1,2Immunology Laboratory, Department of Biochemistry, North-Eastern Hill University, Shillong-793022, Meghalaya, India and
3Department of Biochemistry, Royal Global University, Betkuchi. Guwahati, 781035 India
Corresponding author email: sthitidutta7@gmail.com
Article Publishing History
Received: 09/01/2020
Accepted After Revision: 29/02/2020
The study of antigens on tumor cells and their role in immune response against the tumor is an important aspect of tumor immunology, and the recognition and characterization of novel tumor associated antigens (TAA) is fundamental to the advancement of cancer immunotherapy. In the present investigation an attempt has been made to purify a highly over-expressed membrane surface glycoprotein of approximately 58 kDa molecular size on liver cell of Swiss albino mice exposed to diethylnitrosamine (DEN), an established hepato carcinogen in rodents. Carcinogenesis induction was monitored by assays of gamma glutamyl transpeptidase (GGT), acetylcholine esterase (AChE), glutathione S-transferase (GST) activities and the glutathione level (GSH) in liver. Animals exposed to DEN showed cell distortion and extensive necrosis as observed in the histological examination and transmission electron microscopy (TEM) study of the liver tissues. An over-expressed glycoprotein on the membrane surface of hepatocytes was purified by ion-exchange chromatography, further characterized by SDS-PAGE and identified as TAA. The glycoprotein contains significantly high carbohydrate moieties and targeted for specific active immunotherapy in mice. The preliminary results of our studies suggest that an effective immune response could be achieved against TAA formulated with either alhydrogel or CFA and could eventually be used to counter tumor regression. The results suggest that specific proteins are uniquely susceptible to alterations in expression and carry implications for the further investigation of their potential as therapeutic and prognostic markers.
Carcinogenesis response modulation, cancer immunotherapy diethylnitrosamine, hepatocarcinogen, tumor associated antigen.
Sohkhlet M, Alam A, Dutta S. P. Identification and Molecular Characterization of Tumour-associated antigen Expressed on Hepatocytes in Mice Exposed to Diethyl Nitrosamine. Biosc.Biotech.Res.Comm. 2020;13(1).
Sohkhlet M, Alam A, Dutta S. P. Identification and Molecular Characterization of Tumour-associated antigen Expressed on Hepatocytes in Mice Exposed to Diethyl Nitrosamine. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2UOlja2
Copyright © Sohkhlet et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Hepatocellular carcinoma (HCC) is being reported as the third most frequent cause of cancer-related death worldwide and it accounts for around 90% of primary liver cancers .In the early 1970s, there were outbreaks of liver disorders, including cancer, in various farm animals in Norway. Intensive investigation revealed that all the affected animals had consumed rations containing herring meal, which had been preserved by the addition of relatively large amounts of sodium nitrite. Further investigation showed that the herring meal contained dimethylnitrosamine (DMN), a chemical class of N-nitroso compounds (NOCs). Later it was found that DMN was formed in the fish meal as a result of chemical reaction between dimethylamine, a commonly occurring amine in fish meal, and a nitrosating agent that formed from the sodium nitrate (Liao et al., 2001; Brown, 1999). DEN is known to induce damage in many enzymes involved in DNA repair and is normally used to induce liver cancer in experimental animal models ( Heindryckx et al., 2009 Wong et al., 2016, Santos et al., 2017; Sklavos et al., 2018 Kaltenecker et al., 2019).
Diethyl nitrosamine (DEN) is a well-established hepatocarcinogen over a wide range of doses with or without promotors in experimental animals (Matuoka et al., 1993; Pascale et al., 1993; Williams et al., 1993; Montesano, 1981; Pariat and Sharan, 1995). This derivative of NOCs has very high degree of cell and tissue specificity depending on its systemic distribution (Montesano, 1981). DEN in its parent form is not active carcinogen. It requires metabolic activation induced by cytochrome P450s and Flavin dependent-oxidases that yield electrophiles which readily alkylate nucleophilic sites in DNA. Exposure to DEN has also been associated with hepatocellular accumulation of reactive oxygen species (Hanahan and Weinberg, 2000), which may lead to oxidative damage to DNA and other nucleophiles, that may further enhance DEN-induced hepatocarcinogenesis (Arboatti et al., 2018).
During nitrosamine-induced hepatocarcinogenesis in mice, processes such as gene expression, replication and differentiation are altered in the transformed cells, which might lead either to the expression of a novel antigen unique to that tumor or the differential expression of some membrane surface proteins usually called as tumor-associated antigens (TAA) (Raghupati, 1996). Many studies have suggested that TAA may be used as a potential candidate for active immunotherapy against cancer (Raghupati, 1996; Goydos et al., 1996; Rodolf et al., 1996; Xing et al., 1995). The present investigation was an attempt with an objective to identify tumor-associated antigen (TAA) on liver cells of DEN-exposed mice, its subsequent purification and to study its immunogenicity upon immunization in mice. The interest in the study of these carcinogens stemmed from the finding that N-nitroso compounds are present in industrial occupational hazards. In recent years these compounds are of great concern, not only to industrial workers, but also to the population at large and have emerged as one of the most important classes of environmental carcinogens.
MATERIALS AND METHODS
Acetylcholine chloride, Acrylamide, Albumin bovine, Ammonium per sulphate(APS), Brilliant Blue G, 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB), Ethylenediamine tetra acetic acid (EDTA), L-γ-Glutamyl-p-nitroanilide, Glycylglycine, 2-Mercaptoethanol, N,N’-Methylene bisacrylamide, N-Nitrosodiethylamine (DEN), Tween 20, Sephacryl S-200 HR, Q-Sepharose Fast Flow, Sodium Lauryl Sulphate, N,N,N’,N’-Tetramethylethylene , Triton-X-100, Trizma base. Trypsin,Protein Molecular weight markers, Rabbit anti-mouse IgG-HRP, TMB/H2O2, Freund’s Complete Adjuvant (CFA), Freund’s Incomplete adjuvant (IFA).
Cancer induction- Healthy Swiss albino balb/c mice 6-8 weeks old weighing approximately 25g from inbred colony, maintained at controlled temperature (20 ± 2 o C), provided with standard mouse pellets and drinking water ad libitum (National Research Council, 1996) were administered an aqueous solution of DEN at a dose of 10 mg kg-1 body weight by intravenous route at weekly intervals for a period of 16 weeks. Sham treated age-matched normal Swiss albino balb/c mice served as control.
Tissue preparation for enzyme assays- After completion of DEN treatment, mice were killed by cervical dislocation. The liver was then quickly excised and removed, rinsed in chilled normal saline (0.9 % NaCl), blotted dry and weighed. A 10 % homogenate of the liver was prepared in chilled 0.25 M sucrose and centrifuged at 20,000 x g for 30 min at 4°C. The resulting supernatant was used for the enzyme assays as described below. The total protein content in the supernatant was estimated by Bradford’s method (Bradford, 1976).
γ-Glutamyl transpeptidase assay– The GGT activity was assayed using the method described by Meister (Meister et al., 1975) with slight modification. Acetylcholine esterase assay- AChE activity was assayed according to the method described by Ellman (Ellman et al., 1961) with slight modificationGlutathione-S-transferase assay- GST activity in the liver was determined by the method described by Habig and Jacoby (Habig and Jacoby, 1981) with slight modificationGlutathione assay–GSH levels were determined using the method described by Ellman (Ellman,1959) with slight modifications. The concentration of GSH in each test sample was read from a standard GSH curve.
Histological study- Microtomy technique described by Ratcliffe (Ratcliffe, 1983) was employed for the histological examination of liver tissues of DEN-treated and age-matched normal control mice. Glycoprotein extraction- Glycoproteins extraction from liver tissues of DEN-exposed and normal control mice was based on the method described Liao (Liao et al., 1985). Gel electrophoresis- The final 1-Butanol extracts containing membrane surface glycoprotein were analysed by SDS-PAGE. The sample were run on acrylamide gel (10%) at a current of 60 mA and constant voltage of 200 V using Mini-PROTEINR Electrophoresis Cell. The gels were stained with Coomassie brilliant blue.
Ion-exchange chromatography- The crude 1-butanol extract was dialysed in phosphate buffer (0.05 M, pH 6.5) overnight. The dialysed sample was then loaded on Q-Sepharose (Anion-exchanger) column pre-equilibrated with Tris HCl buffer (0.01 M, pH 7.5). The column was washed with the tris buffer for 60 min and the bound proteins from the column were then eluted with a salt gradient of 0 – 0.1 M NaCl for another 60 min. Fractions of 2 ml were collected and read at 280 nm.
Preparative SDS-PAGE analysis of bound proteins- The fractions corresponding to different peaks in the bound region obtained from anion exchanger were subjected to run on preparative SDS-PAGE for the final purification of the desired protein. A longitudinal section of the gel corresponding to the crude extract lane was cut and stained. This was used as a reference to locate the exact position of the TAA in the rest of the gel. The portion of the gel corresponding to the TAA was cut out of the unstained gel. It was cut into small pieces and homogenized in 3% 1-butanol. The homogenate was centrifuged at 8000 x g for 30 min at 4 ◦C. The supernatant was collected and the pellet was re-suspended in 3% 1-butanol and centrifuged. The supernatant collected was pooled, dialysed against distilled water overnight and lyophilised. It was then run on a SDS-PAGE gel for homogeneity of TAA.
Molecular weight determination– A fresh, filtered solution of Blue Dextran was prepared. This was applied to the column to determine the void volume (Vo), and to check the column packing. The selected calibration proteins were dissolved in the running buffer and applied to the column. The elution volumes (Ve) for the standards were determined by measuring the volume of the eluent from the point of application to the centre of the elution peak. A calibration curve of logarithm of their molecular weights versus Ve/Vo was prepared. The sample is applied in a volume < 2 % of the total column volume (Vt) and the elution volume (Ve) of the molecule of interest is determined. The corresponding molecular weight of the protein was read from the calibration curve after determining its elution volume. Estimation of neutral hexoses– The total neutral hexoses in purified TAA was determined using the method described by Spiro (Spiro, 1966a). Estimation of total sialic acid- Amount of total sialic acid in TAA was determined by Resorcinol-HCl assay (Spiro, 1966b).
Immunization of animals– Three groups of mice consisting of at least six each were immunized separately with three different antigen formulations which are (a) TAA in normal saline, (b) TAA adsorbed on Alhydrogel (1:1) and (c) TAA emulsified with Freund’s adjuvant (1:1) by intra-muscular route. Formulations (a) & (b) were administered thrice at weekly intervals as primary immunization. However animals immunized with formulation (c) received only one injection. The booster injection was given on 30th day after the primary immunization. Mice that were administered only saline served as control. Blood was collected from the animals on 3rd and 7th day after the booster injection. The serum obtained was pooled and stored at 20oC.
ELISA- Microtitre plate wells were coated with 50 μl of 1 μg/ml TAA in coating buffer. The plate was covered and incubated overnight at 4°C. The wells were then washed with washing buffer for 2-3 times. This was followed by blocking the wells with 100 μl of blocking buffer and incubated overnight at 4°C. After washing the wells again, 50 μl of serially diluted serum (in blocking buffer) was applied to each well of the plate and was incubated for 2 hr at 37°C followed by washing with washing buffer. The commercially supplied anti-mouse-HRP conjugate was diluted 1000 times (in blocking buffer) and 50μl of it was added to each well and incubated for 2hr at 37°C followed by washing step. 50μl of the substrate (20-fold diluted in distilled water) was added and incubated in the dark for 10 min at room temperature. The reaction was stopped by adding 50μl of 1M H2SO4. The absorbance of resulting yellow coloured product was read at 450 nm.
RESULT AND DISCUSSION
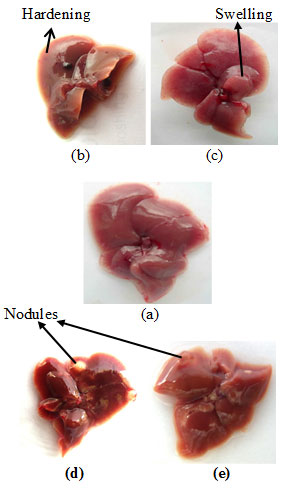
Cancer induction– Upon complete treatment, the mice were sacrificed, and the liver was examined for any visible morphological changes. The liver of DEN-treated mice showed hardening and swelling in some portions of the liver as shown in Fig. I-b & c respectively. However, in some cases nodule formation were also seen in liver (Fig. I-d & e). No such changes observed in the liver of any sham-treated normal control mice.
Figure 1: Liver Photographs: (a) Normal Control Mice; (b), (c), (d) And (e) DEN-Treated Mice. Arrows Indicate The Hardening, Swelling And Nodule Formations In The Liver Of DEN-Exposed Mice.
Marker enzyme activities- The marker enzyme activities viz GGT, AChE, GST and the GSH level in the liver tissues of DEN-treated and that of untreated mice were monitored separately in the supernatant fractions. The GGT and AChE activities were markedly elevated upon DEN exposure in comparison to that of normal control. However, enzyme GST activity in DEN-exposed animals was found significantly low in comparion to normal control. The GSH level was also found elevated significantly in DEN-exposed animals as compared to the normal control (Table-I).
Table 1. Marker Enzyme Activities And GSH Level In The Liver Tissue Of DEN-Treated And Sham-Treated Normal Control Mice. Enzyme Activities Were Measured In The Supernatant Fractions Of Liver Tissue.
| Marker Enzymes | Normal control
Mean ± SEM (n=12) |
DEN-treated
Mean ± SEM (n=12) |
Fold increase / decrease | Test of significance |
| GGT activity (U/mg protein) | 0.0107 ± 0.001 | 0.0179 ± 0.0005 | ~1.7 | P < 0.0003 |
| AChE activity (U/mg protein) | 0.0168 ± 0.002 | 0.0558 ± 0.002 | ~3.3 | P < 0.0001 |
| GST activity (U/mg protein) | 0.5894 ± 0.019 | 0.3705 ± 0.011 | ~1.6 | P < 0.0001 |
| GSH level (mg/gm tissue) | 0.5894 ± 0.003 | 1.7480 ± 0.014 | ~3.0 | P < 0.001 |
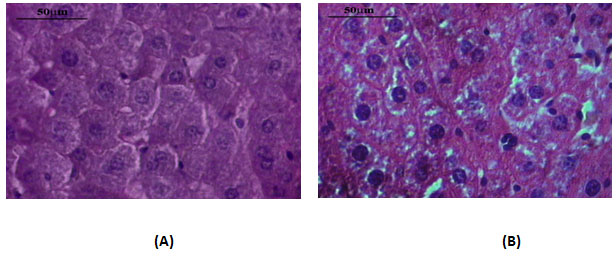
Liver histology- The liver tissues of DEN-exposed mice and age-matched normal control mice were examined microscopically for any morphological differences in liver cells. When compared with the control, DEN-exposed liver micro section showed many changes in liver cells such as loss of regular arrangement, variation in the shape and size, multi-nucleated cells, and loss of contact with the neighbouring cells. In contrast, the liver cells of the untreated normal control showed mono- and bi-nucleated cells only with a regular morphology and well defined outlines (Fig.II).
Figure 2: Microphotographs Of Histological Section Of Liver From Normal Control Mice (A) And DEN-Treated Mice (B). The Slides Were Stained By Haematoxylin And Eosin. Slides Were Examined Microscopically After Drying. Magnification X 40.
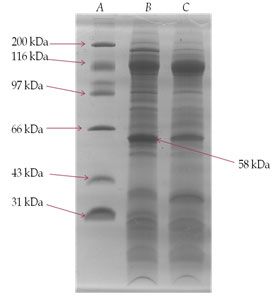
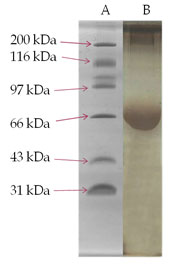
SDS-PAGE analysis of membrane glycoprotein- The membrane glycoproteins 1-butanol extracts obtained from normal control and DEN-exposed mice liver tissues were analysed on SDS-PAGE (Fig. III). A glycoprotein of approximately 58 kDa molecular weight was prominently over-expressed in the treated animals as compared to normal control and was identified as TAA.
Figure 3: SDS-PAGE Analysis Of 1-Butanol Extract Of Membrane Surface Glycoproteins Obtained From Liver Of DEN-Treated And Normal Control Mice. A Gel Consisting Of 10% (W/V) Acrylamide Was Used. Sample Of 25 µg Each Were Run Under The Reduced Condition. The Gel Was Fixed In Methanol/Acetic Acid And Stained With Commassie Brilliant Blue. Lane (A)-Molecular Weight Markers; Lane (B)- DEN-Treated And Lane (C)- Normal Control.
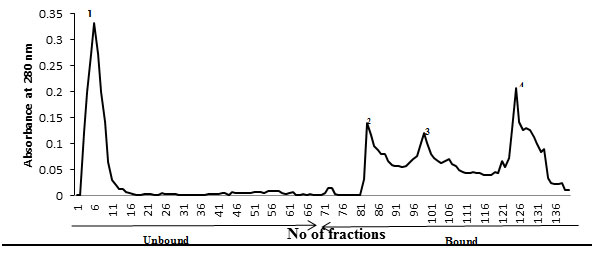
Ion-exchange chromatography- An anion-exchanger (Q-Sepharose Fast Flow) was employed for the purification of the desired glycoprotein. The elution profile of anion-exchanger is shown in Fig. IV. The desired specific glycoprotein eluted out in the bound fraction and present in in all the three peaks 2, 3 and 4 that was confirmed when run on SDS-PAGE. All the peaks were pooled together and run on preparative SDS-PAGE for final purification as discussed in methods and materials section. The glycoprotein was analysed for its recovery from the gel by running it on SDS-PAGE to insure its homogeneity and molecular weight (Fig. V). The purified TAA obtained from gel was subsequently used for molecular weight determination, carbohydrate analysis and immunization.
Figure 4: Q-Sepharose An Anion-Exchange Protein Profile Of Crude Membrane Glycoprotein Extract Of Liver From DEN-Treated Mice. Unbound Glycoprotein Were Eluted With 10 Mm Tris-Hcl Buffer, Ph 7.0 And Bound Glycoprotein Were Eluted With An Increasing Gradient Of 0-0.1M Nacl In The Same Buffer. Fractions Of 2 Ml Were Collected And Read At 280 Nm. The Desired Glycoprotein Was Eluted Out In All Three Peaks Of Bound Fraction Confirmed When Run Of SDS-PAGE.
Figure 5: Preparative SDS-PAGE Purified TAA. The Portion Of The Gel Corresponding To The TAA Was Cut Out Of The Unstained Gel. It Was Cut Into Small Pieces And Homogenized In 3 % 1-Butanol. The Homogenate Was Centrifuged At 8000 X G For 30 Mi At 4 O C. The Supernatant Collected Was Pooled, Dialysed Against Distilled Water Overnight And Lyophilised. Lane (A)- Molecular Weight Markers; Lane (B)- Purified TAA.
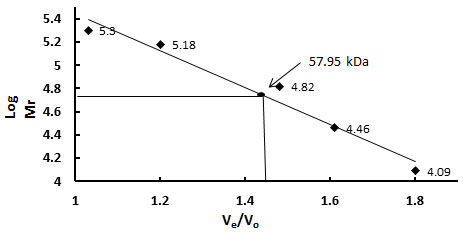
Molecular weight determination- The molecular weight of the purified protein was determined from the plot of log molecular weight versus Ve/Vo of several known calibration standards. The logarithm of their respective molecular weights (Log Mr) was plotted against the ratio of their elution volume to void volume (Ve/Vo). From the calibration curve the molecular weight of the purified glycoprotein sample was calculated to be 57.95 kDa (Fig.VI).
Figure 6: Sephacryl S200 HR Gel Filtration Profile To Determine Molecular Weight Of Purified TAA. Different Calibration Standards Were Used. The Logarithm Of Their Respective Molecular Weights (Log Mr) Was Plotted Against The Ratio Of Their Elution Volume To Void Volume (Ve / Vo). From The Calibration Curve The Molecular Weight Of The Purified Glycoprotein Sample Was Calculated And Found To Be 57.95 Kda.
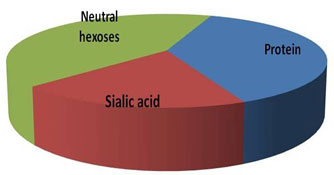
Estimation of neutral hexoses, sialic acid and protein- Neutral hexoses, sialic acid and the polypeptide contents of the glycoprotein were estimated, and the results are shown in Fig. VII. The protein content in the sample was estimated by Bradford’s method, while the amount of sialic acid and neutral hexoses in the sample was calculated as described in method section. The total carbohydrate to peptide ratio was found to be >2:1, which signifies TAA as highly glycosylated protein.
Figure 7: Total Amount Of Neutral Hexoses, Sialic Acid And Protein In Purified TAA Sample Determined As Per The Method Described In Method Section.
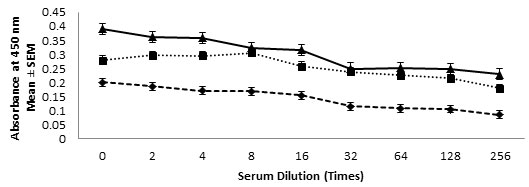
ELISA- Presence of anti-TAA antibody in the immune sera were determined using ELISA. The assay carried out showed significantly very high titer of anti-TAA antibodies in the immune serum obtained from of all the three antigenic formulations. The presence of antibodies in test serum were carried out using ELISA and high titer was observed even up to a dilution of 1:256 as shown in Fig. VIII.
Figure 8:- Anti-TAA Antibody Titres Were Determined Using ELISA In Serum Samples Obtained From Mice Immunized With Various Antigenic Formulation As Described In Material And Method Section. Mice Injected With Saline Alone Served As Normal Control. The Absorbance Measured In Serum Sample Of Normal Control Were Subtracted From The Experimental Sample Readings. Antibody Levels At Various Dilutions In Mice Immunized With TAA-CFA (▲), TAA-Alhydrogel (■) And TAA-Saline (♦) Formulations.
Diethynitrosamine (DEN), an important environmental and food carcinogen was employed as the chemical carcinogen to induce carcinogenesis in Swiss albino mice. DEN is a genotoxic, carcinogenic nitrosamine having its place among N-nitrosodialkylamines (Fausto et al., 2010). DEN was administered intravenously at a weekly dose of 10 mg/kg body weight for a period of 16 weeks. DEN is well known for contributing to the pathogenesis in hepatocarcinogenesis (Chen et al., 2008). Reports available state that the initiation of carcinogenesis especially in the liver is induced only upon coupling it with proliferative stimuli (Ying et.al.,1982), however, this protocol successfully triggered initiation of carcinogenesis in the experimental mice without coupling any proliferative stimulus with DEN. Morphological changes such as swelling, hardening of the tissue and nodule formation seen in the liver of the treated animals signify carcinogenesis induction upon DEN-exposure (Fig.II). Carcinogenesis induction in liver of DEN-exposed mice was followed by enzyme marker assays viz. GGT, AChE, GST and the level of GSH and compared with age-matched normal control animals. These marker enzymes activities in liver have been recognised as a positive marker for hepatocytes which have undergone malignant transformation (Boelsterli, 1979). Increased generation of ROS and abnormal production of antioxidant enzymes in liver tissues have been reported in many models of DEN-induced hepatocellular carcinoma (Sivaramakrishnan et al., 2008).
DEN-treated mice liver showed marked elevation in GGT and AChE activities as compared to the normal control (Table-I). The hyper-activation of these enzymes in DEN-exposed mice signifies hepatocellular transformation. Glutathione-S-transferase (GST) was another marker enzyme studied to monitor cancer induction. It plays a protective role for the cell towards cytotoxic and mutagenic effects of electrophiles and perhaps it evolved to protect cells against reactive oxygen metabolites. Upon DEN-exposure the GST activity in liver drastically decreased as compared to normal control mice (Table-I). The decrease in GST activity in mice exposed to DEN signifies that it leaves the cell vulnerable to these agents. Such alterations have been shown earlier in mice exposed to DBN (Alam et al., 2005).
Intracellular GSH plays an important role in a series of physiological functions in the plasma membrane, particularly in tumor cells. Because of its reducing properties of GSH, it can inactivate some carcinogens, protect DNA against free radicals that are damaging, protect the integrity of different tissues, and prevent lipid peroxidation (Traverso et al., 2013). Reduced glutathione acts as a nucleophile that protects the DNA and other components from attack by the reactive forms of these carcinogens (Moldeus and Jernstrom, 1983). High intracellular levels of glutathione would also prevent oxidative damage. An increase in GSH level was observed in the liver tissue of DEN-exposed mice (Table-I). This increase in GSH level may also be likely due to a rise in the GGT activity, as modulation in cellular GSH levels has been correlated with hyper-activation of GGT. Overproduction of GGT results in increased intracellular GSH synthesis, it plays an important role in the development of resistance to certain chemotherapeutics, such as alkylating agents (Bansal et al., 2018).
The histological examination of liver tissues of DEN-treated mice exhibited that the hepatocytes in liver section were in a neoplastic state. Many changes in hepatocytes such as, irregular arrangement, variation in the shape and size, multi-nucleated cells, and loss of contact with the neighbouring cells were noticed. In contrast, the liver cells of the untreated normal control mice did not show any such changes and were found with a regular morphology and well-defined outlines (Fig.II). Thus, the morphological changes seen in the liver, alterations in the marker enzyme activities, the increase in GSH level and changes in histological section of liver tissue, all these observations support the development of cancer induction in liver of mice upon DEN-exposure.
After having established cellular transformation in mice upon exposure of DEN, identification of TAA was carried out. The SDS-PAGE analysis of the 1-butanol liver extract showed that a membrane surface glycoprotein of ~58 kDa molecular weight identified as TAA, was found to be significantly over-expressed in DEN-treated mice liver cells (Fig. III). Observed alteration in the expression of above glycoprotein clearly indicate that DEN has caused major changes in the membrane of liver cells, which may involve in distortions and alterations of cell membrane during treatment. Attempt was made to purify the above membrane glycoprotein TAA using ion-exchange chromatography followed by gel filtration. The desired glycoprotein was found to be an anionic protein as evident from the elution profile shown in Fig. IV. Further purification of the glycoprotein was carried out by gel filtration chromatography using sephadex G-100 but could not be achieved due to small differences in the molecular weight of other anionic protein eluted with it. Hence was finally purified by preparative SDS-PAGE (Fig. V).
Molecular weight of TAA was determined by gel filtration chromatography. Membrane glycoprotein TAA was analysed for the carbohydrate moities i.e. neutral hexoses and sialic acid and for its protein content. The results are shown in Fig. VII. The total carbohydrate to protein ratio was calculated to be >2:1. Thus, the carbohydrate moieties seem to make up a large portion of the TAA, as is evident from its high content as compared to the protein. The substantially high concentration of sialic acid observed during carbohydrate analysis of TAA also reveal about the anionic nature of TAA. Sialic acid content on membrane surface varies from cancer to cancer and has been associated with malignancy, if not with every stage of carcinogenic process, but starting with initiation, progression and finally metastasis.
The purified TAA was tested for its immunoreactivity in allogenic normal control Swiss albino mice through active immunization using three different TAA-formulations as discussed in method section. The anti-TAA antibody concentrations in immune sera were determined by ELISA as has been shown in Fig. VIII. These observations clearly indicate that the purified TAA is an anionic glycoprotein with very high content of carbohydrate moieties, highly immunogenic, may elicit a significantly high antibody response against it in mice upon immunization and thus could suitably be used as an effective target for active immunotherapy against cancer. The results suggest that specific proteins are uniquely susceptible to alterations in expression and carry implications for the further investigation of their potential as therapeutic and prognostic markers.
ACKNOWLEDGEMENT
This investigation was supported by a project grant from Department of Science and Technology (DST), New Delhi sanctioned to Prof. A. Alam.
REFERENCES
Alam A Singha LI and Singh V (2005) Molecular characterization of tumor associated antigen in mice exposed to a hepatocarcinogen Molecular and Cellular Biochemistry Vol 271 Pages 177-188.
Arboatti AS Lambertucci F Sedlmeier MG Pisani G Monti J Álvarez ML Francés DEA Ronco MT and Carnovale CE (2018) Diethylnitrosamine Increases Proliferation in Early Stages of Hepatic Carcinogenesis in Insulin-Treated Type 1 Diabetic Mice BioMed Research International Vol 2018: 9472939 Pages 1-10.
Bansal A and Simon MC (2018) Glutathione metabolism in cancer progression and treatment resistance Journal of Cell Biology Vol 217 No 7 Pages 2291-2298.
Boelsterli U (1979) Gamma-glutamyl Transpeptidase (GGT) – an early marker of hepatocarcinogenesis in rats Trends in Pharmacological Sciences Vol 1 No 1 Pages 47-49.
Bradford MA (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilising the principle of dye binding. Analytical Biochemistry Vol 72 No7 Page 245.
Brown JL (1999) N-Nitrosamines Occupational Medicine Vol 14 No 4 Pages 839-848.
Chen Qi Y Chan X and Cy (2008) Two-dimensional differential gel electrophoresis/analysis of diethylnitrosamine induced rat hepatocellular carcinoma,International Journal of Cancer Vol 122 Page 2382-2688.
Ellman GL (1959) Tissue sulfhydryl group Archives of Biochemistry and Biophysics Vol 82 No 1 Pages 70–77.
Ellman GL Courtney DK Andres V and Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholine esterase activity Biochemical Pharmacology Vol 7 No 2 Pages 88-95.
Fausto N Campbell J (2010) Mouse Models of Hepatocellular Carcinoma Seminars in Liver Disease Vol 30 No 1 Page 87–98.
Goydos JS Elder E Whitiside TL Finn OJ and Lotze MT (1996) A phase I trial of a synthetic mucine peptide vaccine Journal of Surgical Research Vol 63 No 1 Pages 298-304.
Habig WH and Jakoby WB (1981) Glutathione S-transferases (Rat and Human) Methods in Enzymology Vol 77 Pages 218–237.
Hanahan D and Weinberg RA (2000) The hallmarks of cancer Cell Vol 100 No 1 Pages 57–70.
Heindryckx F Colle I and Vlierberghe HV (2009) Experimental mouse models for hepatocellular carcinoma research International Journal of Clinical and Experimental Pathology Vol 90 No 4 Pages 367–386.
Kaltenecker D Themanns M Mueller KM Spirk K Schwarzl NG Friedbichler K Kenner L Haybaeck J Moriggl R (2019) STAT5 deficiency in hepatocytes reduces diethylnitrosamine-induced liver tumorigenesis in mice Cytokine Vol 124 Pages 154573.
Liao DJ Blanck A Eneroth P Gustafsson JA and Hällström IP (2001) Diethylnitrosamine causes pituitary damage, disturbs hormone levels and reduces sexual dimorphism of certain liver functions in the rat Environmental Health Perspective Vol 109 No 9 Pages 943–947.
Liao S Smith JW and Kwong PC (1984) Selective extraction by 1-butanol of surface glycoprotein antigens from human melanoma cells Cancer Immunology Immunotherapy Vol 17 No 2 Pages 95–99.
Matuoka K Marus I Wong A and Smith GJ (1993) Diethylnitrosamine and partial hepatectomy induced in alpha 2m-globulin mRNA level in the rat liver Journal of Cancer Research and Clinical Oncology Vol 119 No 10 Pages 572-275.
Meister A Tate SS and Griffith OW (1975) γ -Glutamyl transpeptidase Methods in Enzymology Vol 77 Pages 237–251.
Moldeus P and Jernstrom B (1983) In: Interaction of glutathione with reactive intermediates Pp 99-108 (Edited by) Larsson A Orrenivs S Holmgren A and Mannervik B Functions of Glutathione: Biochemical, Physiological, and Clinical Aspects Raven Press New York.
Montesano R (1981) Alkylation of DNA and tissue specificity in nitrosamine carcinogenesis Journal of supramolecular structure and cellular biochemistry Vol 17 No 3 Pages 259-73.
National Research Council (2011) Guide for the care and use of laboratory animals. National Academy Press 39.
Pariat T and Sharan RN (1995) Low dose exposure of diethylnitrosamine affects mice liver thymidine kinase Life Science Vol 57 Pages No 26 Pages 2431-2431.
Pascale RM Simile MM Gaspa L Diano L Seddain MA Pinna G Carta M Zolo P and Feo F (1993) Alterations of ornithine decarboxylase gene during the progression of rat liver carcinogenesis Carcinogenesis Vol 14 No 5 Pages 1077-1080.
Raghupati G (1996) Carbohydrate antigens as targets for active specific immunotherapy Cancer Immunology Immunotherapy Vol 43 No 3 Pages 152-157.
Ratcliffe NA (1983) Practical illustrated histology. The Macmillan Press London and Basingstoke 24-23.
Rodolf M Zilocchi C Melani C Cappetti B Arioli I Parmiani G and Colombo PM (1996) Immunotherapy of experimental metasis by vaccination with interleukin gene-transduced adenocarcinoma cells sharing tumor-associated antigens. Journal of Immunology Vol 157 No12 Pages 5536-5542.
Santos NP Colaço AA and Oliveira PA (2017) Animal models as a tool in hepatocellular carcinoma research: A Review Tumor Biology Vol 39 No 3.
Sivaramakrishnan V Shilpa PNM Praveen Kumar VR and Niranjali DS (2008) Attenuation of N-nitrosodiethylamine-induced hepatocellular carcinogenesis by a novel flavonol-Morin. Chemico-Biological Interactions Vol 171 No 1 Page 79–88.
Sklavos A Poutahidis T and Giakoustidis A (2018) Effects of wnt-1 blockade in DEN-induced hepatocellular adenomas of mice Oncology Letters, Vol 15 No 1 Pages 1211–1219.
Spiro RG (1966a) In: Determination of neutral hexoses Vol 8 Pp 4-5 (Edited by) Neufeld EF and Ginsburg V. Methods in Enzymology Academic Press New York.
Spiro RG (1966b) In: Resorcinol reaction Vol 8 Pp 15-16 (Edited by) Neufeld EF and Ginsburg V. Methods in Enzymology Academic Press New York.
Traverso N Ricciarelli R Nitti M Marengo B Furfaro AL Pronzato MA Marinari UM and Domenicotti C (2013) Role of Glutathione in Cancer Progression and Chemoresistance Oxidative Medicine and Cell Longevity Vol 2013:972913.
Williams GM Gebhardt R Sirma H and Stenback F (1993) Non-linearity of neoplastic conversion induced in rat liver by low exposure to Diethylnitrosamine. Carcinogenesis Vol 14 No 10 Pages 2149-2156.
Wong CR Nguyen MH and Lim JK (2016) Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease World Journal of Gastroenterology Vol 22 No 37 Pages 8294-8303.
Xing P Michael M Apostopoulos V Prenzoska J Marshall C Bishop J and Mckenzie (1995) Phase I study of synthetic MUC1 peptides in breast cancer International Journal of Oncology Vol 6 No 6 Pages 12883-1287.
Ying TS Enomoto K Sharma DSR and Farber E (1982) Effects of delays in the cell cycle on the initiation of preneoplastic lesions in rat liver by 1,2-dimethylhydrazine Cancer Research Vol 42 No 3 Pages 876-880.