1Department of Biology, Faculty of Science, University of Ha’il, Ha’il, Saudi Arabia
3Department of Biology, Faculty of Science, University of Tabuk, Saudi Arabia
4Department of Biology, Faculty of Science, University of Bisha, Saudi Arabia
5Department of Biological Sciences, University of Arkansas, Fayetteville, Arkansas USA
Corresponding author email: abuelhadi@hotmail.com
Article Publishing History
Received: 05/10/2020
Accepted After Revision: 10/12/2020
Red palm weevil (RPW), a dangerous insect of date (Phoenix dactylifera), is an invasive insect not known to occur in the Arab region until recently. Control strategies for RPW include the utilization of pheromone traps and insecticides. The side effects of insecticides on people and the environment have driven researchers and policy makers to look for other methods of controlling RPW. As such, information on the normal adversaries of RPW and the defensive mechanisms of this insect against them is imperative to develop techniques for an integrated pest control. Conventional and molecular techniques were employed in the Hail region to identify and characterize the fungi associated with RPW to assess potential indigenous entomopathogenic fungi for biological management. Conventional identification methods indicated that the genera Aspergillus and Fuzarium spp were highly associated with RPW.
Aspergillus niger contributed to the highest number of fungal isolates among all species, followed by Aspergillus flavus and Fusarium solnum. Other fungal isolates were tentatively identified as Fusarium solani (accession number: MH151017.1 with 100% sequence identity), Fusarium proliferatum (accession number: MK522076.1 with 100% sequence identity) and Nectria haematococca (accession number: DQ535183.1 with 99% sequence identity). Most of the isolated species in this study are saprophytic fungi which normally live in soil. However, some species are pathogenic fungi such some species of Aspergillus which would be potential candidates for biological control of RPW.
Biological Control, DNA Extraction, Mycelium, Defensive Mechanism
Alanazi N. A, Sulieman A. M. E, Alshammari N. I, Al-Azmi M, Aziz A, Elbadri G. A, Stephenson S. L. Fungi Related with the Red Palm Weevil (Rhynchophorus ferrugineus) in the Hail Area, Northern Saudi Arabia. Biosc.Biotech.Res.Comm. 2020;13(4).
Alanazi N. A, Sulieman A. M. E, Alshammari N. I, Al-Azmi M, Aziz A, Elbadri G. A, Stephenson S. L. Fungi Related with the Red Palm Weevil (Rhynchophorus ferrugineus) in the Hail Area, Northern Saudi Arabia. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3kadYwb
Copyright © Alanazi et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The Kingdom of Saudi Arabia occupies an advanced position among the countries of the world in the cultivation of palm trees and the production of dates of all kinds, as the number of palm trees has reached 28.5 million, producing 1.3 million tons of dates annually. The date palm tree is an important part of the religious, cultural, and economic heritage of Semi-Arabian Island. This tree was exposed to grave problems that threatened the continuity of its production. The date palm tree is infected by many insect and fungal pathogens and pathogens, bacteria, nematode diseases, birds, weeds, and others organisms. This insect is considered to be the most dangerous insect, where it feeds its larvae openly soft tissue inside the palm tree.
This insect came from Southeast Asia to the Arab region and has been invading cultivated palms throughout the world. The red palm weevil, (RPW) causes considerable misfortune to date growers. In truth, it has been designated a most serious pest of date in Arab countries (Al-Shawaf et al., 2013). This insect belongs to the Curculionidae family in the order of Coleoptera (beetles and weevils). RPW is a hidden pest and remains inside the palm during the larval development and makes tunnels and then pupates. Subsequently, along with its omnipresent nature, these species are of incredible significance as they have effects on environment, agriculture, food production, biotechnology, and human and animal health (Abdel-Azeem et al. 2019 Qayyum et al 2020).
Biological control is a significant biological system administration and option in contrast boundless pesticide use (Crowder and Jabbour, 2014; Crowder et al., 2010). Biological control can harnessed as an environment administration which enhance hiding the pests from their adversaries such as predators, parasites and pathogens. This pest guideline by common enemies in crops offers natural and suitable conditions as harvest misfortune can be decreased because of over the top utilization of chemical pesticides (Bianchi et al., 2006).Numerous entomogenous fungi are generally normal, frequently inciting epizootics and consequently assaulting terrestrial insects in the groups Hyphomycetes and Entomophthorales. In contrast to other pathogens, the fungi entering the insect exoskeleton (Butt 2002).
The host can be tainted by (a) immediate treatment, (b) even transmission from contaminated insects or dead bodies to healthy insects, and (c) vertical transmission to resulting formative stages by means of the new age of spores. There are several studies which have been conducted to investigate the capability of fungi against insect pests. Beauveriabassiana and Metarhiziumanisopliae are among the most common entomopathogenic fungi which have been utilized in the control of insects throughout the world. Impressive research have been done to utilize entomopathogenic parasites as biocontrol agents against RPW (Sewefy et al., 2009; Demibilo et al, 2010; Guerri-Agullo et al, 2010 Belein 2018 Qayyum et al 2020).
However, such information is lacking in Saudi Arabia, therefore, the purpose of this study was to identify and characterize the fungi associated with RPW, invading date palm trees in Hail province. Understanding the connection between fungi and insects should be helpful in the improvement of biocontrol agents utilizing entomolopathogenic fungi as an elective control system for this genuine insect pest.
MATERIAL AND METHODS
Sampling: One hundred and thirty adults of RPW were gathered from 5 infested farms in the study area (Hail area) during the period of October 2019 to May 2020. These were Al gayed, Jubbah, Helala, Horir and Guthasharagiya, and all of the collecting sites occur within latitude 27.523647 and longitude 41.696632. A pheromone trap was utilized for the collection of RPW. 30 males and 30 females were placed in plastic boxes, then maintained in a freezer at −20 °C at the microbiology laboratory until used for investigating the associated fungi.
Isolation and preliminary identification of fungi:The infected RPW adults were utilized to start stock colonies, and the colonies were periodically supplemented with the introduction of additional wild specimens. Infected plants were assessed for the presence of RPW by visual observation and listening for the feeding sounds the insect by touching the ear to the stem of the palm. Fungal growth was induced by placing the RPW in moisture chambers at 20-22 °C for 24-48 hours, then recovering the fungi by direct and indirect isolation techniques (Zho et al., 2008).
For direct isolation of fungi, small amounts of the visible hyphal mat were inoculated onto a Petri dish containing potato dextrose agar. While for isolating fungi using indirect method specimens were surface sterilized, then dissected, placed in Petri dishes containing PDA and were incubated at 25°C. The presence of growing fungal colonies was observed every day. The PDA plates were checked daily for fungal growth. After 5-10 days of incubation, several fungal colonies were grown on the plates. The mycelium of each fungal colony was transferred and maintained on fresh PDA plates.
For identification of fungi, a small part of pure fungi colony was cut from the colony edge using a sterilized scalpel. The fungi plugs were placed in Petri dishes. The fungal isolates were identified to the genus level based on microscopic features (morphology of hyphae, fruiting bodies, gamic spores, and conidia) and macroscopic features (colony morphology, colour, and growth rate) using the appropriate identification keys.
Frequency of occurrence: Existence of fungi collected from the RPW samples was determined utilizing the following formula: F= (NF/NT) X 100% Where: F = Frequency of occurrence % NT= total number of samples
B- DNA Extraction :The method modified by Dellaporta et al. (1983) was utilized for the DNA extraction as outlined below:Twenty 20 mg of fresh harvested mycelium were ground with a pestle in a 1.5 ml tube, and 33 μl of 20% sodium dodecyl sulfate (SDS, w/v) and 160 μl of 5 M potassium acetate KoAc (Sigma chemicals) were added and vortexed. Then the mixture was centrifuged for 10 min at 10,000 rpm, and 450 μl of supernatant was transferred to a new tube. 450 μl of phenol, chloroform, and isoamyl-alcohol (PCI) were added with a ratio of 25:24:1 and vortexed, then centrifuged for 5 min at 10,000 rpm. 400 μl of the upper phase was then removed to a clean microcent7. The supernatant was removed and the total nucleic acid was precipitated in the bottom of the tube. The pellet were washed with 70% ethanol and spun 5 min at 10,000 rpm. Then the pellet was re-suspended in 100 μl of Double-distilled water (ddH2O).
C- Polymerase Chain Reaction (PCR):For fungal isolates, Two primer pairs, the forward IT5 primer (5′- GGAAGTAAAAGTCGTAACAAGG-3′) and the reverse ITS4 primer (5′-TCCTCCGCTTATTGATATGC-3′) were used to amplify the entire ITS region (White et al., 1990).
The purified PCR products were sequenced by Macrogen Inc., (Korea), and sequencing of the purified isolates was performed in both directions using ITS5 and ITS4 primer pairs. Sequence alignments were edited by MEGA7 (Kumar et al., 2016).
RESULTS AND DISCUSSION
Frequency and identification of fungi associated with the red palm weevil
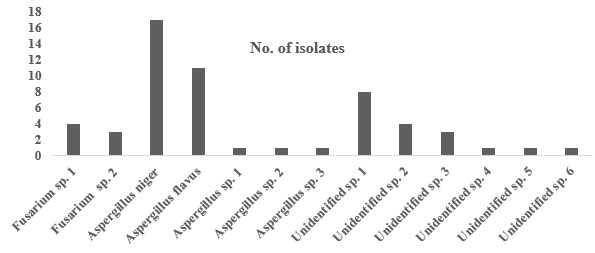
Fifty-six fungal isolates were successfully isolated from the bodies of RPW (Table 1 and figure 1& 2). Using conventional methods, 38 fungi that were identified to the genus level, which incorporated several members of the genera Fusarium and Aspergillus. Among all the isolates the most dominant fungal species was Aspergillus niger (17 isolates), followed by Aspergillus flavus (11 isolates), Fusarium sp. 1 (4 isolates), Fusarium sp. 2 (3 isolates), Aspergillus sp. 1 (1 isolate), Aspergillus sp. 2 (1 isolate), and Aspergillus sp. 3 (1 isolate). In addition, 18 isolates could not be identified.
Table 1. Occurrence of fungal species from RPW in various sites
| Fungal species | No. of isolates | Prevalence (%)* |
| Fusarium sp. 1 | 4 | 7.1 |
| Fusarium sp. 2 | 3 | 5.3 |
| Aspergillus niger | 17 | 30.35 |
| Aspergillus flavus | 11 | 19.6 |
| Aspergillus sp. 1 | 1 | 1.7 |
| Aspergillus sp. 2 | 1 | 1.7 |
| Aspergillus sp. 3 | 1 | 1.7 |
| Unidentified sp. 1 | 8 | 14.28 |
| Unidentified sp. 2 | 4 | 7.1 |
| Unidentified sp. 3 | 3 | 5.3 |
| Unidentified sp. 4 | 1 | 1.7 |
| Unidentified sp. 5 | 1 | 1.7 |
| Unidentified sp. 6 | 1 | 1.7 |
*Prevalence was calculated based on the total number of fungal isolates (N/56)
Figure 1: Frequency of fungi isolated from the bodies of the red palm weevil.
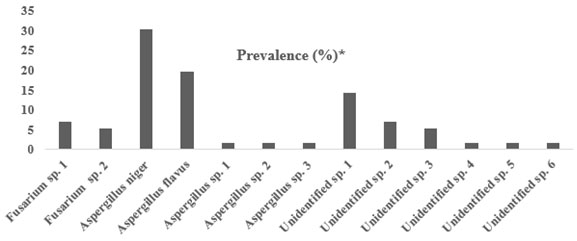
Figure 2: Frequency of fungi Prevalence % from the bodies of the red palm weevil.
Note. Prevalence was calculated based on the total number of fungal isolates (N/56)
Molecular identification of the fungi: The results of using molecular identification showed that of the fungal isolates were tentatively identified as Fusarium solani (accession number: MH151017.1 with 100% sequence identity), Fusarium proliferatum (accession number: MK522076.1 with 100% sequence identity) and Nectria haematococca (accession number: DQ535183.1 with 99% sequence identity) as indicated in Table 2 and Figure 1.
Table 2. Taxa of fungi recorded from RPW in various sites. Note: %ID = percent sequence identity, and SGB = sequence in GenBank.
| Sp. | Taxon | SGB | ID% |
| 1. | Fusarium proliferatum.(Matsush.)Nirenberg | MK522076.1 | 100 |
| 2. | Nectria haematococca. Berk. & Broome | DQ535183.1 | 99 |
| 3. | Fusarium solani. (Mart.) Sacc. | MH151017.1 | 100 |
According to the World Health Organization WHO (2013), there are approximately 23-25 million people infected every year with pesticide toxins, and nearly 20,000 of them die annually
(Schmutterer, 2002). Therefore, there was a need to develop non-toxic, safe, human and animal alternatives as one of its control methods intended to reduce the population of the pests or its harmful effects, to the extent that it does not constitute with severe harm to humans, animals, or their activities. This can be achieved by means of other biological species from insects, nematodes, bacteria, fungi, etc. Pathogenic fungi to insects are one of the important factors due to their wide presence in nature in addition to being invisible, expensive and highly specialized for treating specific pests (Shiff; 2014., Rajesh et. al., 2002).
The outcomes of the present study indicated that the most frequently encountered fungal species associated with RPW collected from various farms in the Hail region was Aspergillus spp., specifically Aspergillus niger and Aspergillus flavus as identified with conventional identification methods. Aspergillus niger constituted 30.35%, whereas Aspergillus flavus constituted 19.6% of the total number of fungal isolates. Furthermore, Fusarium sp. 1 and Fusrium sp. 2 were identified with a percentage 7.1% and 5.3%, respectively. On the other hand, 31.78% of the fungal isolates were unidentified using conventional methods. Using molecular biological techniques, the dominant fungal species were Fusarium solani, Fusarium proliferatum, Fusarium proliferatum and Nectria haematococca, respectively.Aspergillus spp. and Fuzarium spp. were highly associated with the RPW. Aspergillus niger contributed to the highest number of fungal isolates among all species. This suggests that A. niger lives as saprotroph or symbiont with RPW.
Surprisingly, fungi considered as saprotrophs or symbionts just assimilate the organic matter from the host, yet they don’t have capacity to taint the host (Dube 2007). A few different fungal species, which have low number of fungal isolates (e.g., Aspergillus flavus, Fusarium solani and Fusarium proliferatum) were likewise found as saprotrophs on soil, spices, insects, and plants. This shows that RPW have an extraordinary relationship with soil and plant fungi. This could be attributed to the inclination of insects to contact the ground or plant-related fungi. Other than that, the strategy utilized for getting the fungi may most likely mirrored the aftereffects of this study. The thorough review of fungi associated with RPW could be acquired by utilizing distinctive isolation strategies coupled with culture-dependent techniques, which will uncover a few species other than the saprophytic ones.
It is known that saprophytic fungi are the largest group of macro fungi, responsible for breaking down and recycling dead plant and animal material. Therefore, the presence of these fungi is not surprising since saprophytic fungi are generally recognized from the fruiting bodies observed on dead trees, leaf litter, animal bones, even feces. Saprophytic fungi discharge enzymes to separate and digest the lignin, cellulose, or chitin in this material into simple soluble aggravates, that can be consumed by them, and by plants, as supplements. In this manner, they assume an imperative role in diminishing the collection of dead natural material and in reusing fundamental supplements, especially carbon and nitrogen (https://fungimap.org.au/about-fungi/saprophytic-fungi/)
Several species of the fungus Aspergillus are considered biological control agents for many insects, these species included: Aspergillus fumigatus. Aspergillus nidulans and Aspergillus flavus which infect the larvae and adults of honey bees, causing the disease (Brood stone). However, the success of infection depends on the inheritance of the fungus, its growth rate and capacity to exploit the insect’s materials for growth, as well as its ability to produce dermal-degrading enzymes, poisons, and overcoming insect resistance (Cole and Rolinson 1972).Aspergillus niger fungus is one of the most common types of Aspergillus.
It causes diseases to fruits and vegetables called black rot. The black spores of A. niger evidently protects against sunlight and UV irradiation, prompting an upper hand over different microorganisms in their living spaces. These capacities guarantee its progressively presence in warm habitat (Krijgsheld et. al., 2012). Some scientists tend to argue that some strains of Aspergillus niger produce toxins called okratoxin. However, this is not always the case, as some dispute this claim by saying that the previous reports are the result of a misdiagnosis of the fungi species. Recently, evidence has been obtained that strains of Aspergillus niger actually produce octeratoxin (Schuster et. al., 2002).
Aspergillus flavus, the second fungus associated with RPW in the present study, a well-known species related with aflatoxin contamination of agricultural crops (Cotty et al. 1994, Cotty 1997). It was also found that the fungus A. flavus. had an effect on silkworms, as this fungus secreted type 1 aflatoxin (Raper et. al., 1965). The following metabolites in fungus A. Parasiticus may have a role in killing the insect (aflatoxin toxins G2, G1, B2, B1) as well as secreting the following enzymes: Aminopeptidase, N-acetyl-β-glcosaminindase, Alkaline protease, Serineprotenase.
The genus Fusarium is a filamentous fungi, widespread in nature, as it is found in soil is in the decaying organic matter of plants and animals as well as food residues, and many types of waste. The genus presently contains almost under 200 accepted species, and its monetary and authentic significance causes it to stay at center stage in future conversations about terminology and mycological variety. Subsequently, along with its omnipresent nature, these species are of incredible significant effects on environments, agriculture, food production, biotechnology, and human and animal health (Abdel-Azeem et al. 2019).
Fusarium sp. cause many plant diseases such as root rot and vascular wilt. Also, species of this genus are associated with animals such as nematodes, spiders, amphibians and reptiles. Some species were documented as insect parasites and an insect pathogen. Roberts (1981) stated that this fungus has the effect of acting as pesticides against different insects. Fusarium solani is likewise connected with opportunistic infections of humans and other animals, causing systemic infections with a high death rate, as well as restricted infections in the skin and other body parts (Gupta et. al., 2000).
Fusarium solani is considered ubiquitous, while the distribution of some other species depends more on climate conditions (Summerell et al. 2010).Preceding host intrusion, certain fungal attributes assign them harmful or destructive organism. The organism must produce high number of conidia with strong adhesion that allow them to enter into the host through straight forward penetrating structures. Besides, the attacking organism must have the ability to sidestep or beat the host immune system by creating toxins. In future, experiments must be directed to investigate in detail the immune mechanism of RPW that may assist with discovering genes engaged in host defense (Abid et. al., 2013).
Naturally occurring bio-control agents are a desirable choice to switch the utilization of dangerous synthetic insecticides. Among these microbes, the utilization of entomopathogenic fungi was discovered to be promising substitute for insect control. As per a gauge, in excess of 700 species of fungi having a place with various genera, are known to infect insects. Before, the capability of entomopathogenic fungi particularly Beauveria bassiana, Metarhizium anisopliae and Isaria fumosorosea have been assessed against various pests including Aphis craccivora (Saranya et. Al., 2010), Aedes aegypti (García-Munguía et. al., 2011), Bemisia argentifolii (James et al., 2003), Coptotermes formosanus Shiraki (Hussain el al., 2010), Melanoplus sanguinipes (Inglis et al., 1996), Ocinara varians Walker (Hussain et. al. 2009), Odontotermes obesus (Hussain et al. 2011), Periplaneta americana (Mohan et.,al., 1999), Rhynchophorus ferrugineus (Dembilio et. al., 2010), Scolytus scolytus (Doberski 1981), Thrips tabaci (Al-mazra’awi et al 2009).
The accomplishment of these naturally occurring microorganisms principally relies upon the host pathogen interaction.The entrance of entomopathogenic fungi to attack the host is through the cuticle that involves complex biochemical interactions between the host and the pathogen (fungus) before germination, penetration, growth, and reproduction of the fungus. Preceding the host attack, there are certain characteristics of fungi that assign them destructive or a virulent strains (Abid et al 2013).
CONCLUSION
In summary, the successful control of RPW depends largely upon interactions between the host and its pathogens. There is a steady battle among host and a pathogen that eventually leads to the success or failure of the latter. In case of compatible interactions, the pathogen must have strong adhesion capacity that eventually penetrates into the host. Aspergillus app and Fusarium spp. dominated fungal flora of RPW. These fungi might have effects in management of this destructive pest. Most of the isolated fungal species in this study are saprophytic fungi which normally occur in soil. Thus, some species are pathogenic fungi such as Aspergillus spp. which would be potential candidate for biological control of RPW.
Authors’ Contribution: All authors contributed in designing, carrying out, and reporting this research.
Competing Interest: All the authors declare that they have no competing interest that can affect the current study.
ACKNOWLEDGEMENTS
This study was financially by the University of Hail, Project Number RG-191352. We are grateful to the University of Hail KSA for the financial support and to the Ministry of Agriculture, Hail region for technical assistance.
REFRENCES
Abid Hussain, Muhammad Rizwan-ul-Haq and Ahmed M. Al-Jabr. (2013). Red Palm Weevil: Understanding the fungal disease mechanism and host defense. Microbial pathogens and strategies for combating them: science, technology and education (A. Méndez-Vilas, Ed.).
Ahmed M. Abdel-Azeem A. M., Mohamed A. A, Darwish A.G, Nafady N.A, and Ibrahim N.A. (2019). Fusarium: Biodiversity, Ecological Significances, and Industrial Applications in:
N. Yadav et al. (eds.), Recent Advancement in White Biotechnology Through Fungi, Fungal Biology. Springer Nature Switzerland. https://doi.org/10.1007/978-3-030-10480- 1_6.
Al-mazra’awi MS, Al-Abbadi A, Shatnawi MA, Ateyyat M. (2009). Effect of application method on the interaction between Beauveria bassiana and neem tree extract when combined for Thrips tabaci (Thysanoptera: Thripidae) control. Journal of Food Agriculture and Environment. 2009; 7(2): 869-873.
Al-Shawaf A, Al-Shagag A., Al-Bagshi M, Al-Saroj S. Al-Bather S., Al-Dandan A., Ben A. and Faleiro J. R. (2013). A quarantine protocol against red palm weevil RED PALM WEEVIL Rhychophorus ferrugineus (Olivier) Coleptera: curculiondae) in Date palm. Journal of Plant Protection Research 53 (4), 2013.
Belein T (2018) Entomopathogenic nematodes as biocontrol agents of insect pests in orchards doi: 10.1079/PAVSNNR201813058 http://www.cabi.org/cabreviews © CAB International 2018 (Online ISSN 1749-8848)
Bianchi F. J., Booij C.J. and Tscharntke T. (2006). Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proceedings of the Royal Society B: Biological Sciences 273(1595):1715-27.DOI: 10.1098/rspb.2006.3530
Booth, C. (1971). The Genus Fusarium. Commonwealth Mycological Institute, Surrey, England.
Butt T.M., (2002). Use of entomogenous fungi for the control of insect pests, In: Esser K., and Bennett J.W. (eds.), Mycota, Springer, Berlin, pp.111 -134.
Cole, M and Rolinson N. (1972). Microbial metabolites with insecticidal properties. Appl Microbiol. 1972 24(4):660-2.
Cotty PJ (1997). Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycological Research 101: 698–704.
Cotty PJ, Bayman DS, Egel DS, Elias KS (1994). Agriculture, aflatoxins and Aspergillus. In: The genus Aspergillus: from taxonomy and genetics to industrial applications. (Powell KA, Renwick A, Peberdy JF, eds.) New York. Plenum Press: 1–
Cotty PJ, Bayman P (1993). Competitive exclusion of a toxigenic strain of Aspergillus flavus by an atoxigenic strain. Phytopathology 83: 1283–1287.
Crowder D. and Randa Jabbour R. (2014). Relationships between biodiversity and biological control in agroecosystems: Current status and future challenges. Biological Control 75 (2014) 8–17 Elsevier Inc. http://dx.doi.org/10.1016/j.biocontrol.2013.10.010
Crowder, D.W., Northfield, T.D., Strand, M.R., Snyder, W.E., 2010. Organic agriculture promotes evenness and natural pest control. Nature 466, 109–112.
Dellaporta S, J Wood, and JB Hicks. (1983). A plant DNA mini preparation: version II. Plant Mol Biol Rept 1:19–21.
Dembilio O., Quesada-Moraga E., Santiago-Alvarez, C., Jacas, J.A. (2010). Journal of invertebrate pathology. 104, 214-221.
Dembilio O´, Quesada-Moraga E, Santiago-Alvarez C, Jacas JA. (2010). Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the Red Palm Weevil, Rhynchophorus ferrugineus. Journal of Invertebrate Pathology. 2010; 104(3): 214-221.
Doberski JW. (1981). Comparative laboratory studies on three fungal pathogens of the elm bark beetle Scolytus scolytus: Effect of temperature and humidity on infection by Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. Journal of Invertebrate Pathology. 1981; 37(2): 195-200.
Dube H. C (2007). Fungi, Bacteria and Viruses, 3rd Edition Jodhpur, Agrobios
García-Munguía AM, Garza-Hernández JA, Rebollar-Tellez EA, Rodríguez-Pérez MA, Reyes-Villanueva F. Transmission of Beauveria bassiana from male to female Aedes aegypti mosquitoes. Parasites and Vectors. 2011; 4: 24.
Guerri-Agullo, B., Gomez-Vidal, S., Asensio, L., Barranco, P., Lopez-Llroka L.V. (2010). Microscopy Research and Technique. 73: 714-725.
Gupta, A. K., R. Baran, and R. C. Summerbell. (2000). Fusarium infections of the skin. Curr. Opin. Infect. Dis. 13:121–128.
(https://fungimap.org.au/about-fungi/saprophytic-fungi/j
Hussain A, Ahmed S, Shahid M. (2011). Laboratory and field evaluation of Metarhizium anisopliae var. anisopliae for controlling subterranean termites. Neotropical Entomology. 2011; 40(2): 244-50.
Hussain A, Tian MY, He YR, Ahmed S.(2009). Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians Walker (Lepidoptera: Bombycidae) larvae. Insect Science. 2009; 16: 511-517.
Hussain A, Tian MY, He YR, Bland JM, Gu WX. (2010). Behavioral and electrophysiological responses of C. formosanus towards entomopathogenic fungal volatiles. Biological Control. 2010; 55: 166-173.
Inglis G.D., Johnson D.L., Goettel M.S. (1996). Effects of temperature and thermo-regulation on mycosis by Beauveria bassiana in Grasshoppers. Biological Control. 1996; 7: 131-139.
James RR, Buckner JS, Freeman TP. (2003). Cuticular lipids and silverleaf whitefly stage affect conidial germination of Beauveria bassiana and Paecilomyces fumosoroseus. Journal of Invertebrate Pathology. 2003; 84(2): 67-74.
Krijgsheld, P.; Altelaar, A. F. M.; Post, H.; Ringrose, J. H.; Müller, W. H.; Heck, A. J. R.; Wösten, H. A.; J. Proteome Res. 2012, 11, 2807.
Kumar S, Stecher G., Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874.
Mohan C.M, Lakshmi KA, Devi K.U. (1999). Laboratory evaluation of the pathogenicity of three isolates of the entomopathogenic fungus Beauveria bassiana (Bals.) Vuillemin on the American cockroach (Periplaneta americana). Biocontrol Science and Technology. 1999; 9(1): 29-33.
Qayyum MA, Muhammad Asad Saleem, ShafqatSaeed, Waqas Wakil et al (2020) Integration of entomopathogenic fungi and eco-friendly insecticides for management of red palm weevil, Rhynchophorus ferrugineus (Olivier) Saudi Journal of Biological Sciences Volume 27, Issue 7 Pages 1811-1817
Rajesh ,K.; Dhanasekaran,D. and Tyagi,K. (2014). Mosquito survey and larvicidal activity of actinobacterial isolates against 91 Culex larvae (Diptera: Culicidae), Journal of the Saudi Society of Agricultural Sciences. 2 (6): 233-239 .
Raper, K.B. and Fennell, D.I. (1965). The genus Aspergillus. Baltimore: The Williams and Wilkins Company Research.99:441- 446.
Roberts, H.A. (1981). Seed banks in the soil. Advances in Applied Biology, Cambridge, Academic Press.v.6, 55p.
Saranya S., Ushakumari R, Jacob S, Philip B.M. (2010). Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). Journal of Biopesticides. 2010; 3(1 Special Issue): 138-142.
Schuster, E.; Dunn-Coleman, N.; Frisvad, J. C. P.; van Dijck, W. M. (2002). Appl. Microbiol. Biotechnol. 2002, 59, 426.
Sewefy, G. H., Belal, M.H., Alwash S.A. (2009). Egyptian journal of Biology Pest Control. 19 (2): 157-161.
Shiff ,C. (2002). Integrated approach to malaria control. Clin. Microbiol.Rev. 15: 278-293.
Summerell B.A., Laurence M.H., Liew E. C., Leslie J.F. (2010) Biogeography and phylogeography of Fusarium: a review. Fungal Divers 44:3–13.https://doi.org/10.1007/s13225-010-0060-
White, T. J., Bruns, T., Lee, S. J. W. T., & Taylor, J. W. (1990). Amplifi cation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications, 18(1), 315-322.
World Health Organization WHO (2013). The World Health Report 2013: research for universal health coverage. World Health Organization (contributor). 2013. ISBN 9789241564595. http://www.who.int/whr/2013/report/en/index.html.
Zhou X D., Jacobs K., Kiristis, T., Chhetri.D.B., Wingfield, M. J. (2008).Persoonia. 21,1-8.