Jollow DJ Mitchell JR Zampaglione NA Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3) Pages 151-69.
Kapoor U Srivastava MK Bhardwaj S Srivastava LP (2010) Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its No Observed Effect Level (NOEL). The Journal of toxicological sciences 1; 35 (4) Pages 577-81.
Kim WK and Jung J (2016) In situ impact assessment of wastewater effluents by integrating multi-level biomarker responses in the pale chub (Zacco platypus). Ecotoxicology and Environmental Safety 128 Pages 246-251.
Kroon F Streten C and Harries S (2017) A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PloS one, 12(4) Pages 0174762.
Kucukler S Benzer F Yildirim S Gur C Kandemir FM Bengu AS Ayna A Caglayan C Dortbudak MB (2020) Protective Effects of Chrysin Against Oxidative Stress and Inflammation Induced by Lead Acetate in Rat Kidneys: a Biochemical and Histopathological Approach. Biological Trace Element Research 29 Pages 1-4.
Kumar D Malik DS Patel SL and Gupta V (2019) Human health risk assessment and mitigation of heavy metal pollution in agriculture and environment. Contaminants in Agriculture and Environment: Health Risks and Remediation, 1 Page 66.
Kumar M Gupta N Ratn A Awasthi Y Prasad R Trivedi A and Trivedi SP (2020) Biomonitoring of heavy metals in river ganga water, sediments, plant, and fishes of different trophic levels. Biological trace element research 193(2) Pages 536-547.
Kumar N Krishnani KK and Singh NP (2019) Oxidative and cellular metabolic stress of fish: an appealing tool for biomonitoring of metal contamination in the Kolkata wetland, a Ramsar site. Archives of environmental contamination and toxicology, 76(3), pp.469-482.
Kumar N Krishnani KK Gupta SK Singh NP (2017) Cellular stress and histopathological tools used as biomarkers in Oreochromis mossambicus for assessing metal contamination. Environmental Toxicology and Pharmacology 49 Pages 137-47.
Kumari M and Kumar A (2020) Identification of component-based approach for prediction of joint chemical mixture toxicity risk assessment with respect to human health: A critical review. Food and Chemical Toxicology Pages 111458.
Lackner R (1998) Oxidative stress in fish by environmental pollutants. In: Fish ecotoxicology Birkhäuser Basel Pages 203-224.
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine pollution bulletin, 42(8) Pages 656-666.
Louiz I Palluel O Ben-Attia M Aït-Aïssa S Hassine OK (2018) Liver histopathology and biochemical biomarkers in Gobius niger and Zosterisessor ophiocephalus from polluted and non-polluted Tunisian lagoons (Southern Mediterranean Sea). Marine pollution bulletin1 (128) Pages 248-58.
Marklund S Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European journal of biochemistry 47(3) Pages 469-74.
Mohamed AA El-Houseiny W Abd Elhakeem EM Ebraheim LL Ahmed AI Abd El-Hakim YM (2020) Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicology and environmental safety 188 Pages109890.
MPCB (2019) Report on prevention & control of pollution in River Chambal. An action plan for rejuvenation. http://www.mppcb.mp.gov.in/proc/poll-st A PLAN% 2019/ NGT Chambal.pdf.
Nofal MI (2019) Effects of heavy metal pollution on Nile tilapia in Manzala farm: Oxidative stress biomarkers and histopathological findings. International Journal of Fisheries and Aquatic Studies 7(5): 315-328.
Pabst MJ Habig WH Jakoby WB (1974) Glutathione S-transferase a novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. Journal of Biological Chemistry. 1974 Nov 25; 249(22):7140-8.
Ratn A Prasad R Awasthi Y Kumar M Misra A Trivedi SP (2018) Zn2+ induced molecular responses associated with oxidative stress, DNA damage and histopathological lesions in liver and kidney of the fish, Channa punctatus (Bloch, 1793). Ecotoxicology and environmental safety 30; 151 Pages 10-20.
Reddy PB (2012a) Histopathogical studies as potential and direct biomarkers of pollution. Trends in Life sciences, 1(1) Pages 27-31.
Reddy PB (2012b) Evaluation of potential biomarkers for effluent induced hepatotoxicity. International Journal on Applied Bioengineering 6(2).
Reddy PB (2016) Study of pollution induced oxidative stress in a cat fish (Mystus tengara) European Journal of Biomedical, 3(12) Pages 595-600.
Reddy PB (2017) Study on the toxic effects of wastewater in catfish (Heteropneustes fossilis Life Sciences International Research 5(2) Pages 165-174.
Reddy PB (2017) Study on the toxic effects of wastewater in catfish (Heteropneustes fossilis. Life Sciences International Research 5(2) Pages 165-74.
Reddy PB and Baghel BS (2012) Impact of Industrial waste water on the Chambal River and Biomarker responses in fish due to pollution at Nagda. MP India. DAV Int. J. Sci, 1(1) Pages 86-91.
Reddy PB and Rawat SS (2013) Assessment of aquatic pollution using histopathology in fish as a protocol. International Research Journal of Environment Sciences, 2(8) Pages 79-82.
Reddy PB Singh RK (2011) Biomarker responses in fish exposed to industrial effluent. In: International Conference on Green technology and environmental Conservation (GTEC-2011) IEEE Pages 191-204.
Renuka S Umamaheswari S Shobana C Ramesh M and Poopal RK (2019) Response of antioxidants to semisynthetic bacteriostatic antibiotic (erythromycin) concentrations: A study on freshwater fish. Acta Ecologica Sinica 39 (2) Pages 166-172.
Samanta P Im H Lee H Hwang SJ Kim W Ghosh AR Jung J (2016) Impact assessment of sewage effluent on freshwater crucian carp Carassius auratus using biochemical and histopathological biomarkers. J Kor Soc Water Environ 32 (5) Pages 419-32.
Samanta P Im H Na J Jung J (2018) Ecological risk assessment of a contaminated stream using multi-level integrated biomarker response in Carassius auratus. Environmental Pollution 1; 233 pages 429-38.
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of botany Pages 1-27.
Singh N Kumar A Gupta VK Sharma B (2018) Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chemical Research in Toxicology. 2018 Sep 4; 31(10) Pages 1009-21.
Srivastava B, Reddy PB (2017) Lipid peroxidation and DNA damage as biomarkers of pollution induced oxidative stress (OS) in fish. Life Sciences International Journal 4 (1) Pages 194-8.
Srivastava Bhawna and Reddy PB (2019) Haematological profile in fish as an effective and sensitive index in aquatic pollution. In: Proceedings of International multidisciplinary Academic Conference, Asian Institute of Technology, Bangkok. May, Pages 51-57.
Sutradhar S Deb A Singh SS (2020) Melatonin attenuates diabetes-induced oxidative stress in spleen and suppression of splenocyte proliferation in laboratory mice. Archives of Physiology and Biochemistry 3: Pages 1-2.
Tan BL Norhaizan ME Liew WP Sulaiman Rahman H (2018) Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Frontiers in pharmacology16; 9 pages 1162.
Tenji D Micic B Sipos S Miljanovic B Teodorovic I Kaisarevic S (2020) Fish biomarkers from a different perspective: evidence of adaptive strategy of Abramis brama (L.) to chemical stress. Environmental Sciences Europe 32(1) Pages 1-5.
Tyor AK and Pahwa K (2017) Pollutants induced oxidative stress, DNA damage and cellular deformities in Clarias gariepinus (burchell), from river Yamuna in Delhi region, India. Bulletin of environmental contamination and toxicology, 99(1) Pages 33-38.
Verma PK Raina R Sultana M Singh M Kumar P (2016) Total antioxidant and oxidant status of plasma and renal tissue of cisplatin-induced nephrotoxic rats: protection by floral extracts of Calendula officinalis Linn. Renal failure 2; 38 (1) Pages 142-50.
Weber AA Sales CF de Souza Faria F Melo RM Bazzoli N Rizzo E (2020) Effects of metal contamination on liver in two fish species from a highly impacted neotropical river: A case study of the Fundão dam, Brazil. Ecotoxicology and Environmental Safety 1; 190 Pages 110165.
Wei J Wang B Wang H Meng L Zhao Q Li X Xin Y Jiang X (2019) Radiation-induced normal tissue damage: oxidative stress and epigenetic mechanisms. Oxidative medicine and cellular longevity 2019 Pages 1-12.
Yadav SS Kumar R Khare P Tripathi M (2015) Oxidative stress biomarkers in the freshwater fish Heteropneustes fossilis (Bloch) exposed to sodium fluoride: antioxidant defense and role of ascorbic acid. Toxicology international 22 (1) Pages 71.