1Research Scholar, Department of Biotechnology, Patna University, Patna 800005, India
2Head, Department of Zoology, Patna Women’s College, Patna 800001, India
Corresponding author email:shahla_apex@yahoo.co.in
Article Publishing History
Received: 11/10/2019
Accepted After Revision: 19/12/2019
The present study reports about the fluoride containing minerals present in the rock and soil samples collected from the fluoride endemic region of Gaya district of Bihar. Fluoride was measured in 77 water samples collected from fluoride endemic villages and 69 samples contained fluoride > 1.5 mg/L. A positive correlation was found between pH and fluoride (r=0.24), suggesting geogenic contamination of fluoride into groundwater. X-ray diffraction (XRD) analyses of rocks and soil samples were done. Results showed the presence of biotite in the rock and soil samples, suggesting that F might be leaching from biotite into the groundwater.
Biotite; Fluoride; Health Effects; Xrd Analysis
Ranjan S, Yasmin S. Geological Source of Fluoride in Fluoride Endemic Region of Gaya District, Bihar, India. Biosc.Biotech.Res.Comm. 2019;12(4).
Ranjan S, Yasmin S. Geological Source of Fluoride in Fluoride Endemic Region of Gaya District, Bihar, India. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2szjRy1
Copyright © Ranjan and Yasmin This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Fluorine occurs in many common rock-forming minerals, including fluorite CaF2, which occurs in both igneous and sedimentary rocks. Fluorine is the most abundant halogen in the sedimentary rocks. Clastic sediments can contain higher level of fluorine, while biogenic and chemical sediments may contain from <5 to >800 mg/ kg Fluorine (Wedepohl, 1978). The average Fluorine content of rocks is: 1000 mg /kg in alkalic rocks, 400 mg /kg in intermediate rocks, dropping to 100/ mg /kg in ultramafic rocks deficient in SiO2 (Wedepohl, 1978). Fluorine is released as fluoride (F) during the process of weathering. F is released into the groundwater from F bearing minerals such as fluorite, fluoroapatite, biotite, amphinole, montmorillonite and some micas weathered from igneous and sedimentary rocks (Adimalla, 2018). F has the tendency to complex with other ions. In groundwater F may easily dissolve and form complexes with Ca2+, Al3+, Fe3+, PO4-. Excess intake of fluoride (maximum permissible limit recommended by WHO=1.5 mg/L) can cause dental and skeletal fluorosis (Rao et al., 2017; Li et al., 2018; Adimalla and Quain, 2019).
However, the exposure dose of F may also depend on the amount of water intake which further depends on the local climatic condition, type of food and its preparation methods (Khan et al., 2004; Ranjan and Yasmin, 2015). Most states in India have their groundwater contaminated with fluoride and around 62 million people and 6 million children are facing health problems (fluorosis) due to consumption of F contaminated drinking water (Susheela, 1999; Jacks et al., 2005; Adimalla and Venkatyogi, 2018). Water quality assessment of Gaya district, Bihar was done between 2012-2014 and three villages were found to be fluoride endemic (Ranjan and Yasmin, 2012). Health survey was also conducted and the residents of these villages were found to suffer from dental, skeletal and non-skeletal fluorosis (Ranjan and Yasmin, 2012). Anomalies were also found in the thyroid function (Yasmin et al., 2013) and haematological parameters (Yasmin et al., 2014). A detailed investigation found around 50% of fluoride intake was through drinking water, while the rest 50% came through food crops grown locally and irrigated with the same fluoride rich groundwater (Ranjan and Yasmin, 2015). Analysis of composition of rock and soil samples can reveal the source of fluoride in the groundwater of the region. A study on this aspect has not been conducted so far. Therefore, XRD analysis of rock and soil was done to confirm the presence of F contributing minerals.
MATERIAL AND METHODS
Gaya district is located at 84.4 ° E to 85.5 ° E Longitude and 24.5 ° N to 25.1° N Latitude and has total area of 487607.83 sq km. Gaya experiences subtropical climatic condition with extremely hot summer (maximum temperature shoots to 460C) and cold winter (mercury drops to as low as 40C). Average rainfall during rainy season is approx 338.4 mm. According to Adimalla and Venkatyogi (2018) higher evaporation and less precipitation rate in arid and semi arid region can increase F accumulation in groundwater. Till now there is no provision for rain water harvesting. The greater part of Gaya district is occupied by the Gangetic alluvium, but older rocks are found, chiefly in the south and east. These rocks are mostly composed of foliated gneiss, a subdivision of the Archean system which contains the oldest rocks of the earth’s crust (O’malley, 2007). The south east corner of district is situated in the middle of rich mica belt. The foliated gneisses of oldest formations would have undergone maximum weathering. Weathering and leaching of fluoride bearing minerals lying in the joints, fractures and faults of such rocks under alkaline environment leads to the enrichment of fluoride in the groundwater (Raju et al., 2009). Similar findings were also reported by Sumalatha et al., (1999).
F in water samples were measured by using Fluoride Ion selective electrode Orion 9690 BNWP with PCD 650 cyber scan portable meter (range 0–500 mg/L, calibrated at 0.1, 1.0, 5.0, 10.0, 20.0 mg/L). Ion strength adjusting buffer (ISA I) was added to the samples before the measurement of F. Similarly, F concentration was estimated in the soil and crop samples as described previously (Ranjan and Yasmin, 2015).Rock and soil samples were collected from the F endemic areas. Soil surface was dug at the depth of 10 cm and sample was stored in clean plastic packets. The Rock samples were collected after breaking it with the help of hammer. Samples were brought to the lab and were grinded in MP100 ball mill, Retsech, Germany to get fine powder. Stainless steel jars and balls were used for making the fine powder. XRD analyses of the powdered samples were done with the help of Miniflex 600 XRD, Rigaku, Japan. The X- Ray source was Cu filament. 2θ range was 50-800. Scan rate was 10/min. The step size was 0.02. Goniometer radius was 240 mm. The graph obtained was sent to IIT Mumbai for analysis with the help of PAN analytical software.
RESULTS AND DISCUSSION
An account of F level in groundwater, soil, food crops and major human health problems in F endemic areas are shown in Table 1. According to Wu et al., (2019) correlation plots provide geochemical factors for F accumulation in groundwater. Correlation matrix of physico-chemical parameters shows a positive correlation between pH and F (r=0.24) (Table 2). Similar results were reported by Narsimha and Sudarshan (2017) and Narsimha and Ranjitha (2018). A negative correlation was found between F and Ca (r=-0.38) which clearly indicates that the presence of high calcium results in lowering F concentration by precipitations. Similar findings were reported by Li et al., (2018) and Adimalla et al., (2018). Water soluble fluoride (WSF) of the soil of F-endemic area was high. Similarly, WSF and Total fluoride (TF) of crops grown in fluoride endemic areas were also high (Table 1).
Table 1. Fluoride concentration in groundwater, soil and vegetation of F endemic areas and related health complaints
| Villages | F Level in Water (mg/L) | F in Soil (mg/kg) | F in Food crops (mg/kg) | Health Complaints (%) | ||||||||
| Range | Mean | Water Soluble F | Total F | Water Soluble F | Total F | Sample size | Dental Fluorosis | Skeletal Fluorosis | Dental Caries | Gastro-Intestinal Problems | Joint Pain | |
| Bhupnagar | 1.7-7.2
(N=22) |
3.5± 0.2 | 2.5±0.3
(N=19) |
154.6±13.6 | 1.4±0.1
(N=48) |
6.3±0.5 | N=141 | 99
(67%) |
41 (29%) | 42
(29%) |
88
(62%) |
90
(63%) |
| Bhaktauri | 1.1-5.1
(N=29) |
2.3± 0.1 | 2.3±0.3
(N=18) |
157.1±15.8 | 3.3±0.7
(N=29) |
10.4±1.4 | N=160 | 102
(63%) |
65
(40%) |
26
(16%) |
90
(56%) |
95
(59%) |
| Dhaneta | 0.6-6.2
(N=26) |
2.7± 0.3 | 2.4±0.3
(N=19) |
149±10.9 | 1.8±0.5
(N=33) |
8.3±1.1 | N=133 | 97
(72%) |
73
(54%) |
48
(36%) |
51
(38%) |
80
(60%) |
Red and black rocks are predominant in F endemic villages of Gaya district. XRD pattern of black rock, red rock and soil samples are shown in Figure 1, 2 and 3 respectively. The graphs indicate the predominance of biotite in the rock and soil samples. Detailed mineral composition of black rock, red rock and soil samples are presented in Tables 3, 4 and 5 respectively.
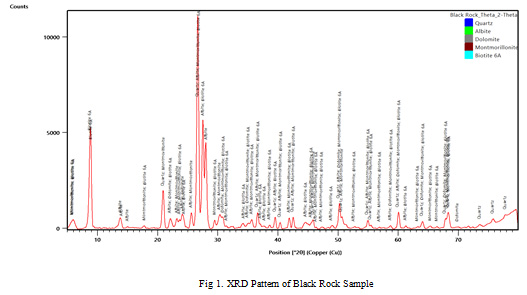 |
Figure 1: XRD Pattern of Black Rock Sample |
Table 2: Correlation between physico-chemical parameters in the F endemic region (N= 77)
| Turbidity | pH | EC | TDS | Fluoride | TH | Calcium | Iron | Mg | |
| Turbidity | 1 | ||||||||
| pH | 0.04 | 1 | |||||||
| EC | -0.23 | 0.28 | 1 | ||||||
| TDS | -0.24 | 0.29 | 0.99 | 1 | |||||
| Fluoride | -0.14 | 0.24 | 0.06 | 0.05 | 1 | ||||
| TH | -0.12 | 0.24 | 0.40 | 0.40 | -0.33 | 1 | |||
| Calcium | 0.17 | -0.27 | -0.11 | -0.09 | -0.38 | 0.40 | 1 | ||
| Iron | 0.23 | -0.22 | -0.47 | -0.44 | -0.03 | -0.10 | 0.22 | 1 | |
| Mg | 0.23 | 0.00 | 0.08 | 0.09 | -0.29 | 0.09 | 0.33 | 0.15 | 1 |
A positive correlation between pH and F in the water samples of fluoride endemic areas suggests that alkaline nature of water promotes leaching of fluoride from the rocks in these areas. The alkaline water most probably mobilizes fluoride from rocks with precipitation of calcium carbonate because the solubility of fluorite (CaF2) increases with an increase in NaHCO3 rather than with other salts (Handa, 1975; Saxena and Ahmed, 2001).
High level of fluoride in the soil may be due to geologic origin or due to irrigation with fluoride contaminated water. Similarly, WSF and Total fluoride (TF) of crops grown in F endemic areas were also high due to irrigation of crops with fluoride rich water. Fluoride was measured in the following crops: wheat (Triticum aestivum), rice (Oryza sativa), pigeon pea (Cajanus cajan), chickpea (Cicer arietinum), mustard (Brassica nigra) and vegetables including potato (Solanum tuberosum), spinach (Spinacia oleracea), tomato (Solanum lycopersicum), coriander (Coriandrum sativum).
Table 3. XRD analysis of Black rock
| Reference Code | Score | Compound Name | Scale Factor | Chemical Formula |
| 98-016-2490 | 54 | Quartz | 0.982 | O2 Si1 |
| 98-008-7654 | 39 | Albite | 0.286 | Al1 Na1 O8 Si3 |
| 98-017-1523 | 21 | Dolomite | 0.066 | C2 Ca1 Mg1 O6 |
| 98-016-1171 | 13 | Montmorillonite | 0.049 | H1 Al2 Ca0.5 O12 Si4 |
| 98-002-4167 | 33 | Biotite 6A | 0.618 | H1 Al1 F1 K1 Mg3 O11 Si3 |
Table 4. XRD analysis of Red rock
| Reference Code | Score | Compound Name | Scale Factor | Chemical Formula |
| 98-016-2490 | 59 | Quartz | 0.986 | O2 Si1 |
| 98-009-0142 | 39 | Albite | 0.171 | Al1.02 Ca0.02 Na0.98 O8 Si2.98 |
| 98-016-1171 | 13 | Montmorillonite | 0.033 | H1 Al2 Ca0.5 O12 Si4 |
| 98-009-8154 | 30 | Biotite 1M | 0.303 | H1.47 Al1.92 F1.98 Fe2.59 K2 Mg3.15 Mn0.09 O21.47 Si5.98 Ti0.27 |
| 98-020-2423 | 45 | Microcline (maximum) | 0.198 | Al1 K0.95 Na0.05 O8 Si3 |
Table 5. XRD analysis of soil
| Reference Code | Score | Compound Name | Scale Factor | Chemical Formula |
| 98-015-6196 | 68 | Quartz | 0.956 | O2 Si1 |
| 98-009-0142 | 39 | Albite | 0.179 | Al1.02 Ca0.02 Na0.98 O8 Si2.98 |
| 98-002-4167 | 34 | Biotite 6A | 0.295 | H1 Al1 F1 K1 Mg3 O11 Si3 |
| 98-003-4787 | 31 | Microcline (intermediate) | 0.162 | Al0.99 K0.94 Na0.06 O8 Si3.01 |
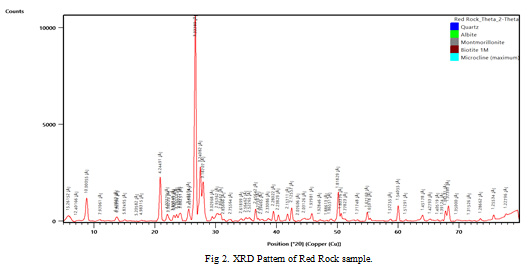 |
Figure 2: XRD Pattern of Red Rock sample |
The XRD analyses indicate the predominance of biotite in the rock and soil samples. Furthermore, XRD peaks also showed the presence of Quartz, Albite, Dolomite, Montmorillonite and Microcline. Biotite is a common phyllosilicate mineral within the mica group, with the approximate chemical formula K(Mg,Fe)3AlSi3O10(F,OH)2. Biotite contributes upto 3400 ppm of fluoride into groundwater (Deer et al., 1992). In metamorphic terrain, high fluoride concentration in groundwater is due to the dissolution of Biotite, which may contain fluorine at the -OH sites of their octahedral sheet (Nordstorm et al., 1989; Li et al., 2003; Dharmagunawardhana, 2004).
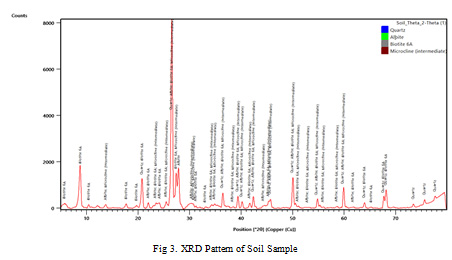 |
Figure 3: XRD Pattern of Soil Sample |
F mainly accumulates in aquifers from fluoride bearing minerals found in igneous and sedimentary rocks. These are the main source of fluoride contamination in groundwater, because longer contact time between rock and water with alkaline activities results in leaching of fluoride in fractured zones (Narsimha and Sudarshan, 2018). High concentration of F in groundwater results from dissolution of biotite because the position of fluorine in the octahedral sheet increases its capacity to dissolve in groundwater (Nordstorm et al., 1989; Li et al., 2003; Subba Rao and Devdas, 2003; Chae et al., 2006; Msonda et al., 2007). In the rocks, biotite appears as tiny grains, providing large contact area with ground water and this enhances their solubility (Deutsch 1997). Various studies report that mineral rocks like syenites, granites, granite gneisses, quartz monzonites, granodiorites, felsic and biotite gnessis contributes high level of fluoride into soil and groundwater, (Handa, 1975; Apambire et al., 1997; Chae et al., 2007). The process of weathering of rock releases fluoride in soil and groundwater through anion exchange (OH– for F–) (Rankama and Edgington 1946; Abdelgwad et al 2009). According to Chae et al (2007), presence of biotite mineral in rocks may alone contribute to F concentration above 4mg/l. It may be due to similarity of ionic radii between F– and OH– group (Evans, 1995; Brownslow, 1996).
The fluoride contamination in groundwater in Gaya district is not due to anthropogenic origin as there are no industries in and around. Rather the contamination is due to geogenic origin. The inhabitants of the F endemic region of Gaya are underprivileged partly because the area is a Naxalite hit belt. They are economically poor (monthly income per family is INR 1750/ USD 25) and their main occupation is farming and firewood collection from nearby forests to sell in the market. As they spend most of their time in the field, therefore their water intake (hence, F intake) is high. The F exposure dose of the inhabitants has been discussed earlier (Ranjan and Yasmin, 2015). Public Heath and engineering department (PHED) of Bihar Government has installed filtration units in the F endemic areas for safe drinking water, but regular maintenance of such units are required. Provision for alternative safe water for irrigation and household purposes should also be made through supply from neighbouring F non-endemic regions. Another probable solution to the problem faced by fluorotic inhabtants of the area is diet counselling and diet editing (Ranjan and Yasmin, 2015; Susheela and Toteja, 2018)
ACKNOWLEDGEMENTS
The authors are thankful to the Central Research Laboratory of Patna Women’s College for providing the laboratory facilities and to Dr. Trupti Chandrasekhar of Department of Earth Sciences, Indian Institute of Technology, Mumbai for analysing the XRD results. Special thanks to Dr. Momina Ahmad for the helpful support.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
Adimalla, N., Qian, H. (2019). Hydrogeochemistry and fuoride contamination in the hard rock terrain of central Telangana, India: analyses of its spatial distribution and health risk. SN Appl. Sci., 1(3):202.
Adimalla, N., Vasa, S.K., Li, P. (2018). Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: an insight of controlling factors of fluoride enrichment model. Earth Syst. Environ., 4(2):841–852.
Adimalla, N. Venkatayogi, S. (2018). Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl. Water Sci., 8:44.
Adimalla, N., Venkatayogi, S. (2017). Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environ. Earth Sci., 76:45
Apambire, W. M., Boyle, D. R., Michel F. A. (1997). Geochemistry, genesis, and health implications of fluoriferous ground waters in the upper regions of Ghana. Environmental Geology, 35: 13–24.
Abdelgawad, A. M., Watanabe, K., Takeuchi, S., Mizuno, T. (2009). The origin of fluoride-rich groundwater in Mizunami area, Japan-mineralogy and geochemistry implications. Engineering Geology, 108: 76–85.
Brownslow, A. H. (1996). Geochemistry, New Jersey: Prentice Hall.
Chae, G. T., Yun, S.T. , Kwon, M. J. , Kim, S.Y. , Mayer B. (2006). Batch dissolution of granite and biotite in water: Implication for fluorine geochemistry in groundwater. Geochemical Journal, 40: 95–102.
Chae, G. T., Yun, S. T. , Bernhard, M., Kim, K.H. , Kim, S.Y. , Kwon, J. S., Kwon, K. , Koh Y. K. (2007). Fluorine geochemistry in bedrock groundwater of South Korea. The Science of Total Environment, 385: 272-283.
Deutsch, W. J. (1997). Groundwater geochemistry: Fundamentals and applications to contamination. Boca Raton, FL: Lewis Publishers.
Deer, W. A., Howie, R. A. , Zussman J. (1992). An Introduction to the Rock Forming Minerals, ELBS, Essex, UK, 2nd edition.
Dharmagunawardhana, H. A. (2004). Fluoride in Groundwater, Surface water, Rocks and Soils of an area of endemic fluorosis in the dry zone of Sri Lanka. In: Proceedings of the 4th International Workshop on Fluorine Prevention and Defluoridation of Water. P7
Evans, H. T. Jr (1995). Ionic radii in crystals. In CRS handbook of chemistry and physics (75th ed). CRC Press.
Handa, B. K. (1975). Geochemistry and genesis of fluoride containing groundwater in India. Ground Water, 13: 275–281.
Jacks G, Bhattacharya P , Chaudhary P, Singh KP (2005). Controls on some genesis of some high fluoride ground waters in India. Applied Geochemistry, 20: 221-228.
Khan, A. A., Whelton, H. , O’Mullane D. (2004). Determining the optical concentration of fluoride in drinking water in Pakistan. Community Dentistry and Oral Epidemiology, 32: 166-172.
Li, Z., Tainosho, Y., Shiraishi, K., Owada, M. (2003). Chemical characteristics of fluorine-bearing biotite of early Paleozoic plutonic rocks from the Sor Rondane Mountains, East Antarctica. Geochemical Journal, 37 (2): 145–161.
Li, P., He, X., Li, Y. (2018). Occurrence and health implication of Fluoride in groundwater of Loess aquifer in the Chinese Loess plateau: a case study of Tongchuan. Expo Health, Northwest China.
Msonda, K. W. M., Masamba, W. R. L. Fabiano E. (2007). A study of fluoride groundwater occurrence in Nathenje, Lilongwe, Malawi. Physics and Chemistry of the Earth, 32: 1178–1184.
Narsimha, A., Sudarshan, V. (2018). Drinking water pollution with respective of fluoride in the semi-arid region of Basara, Nirmal district, Telangana State, India. Data Brief, 16:752–757.
Nordstrom, D.K., Ball, J. W. , Donahoe, R. J. , Whittemore D. (1989). Groundwater chemistry and water rock interactions at Stripa. Geochimica et Cosmochimica Acta, 53: 1727-1740.
Narsimha, A., Rajitha, S. (2018). Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Hum. Ecol. Risk. Assess. Int. J., 24(8):2119–2132.
Narsimha, A., Sudarshan, V. (2013). Hydrogeochemistry of groundwater in Basara area, Adilabad District, Andhra Pradesh, India. J. Appl. Geochem., 15(2):224–237
Narsimha, A., Sudarshan, V. (2017). Assessment of fluoride contamination in groundwater from Basara, Adilabad District, Telangana State, India. Appl. Water. Sci., 7(6):2717–2725.
O’malley LSS (2007). Bengal District Gazetteer:Gaya, Concept Publishing Company.
Rankama, K., Edgington G. (1946). Fluorine in soils. Soil Science, 61: 341–353.
Raju, N., Dey, S. Das K. (2009). Fluoride contamination in ground waters of Sonbhadra District, Uttar Pradesh, India. Current Science, 96 (7):979–985.
Rao, N.S., Rao, P.S., Dinakar, A., Rao, P.V.N., Marghade, D. (2017). Fluoride occurrence in the groundwater in a coastal region of Andhra Pradesh, India. Appl. Water Sci., 7(3):1467–1478
Ranjan S, Yasmin S (2012). Assessment of groundwater quality of Gaya region with respect to Fluoride. Journal of Ecophysiology and Occupational Health, 12: 21-25.
Ranjan, S., Yasmin S. (2015). Assessment of Fluoride intake through Food chain and mapping of endemic areas of Gaya district of Bihar. India. Bulletin of Environmental Contamination and Toxicology, 94 (2): 220-224.
Ranjan, Sumeet & Yasmin, Shahla. (2015). Amelioration of fluoride toxicity using amla (Emblica officinalis). Current Science, 108. 2094-2098.
Saxena, V. K., Ahmed S. (2001). Dissolution of fluoride in groundwater: a water-rock interaction study. Environmental Geology, 40: 1084–1087.
Subba Rao, N, Devadas D. J. (2003). Fluoride incidence in groundwater in a area of Peninsula India. Environmental Geology, 45: 243–251.
Sumalatha, S., Ambika, S. R. A. ,Prasad S J. (1999). Fluoride concentration status of groundwater in Karnataka, India. Current Science, 76: 730–734.
Susheela A K (1999). Fluorosis management programme in India. Current Science, 77 (10): 1250-1256.
Susheela AK, Toteja GS (2018). Prevention & control of fluorosis & linked disorders: Developments in the 21st Century – Reaching out to patients in the community & hospital settings for recovery. Indian J Med Res, 148:539-47.
Wedepohl, K. H. (1978). Handbook of Geochemistry, Vol. II/5. XXXII + 1546 S., 266 Abb. 576 Tab. Berlin Heidelberg. New York.
Wu, J., Li, P., Wang, D., Ren, X., Wei, M. (2019). Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk. Assess, https://doi. org/10.1080/10807039.2019.1594156
Yasmin, S., Ranjan, S. , Hilaluddin, D’Souza D. (2013). Effect of excess fluoride ingestion on human thyroid function in Gaya region, Bihar, India. Toxicological and Environmental Chemistry, 95 (7): 1235-1243.
Yasmin, S., Ranjan, S. , D’Souza D. (2014). Hematological changes in fluorotic adults and children in fluoride endemic regions of Gaya district, Bihar, India. Environmental Geochemistry and Health, 36 (3): 421-425.


