1Department of Pharmacognosy, R.C.Patel Institute of Pharmaceutical Education and Research, Shirpur, 425405 Maharashtra, India
.2Department of Pharmaceutics, R.C.Patel Institute of Pharmaceutical Education and Research, Shirpur, 425405 Maharashtra, India.
Corresponding author Email: aniltatiya12171@gmail.com
Article Publishing History
Received: 04/04/2019
Accepted After Revision: 28/05/2019
Glycyrrhiza glabra contains the triterpenoid saponins which are hydrophilic therefore it is essential to provide lipid environment or these molecules should be micro or nanosized so easily diffuse within the aqueous regions near the outer surface of intracellular keratin filaments. The purpose of this study was to enhance the potential of Glycyrrhiza glabra extract (GGE) by preparing nanoemulsion formulation for transdermal application. Nanoemulsion system was developed with Tween 80 as surfactants and IPA as co-solvent and iso propyl myristate as oil for transdermal delivery of Glycyrrhiza glabra extract. Region of nanoemulsion was found in the pseudo-ternary phase diagrams developed at various Tween 80 and IPA ratios. The optimal nanoemulsion formulation consisted ofwater (14.28%),Tween 80 (38.10%), IPA (19.04%)and IPM (28.57%). Invitro and Ex vivo diffusion study shows that absorption of Glycyrrhiza glabra extract /(MAG) was found to be fairly rapid, as compared to aqueous solution of extract.Nanoemulsion in corporate gel (NEIG) was prepared by using Carbopol 93 4 as a gelling agent. In vitro diffusion study showed that there was increase in permeation of GG extract from NEIG; also improve bioavailability of the drug. Our findings suggest that such novel w/o nanoemulsion exhibited significant antimicrobial property and NEIG useful as anti-inflammatory gel and possible alternative to traditional topical formulations with bioavailability issues.
Nanoemulsion,transdermal drug delivery, nanoemulsion gel,Glycyrrhiza glabra extract
Tatiya A, Bhavsar S, Mahajan H, Surana S. Experimental Design and Characterization of Nanoemulsion Based Topical Herbal Gel Developed for Site-Specific Activity of Glycyrrhiza Glabra Extract: in Vitro And Ex- Vivo Studies. Biosc.Biotech.Res.Comm. 2019;12(2).
Tatiya A, Bhavsar S, Mahajan H, Surana S. Experimental Design and Characterization of Nanoemulsion Based Topical Herbal Gel Developed for Site-Specific Activity of Glycyrrhiza Glabra Extract: in Vitro And Ex- Vivo Studies. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2MxGA7d
Copyright © Tatiya et al.,, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Over the ancient times, natural origin compounds and their formulations recognized its biological activities and health benefits. Most of the biologically active components of plants are polar like tannins, flavonoids,terpenoids etc. on the other hand, they are weakly absorbed due to their large molecular size which cannot absorb by passive diffusion and due to their pitiable lipid solubility resulting their pitiable bioavailability (Krishna and Gejjalagere 2018). Nanoemulsion has the ability to solubilize hydrophobic as well as hydrophilic drugs in their nanostructure. As nanoemulsion contain surfactants in it’s composition, the application on the skin surface usually produces an increase in the membrane permeability smooth the progress of transdermal transport (Seema 2014, Chang 2013).A Nanoemulsion is a thermodynamically or kinetically stable liquid formulation. It is a dispersion of an oil phase and a water phase, in combination with a surfactant. The dispersed phase usually consist of small particles or droplets, with a size range of 5 nm-200 nm (Devarajan 2011).The o/w nanoemulsion is used for improving delivery of hydrophobic drugs, whereas w/o nanoemulsion preferred for incorporating hydrophilic drugs (Yen,et.al.,2018.)
Nanoemulsion is of great interest as pharmaceutical, drugs, nutraceuticals, and food products & cosmetics formulation. They are used for administration through various routes like parentral, oral, topical, ocular, pulmonary, mucosal, cosmetic, transdermal, controlled and target (Rachmawati et.al., 2015 Eid et.al., 2013). Nanoemulsions have improved transdermal permeation of many drugs over the conventional topical formulations such as emulsions and gel (Ghosh et.al., 2013, Zhou et.al.,2010 , Sonneville-Aubrun 2018).
Glycyrrhiza glabra contains the triterpenoid saponins which are hydrophilic so it is essential to provide lipid environment or these molecules should be micro or nanosized that can easily diffuse within the aqueous regions near the outer surface of intracellular keratin filaments. The main objective of this study is to formulate nanoemulsion of Glycyrrhiza glabra extract which may increase the permeation through skin and its anti-inflammatory potential.
Materials and Methods
Glycyrrhiza glabra extract was received as a gift sample from Amsar Pvt.Ltd. Indore India. Tween 80, IPA, isopropyl myristate and methanol were procured from LobaChemie, Mumbai, India. All other reagents used were of analytical grade. Solubility of extract and screening of oils and surfactants for nanoemulsion:The equilibrium solubility study was performed by adding an excess amount of Glycyrrhiza glabra extract in 2 mL of various oils (soybean oil, sunflower oil, coconut oil, olive oil, iso propyl myristate), surfactants (Tween 80, Tween 20, span 80, SLS, Transcutol) and cosurfactants (IPA, ethanol, n-amyl alcohol) in 5 mL capacity vials each separately vortexed using a Cyclo mixer [CM 101, REMI (INDIA)].The solubility of Glycyrrhiza glabra extract in various oils and surfactants was determined using the following method. Briefly, an excess amount (approximately 200 mg) of Glycyrrhizaglabra was placed in a 2 mL microtube containing 1 mL of each oil. Then, the vortexed mixture was and kept for 3 days at 37o C in a shaking water bath to facilitate the solubilization. This mixture was filtered using membrane filter (Nylon, 0.45 µm, Gelman, USA).The resulting nanoemulsion were tightly sealed and stored at ambient temperature and physical stability was measured by observing periodically for the occurrence of phase separation. On basis of clarity and transparency of resultant system suitable oil, surfactant and co surfactant were used in the preparation (Rachmawati et.al., 2015 Eid et.al., 2013).
Construction of pseudo-ternary phase diagrams: In order to find out the concentration range of components for nanoemulsion, pseudo-ternary phase diagrams were constructed using oil titration method at ambient temperature (25 0C). On the basis of the solubility studies, IPM was selected as the oil phase. Tween 80 and TPA were selected as surfactant and co surfactant, respectively. Distilled water was used as an aqueous phase. Surfactant and co surfactant (Smix) were mixed at different mass ratios such as 9:1,8:2,7:3,6:4,5:5,4:6,3:7,2:8,1:9.These ratios were chosen in decreasing concentration of surfactant with respect to co surfactant for a detailed study of the phase diagrams. For each phase diagram, oil and Smix at a specific ratio was mixed thoroughly at different mass ratios from 1:1 to 2:1 in different glass vials. Different combinations of oil and Smix were made so that maximum ratios were covered for the study to define the limits of phases precisely formed in the phase diagrams. Pseudo ternary phase diagrams of oil, Smix and aqueous phase were developed using the aqueous titration method. Slow titration with aqueous phase was performed for each mass ratio of oil and Smix and visual observations were made for transparent and easily flow able o/w nanoemulsion. For convenience, the phase diagrams were constructed by drawing “water dilution lines” representing increasing water content and decreasing surfactant-co surfactant levels. The water was titrated along dilution lines drawn from the surfactant-co surfactant to the opposite oil side of the triangle. The line was arbitrarily denoted as the value of the line intersection with the oil scale (eg, 20:80, 30:70). If turbidity appeared followed by a phase separation, the samples were considered to be biphasic. If clear and transparent mixtures were visualized after stirring, the samples were considered monophasic. The samples were marked as points in the phase diagram. The area covered by these points was considered to be the nanoemulsion region of existence (Rachmawatiet.al., 2015 Eid et.al., 2013, Ghosh et.al.,2013).
Formulation development of herbal nanoemulsion: Phase diagram demonstrate the surfactant /co surfactant in ratio 2:1 suitable for nanoemulsion formulation The liquid nanoemulsion was prepared by dissolving extract in double distilled water (0.5-1 %) to the resultant solution added drop wise the mixture of Tween 80 (38.10%), IPA (19.04%) and then IPM (28.57%) with continuous stirring on vortex mixer. No heat is supplied during formulation. The resulting nanoemulsion was tightly sealed and stored at ambient temperature and their physical stability was measured by observing periodically for the occurrence of phase separation; nanoemulsion was also subjected for characterization. Characterization of Nanoemulsion: Dilution test -Small amount of nanoemulsion was placed on a clean glass slide. A drop of water added to the nanoemulsion and was mixed with the help of glass rod and their transparency was assessed visually. If the emulsion is o/w type and it’s diluted with water, it will remain stable as water is the dispersion medium; but if it is diluted with oil, the emulsion will break as oil and water are not miscible with each other, (Shivhare et.al.,2009).
Dye test: Add oil soluble dye such as sudan red III in prepare a nanoemulsion with oil and surfactant Observed a drop of nanoemulsion under microscope and under motic microscope supported with Motic images version 2. Effect of dilution was observed visually and the pH of nanoemulsionmeasured directly on digital pH meter. Droplet Particle size and Polydispersity: Droplet Particle size was measured by dynamic light scattering with zetasize ZS-90 (Malvern instruments ltd., Malvern, U.K) at Temperature 250C, for 30 second duration.Data analysis was conducted using a software package (ELS-8000 software) provided by the manufacturer. Polydispersity index is the ratio of standard deviation to mean droplet size. Polydispersity index (PI) is a measure of particle homogeneity and it varies from zero to 1.0. Zeta potential: Zeta potential of nanoemulsion was determined using zetasizerZS-90 (Malvern instrument ltd., UK). Samples were placed in clear disposable zeta cells and results were recorded. Aliquots of nanoemulsion were sampled in disposable zeta cells and zeta potentials were determined on the basis of electrophoretic mobility under an applied electrical field.
Viscosity :Low viscosity is required to make them good in appearance and easy to handle and packed. Also provide good spray ability. Determine by using Brookfield Viscometer at single mode (spindle C-15) (Stoughton, A) at 100 rpm each sample evaluated in triplicate at a temperature of 250C, and results were presented as average ± standard deviation. Stability Study: The physical stability of the nanoemulsion must be determined under different storage conditions (4, 25 and 40 °C) during study cycle. Fresh preparations that have been kept under various stress conditions for extended period of time are subjected to droplet size distribution analysis and observed for any evidences of phase separation, flocculation or precipitation. Effect of surfactant and their concentration on size of droplet is also being studied. In order to estimate metastable systems, the optimized nanoemulsion formulation was diluted with purified distilled water and was centrifuged (Remi laboratories, Mumbai, India) at 3500 rpm for 30 minute at room temperature and observed for any change in homogenecity of nanoemulsion.
Drug content of nanoemulsion: The drug content of optimized formulation was determined by UV spectrophotometer. 10 mg equivalent of extract containing nanoemulsion was dissolved in 10 ml of methanol. The concentration of solution was found to be 100µg/ml. The Mono amino glycerrhizinate content was estimated at 248nm using UV-Visible spectrophotometer UV-1700. In vitro diffusion studies: In vitro diffusion of nanoemulsion and aqueous solution of extract was carried out by Franz diffusion cell having 3.0 cm diameter and 25 ml capacity. Dialysis membrane (Hi-media) having molecular weight cut off range 12000 – 14000 kDa was used as diffusion membrane. Dialysis membrane was soaked in phosphate buffer pH 5.8 for 24 hrs prior to experiment. Diffusion cell was filled with phosphate buffer pH 5.8 and dialysis membrane was mounted on cell. The temperature was maintained at 32.5oC. After a pre-incubation time of 20 minutes, the nanoemulsionand aqueous solution containing extract equivalent to 10 mg were placed in the donor chamber. Samples were periodically withdrawn from the receptor compartment for 6 hours and replaced with the same amount of fresh phosphate buffer solution, and assayed by a spectrophotometer at 248 nm.
Development of Nanoemulsion based gel :Gel base was prepared by dispersing the 1 g of the Carbopol 934 in a sufficient quantity of distilled water (100ml) after complete dispersion.Carbopol 934 solution was kept for 24 hours till complete swelling. Then the Glycyrrhiza glabra extract loaded nanoemulsion was slowly added to the above prepared gel baseunder magnetic stirring for the development of nanoemulsion based gel ( Modi JD, Patel JK 2011)
Physicochemical Evaluation of gel:Gel was tested for homogenecity by visual inspection after the gel has been set in container they were tested for their appearance of any aggregates.The pH of various gel formulations was determined by using digital pH meter.. The measurement of pH of each formulation was done in triplicate and average values are calculated. Viscosity of the prepared gel was measured using Brook field viscometer at single mode (spindle C-15) (Stoughton, A) at 100 rpm each sample evaluated in triplicate at a temperature of 250C, and results were presented as average ± standard deviation.One of the criteria for a gel to meet the ideal qualities is that it should possess good spread facility. It is the term expressed to denote the extent of area to which gel yarely spreads on application to skin or affected part. The therapeutic efficacy of a formulation additionally depends upon its spreading value. The spredability of gel was tenacious utilizing the following method: Gel (0.5 g) was placed within circle of 1 cm diameter remarked on a glass plate over which a second glass plate was placed. A weight of 500g was sanctioned reposing on the upper glass plate for 5 min. The incrementation in diameter due to spreading of gel was noted (Karri et.al.,2015).
Extrudability study: The formulations were filled in the collapsible tubes after the gels were set in the container. The extrudability of the formulation was determined in terms of weight in grams required to extrude a 0.5 cm. ribbon of gel in 10 second. In vitro drug release: In-vitro diffusion study of nanoemulsion incorporated gel (NEIG) and extract in gel base was carried out by Franz diffusion cell having 3.0 cm diameter and 25 ml capacity. Dialysis membrane (Hi-media) having molecular weight cut off range 12000 – 14000 kDa was used as diffusion membrane. Dialysis membrane was soaked in phosphate buffer (PB) pH 5.8 for 24 hrs prior to experiment. Diffusion cell was filled with phosphate buffer pH 5.8 and dialysis membrane was mounted on cell. The temperature was maintained at 37oC. After a pre-incubation time of 20 minutes, the NEIG and extract gel containing extract equivalent to 10 mg were placed in the donor chamber. Samples were periodically withdrawn from the receptor compartment for 4-6 hours and replaced with the same amount of fresh phosphate buffer solution, and assayed by a spectrophotometer at 248 nm.
Ex Vivo Skin Permeation Studies :The hairs of rat abdominal region were clipped with animal clippers. Full thickness dorsal skin was carefully excised and subcutaneous fat was removed with a dull scalpel then soaked in phosphate buffer pH 5.8. Diffusion cell was filled with phosphate buffer pH 5.8. The skin samples were mounted diffusion cell with stratum corneum side up. Diffusion cell was filled with phosphate buffer pH 5.8 and dialysis membrane was mounted on cell. The temperature was maintained at 32.5oC. After a pre-incubation time of 20 minutes, the NEIG and extract in gel base equivalent to 10 mg were placed in the donor chamber. Samples were periodically withdrawn from the receptor compartment for 4-6 hours and replaced with the same amount of fresh phosphate buffer solution, and assayed by a spectrophotometer at 248 nm.
Skin Irritation studies: The skin irritation study for nanoemulsion based gel was carried out on Swiss albino mice weighing 25-35 g. The experiments were carried as protocol approved from Institutional Animal ethical committee. The animals were kept under standard laboratory conditions and housed in polypropylene cages. The animals were divided into three groups. Group I was taken as negative control received normal saline and Group II served as positive control received formalin solution and Group III applied with nanoemulsion based gel formulations. A single dose of the NEIG was applied to right ear of the mice keeping left as control in Group III. In group II formalin solution was applied; development of erythema or skin irritation was visualized after a total period of 21 days (Sonia K et.al., 2011)
In-Vivo Pharmacodynamics Study Using Carrageenan-Induced Rat Paw Edema
Anti-inflammatory activity: It was evaluated on the basis of the inhibition of the carrageenan-induced hind paw edema.The rats of either sex (180-200) were divided into four groups each group containing six animals .The rats were fasted for 12 hrs prior to induction of edema however water was provided. To ensure uniform hydration, the rats receives 5ml of water by stomach tube.Thirty minutes later acute inflammation was induced by sub‐planter injection of 0.1 ml of freshly prepared 1 % suspension of carrageenan in normal saline in left hind paw of the rats The NEIG formulation (0.25g) or base was applied topically with gentle rubbing to the paw of each rat of test group one hour before and one hour after the carrageenan challenge. Rats of the control groups received only the gel base and standard marketed formulation diclofenac gel BP applied in the same way as a reference standard. The paw volume was measured at 0, 1, 2, 3, 4, 5 and 6 hour using the digital Plethysmometer (Ugo Basile, Italy). The percentage inhibition of paw volume in test groups was compared with the control group (Prakash PR et.al.,2010)
Percentage inhibition of edema = (1−Vt/Vc) × 100
Where, Vt is the inflammatory increase in paw volume in test groups and Vc is the inflammatory increase in paw volume in normal control group of rats. Percentage inhibition of edema is proportional to anti-inflammatory activity.
Antimicrobial Study: Broth macro dilution Assay: Broth dilution is a technique in which a suspension of bacterium of a appropriate concentration is tested against varying concentrations of an antimicrobial agent. As nanoemulsion o/w it will break if directly mixed with broth media which was prepared in water so the 1% and 2% nanoemulsion was dissolved in DMSO so that final concentration was 2.5mg/ ml and 5mg/ml the and Glycyrrhiza glabra extract 5mg/ml and 10 mg/ml was prepared in DMSO. A suspension of different bacterium and fungi was poured in above test tubes. The tubes with bacterial suspension were incubated at 37 0C for 24 hours and with fungal culture were incubated at 300C for 48 hours.After incubation observed for growth or turbidity (Das K et.al.,2010).
MIC by Micro titer plate method
Different concentrations of nanoemulsion and extract were prepared in DMSO. An equal volume i.e 1µl of bacterial suspension/fugal suspension and 1µl each concentration was added to the wells initial absorbance was measured at 620nm and plates were incubated at 37°C for 24 h after incubation, plates are examined for changes in turbidity as an indicator of growth. Initial absorbance and absorbance after incubation were compared. The lowest concentration of drug that reduces, by more than 50% or Concentration with sharp decline in absorbance value is the MIC (Hasan et.al., 2010,Selvamohan et.al., 2012 ,Dasari et.al.,2014).
Results and Discussion
Development of nanoemulsion formulation depends on physicochemical properties of drugs. The solubility of the drug is most important factor as the ability of nanoemulsion to maintain the drug in solubilised form. Solubility of the lipophilic drugs in the oil phase and hydrophilic drugs in aqueous phase is an important criterion for the selection of oils and water respectively. GGE and MAG are hydrophilic in nature. The solubility of MAG in different oils was determined since solubility of MAG was higher in aqueous phase as compared to oil phase, w/o nanoemulsion was developed for transdermal delivery of MAG. In order to select suitable oils and surfactants for good solubilizing of herbal extract of Glycyrrhizaglabra in various oil and surfactant were determined. Medium and long chain triglyceride oils with different degree of saturation have been tried in the development of nanoemulsion. Oils tried were soybean oil, sunflower oil, coconut oil, olive oil, iso propyl myristate (IPM) etc. Surfactants employed were Tween 80, Tween 20, span 80, SLS, Transcutol and co-surfactant was IPA, ethanol, n-amyl alcohol.
The solubility of Glycyrrhiza glabra was determined indifferent oils. It was evident that Glycyrrhiza glabra exhibited highest solubility in isopropyl myristate (195.71±0.91 mg 2 mL-1) as polarity of the poorly soluble drugs favors their solubilization in small/medium molar volume oils. Edible oils cannot depict large micro emulsion region due to their rancid nature. IPM was selected for the preparation of nanoemulsion due to its well-known permeation enhancing property and biocompatibility.The most critical problem related in the development of nanoemulsion based drug delivery systems is the toxicity of the surfactants. Large amounts of surfactants may cause skin irritation when administered transdermally. It is therefore important to determine the surfactant concentration properly and use the minimum concentration in the development of nanoemulsion formulation (Alam et.al.,2010).
Amongst various surfactants screened, Tween 80 exhibited (96.8±1.47 mg 2 mL-1) highest solubilization capacity of GGE. Whereas various co-surfactants were screened for solubility as well miscibility with surfactant, Isopropyl alcohol IPA was revealed greater solubilization capacity (101.98±1.02 mg 2 mL−1) as well as it forms transparent system (99.87 %T).The screening of surfactant and co-surfactants on the basis of solubility is difficult because all surfactant and co surfactant cannot solubilise all type of oil phase. Surfactant chosen must be able to lower the interfacial tension to a very small value to aid the dispersion process during the preparation of the nanoemulsion, provide a flexible film that can readily deform around droplets. aqueous phase, the second representing oil and the third representing a mixture of surfactant and co surfactant at a fixed mass ratio.
Table 1: Formulation composition of Nanoemulsion Batch 1
| Ingredients | Formulations composition (% w/v) | ||||
| FA1 | FB1 | FC1 | FD1 | FE1 | |
| Distilled Water | 5.5 | 5.5 | 25.0 | 14.28 | 5.0 |
| Tween 80 | 25.00 | 25.00 | 38.89 | 38.10 | 30.0 |
| IPA | 25.00 | 25.00 | 19.44 | 19.04 | 15.0 |
| IPM | 44.44 | 44.44 | 16.66 | 28.57 | 50 |
| Extract | 0.0 | 0.5 | 0.5 | 0.5 | 0.5 |
Table 2: Formulation composition of Nanoemulsion Batch 2
| Ingredients | Formulations composition (% w/v) | ||||
| FA2 | FB2 | FC2 | FD2 | FE2 | |
| Distilled Water | 5.0 | 5.0 | 5.0 | 14.28 | 14.28 |
| Tween 80 | 30.0 | 30.0 | 30.0 | 38.10 | 38.10 |
| IPA | 15.0 | 15.0 | 15.0 | 19.04 | 19.04 |
| IPM | 50 | 50 | 50 | 28.57 | 28.57 |
| Extract | 0.25 | 0.50 | 0.75 | 1.0 | 0.50 |
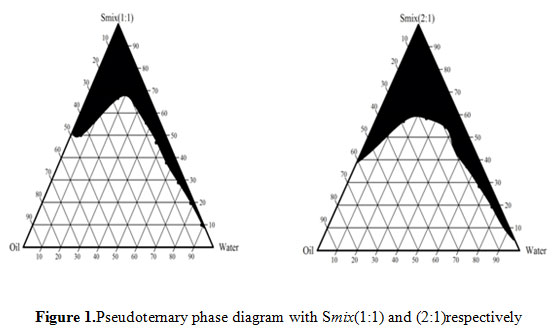 |
Figure 1: Pseudoternary phase diagram with Smix(1:1) and (2:1)respectively |
Figures1 represent the pseudo ternary phase diagrams for nanoemulsion systems along with the ratios of surfactant and co surfactant, as 1:1 and 2:1. The change in the area of nanoemulsion region can be very well observed in the ternary phase diagram as the ratio of surfactant to co surfactant was changed from 1:1 to 2:1. When ratio of surfactant to co surfactant was 2:1, there was increased in nanoemulsion region, because of high concentration of surfactant.Nanoemulsions are considered to be thermodynamically stable systems that are formed at a particular concentration of oil,surfactant, and water, with no phase separation, creaming, or cracking. Selected formulations from phase diagram were subjected to different stress stability testing like heating cooling cycle, centrifugation, and freeze-thaw cycle. During physical stability testing, some formulations became turbid and in some phase separation occurred. One reason of this instability in nanoemulsions may be due to the Ostwald ripening. in which molecules move as a monomer and coalescence of small droplets takes place, resulting in the formation of large droplets by diffusion processes driven by the gain in surface free energy. The other reason may be that when temperature quench occurs during stress stability study, instability of nanoemulsion occurs due to separation of oil phase and droplet distribution of smaller size is favoured by the change in curvature free energy. Only those formulations, which showed no phase separation, creaming, cracking, coalescence, and phase inversion during stress stability tests, were selected for further studies (Osanloo et.al.,2018).
Novel nanoemulsion of Glycyrrhiza glabra extract was prepared by the spontaneous emulsification method (oil phase titration method), Isopropyl myristate was used as oil phase components. Optimized nanoemulsion consists of water (14.28%), Tween 80 (38.10%), Isopropyl alcohol (19.04%) and Isopropyl myristate (28.57%).Nanoemulsion developed was clear and transparent. From dilution and dye test it can be concluded that the system was w/o type with pH 5.59±0.01, 5.78± 0.005.
 |
Figure 2: Photomicrographof W/O type of nanoemulsion. |
As drug loading increased 0.5,1% the particle size of optimized nanoemulsion was varies, 104.2 nm 185.3 nm but almost it is in range. It has been reported that the smaller particle size of the emulsion droplets may lead to more rapid absorption and improve the bioavailability Polydispersity is the ratio of standard deviation to mean droplet size, so it indicates the uniformity of dispersity. Polydispersity index (PDI) is a measure of particle homogenicity and it varies from 0.0 to 1.0. PDI 0.186 – 0.451 found it closer to zero so the more homogenous are the particles. Polydispersity signifies the uniformity of droplet size within the formulation. The polydispersity value of the formulations was very low (<0.4) which indicated uniformity of droplet size within the formulation. The more negative zeta potential, greater the net charge of droplets and more stable the emulsion. Zeta potential values lower than -30 mv generally indicate a high degree of physical stability the zeta potential found -9.5 to -13.8.It shows little change with increase in concentration of extract but all the values are in range and the nanoemulsion was found to be stable on thermodynamic stability study. The viscosity of nanoemulsion at room temperature was 67.43± 0.15cP. Generally, it was observed that the viscosity of the nanoemulsion formulations was very low. Lower viscosity is one of the characteristics of nanoemulsion formulations (Gurpreet & Singh 2018.) The drug content was found to be 97.89% (Table 3, 4)
Table 3: The influence of mixture components on characterization of prepared nanoemulsion Batch -1
| Parameters | FA1 | FB1 | FC1 | FD1 | FE1 | ||||||||
| Initial | After 1 month | Initial | After 1 month | Initial | After1 month | Initial | After 1 month | Initial | After 1month | ||||
| Zeta size | 353.4 | Unstable | 247.1 | Unstable | 203.2 | Unstable | 245.4 | 206.3 | 236.5 | 183 | |||
| PDI | 0.568 | 0.339 | 0.26 | 0.249 | 0.384 | 0.249 | 0.204 | ||||||
| Zeta potential | -44.7 |
|
|
|
-16 | -34.4 | -27.4 |
Table 4: The influence of mixture components on characterization of prepared nanoemulsion batch-2
| Parameters | FA2 | FB2 | FC2 | FD2 | FE2 | |
| Zeta size | 208 | 175.1 | 183.5 | 185.1 | 104.2 | |
| PDI | 0.202 | 0.198 | 0.234 | 0.451 |
|
|
| Zeta potential | -35.3 | -33.6 | -19.2 | -13.8 | -9.5 |
Evaluation of prepared nanoemulsion was carried out for its physicochemical parameters. It was found that w/o nanoemulsion was thermodynamically stable and no or little effect of drug concentration on it. The formulation is safe for topical application.
In vitro diffusion through the dialysis membrane of formulation shown in Table 5, Figure 3.
Table 5: In Vitro Drug Diffusion study of Nanoemulsion and Extract
| Time (Minutes) | % Cumulative Drug Release (Mean ± SD) of Nanoemulsion | % Cumulative Drug Release (Mean ± SD) of Extract |
| 15 | 17.30 ± 0.41 | 8.083±0.60 |
| 30 | 21.57 ±0.62 | 10.85±0.98 |
| 60 | 26.22 ±1.42 | 13.35±0.61 |
| 90 | 38.64 ±1.20 | 16.75±0.18 |
| 120 | 54.66 ±0.72 | 20.00±0.62 |
| 150 | 68.80 ±1.09 | 24.18±0.23 |
| 180 | 82.58 ±0.60 | 28.56±2.28 |
| 210 | 95.30 ±0.97 | 33.17±1.25 |
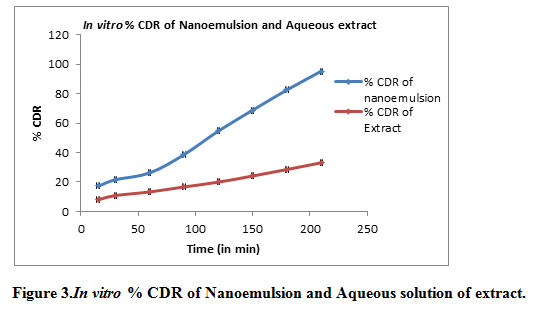 |
Figure 3: In vitro % CDR of Nanoemulsion and Aqueous solution of extract |
After 3.5 hours drug release from the nanoemulsionwas 95.30% while drug release from aqueous solution of extract was 33.17% which is much less as compared to NE. It was observed that rate of diffusion was improved for nanoemulsion attributed to their nano range size. The significant difference in permeation between nanoemulsion formulations and aqueous solution could be due to the mean size of droplets.
Ex vivo permeation through rat skin of formulation shown as
Table 6: Ex-vivo Drug permeation study data
| Time (Minutes) | % Cumulative Drug Release (Mean ± SD) of Nanoemulsion | % Cumulative Drug Release (Mean ± SD) of Extract |
| 15 | 16.77±0.12 | 9.25±0.72 |
| 30 | 20.12±0.29 | 11.11±0.53 |
| 60 | 23.85±0.25 | 13.31±0.27 |
| 90 | 36.60±0.98 | 14.96±0.23 |
| 120 | 48.23±0.40 | 21.37±0.54 |
| 150 | 65.36±0.95 | 25.23±0.19 |
| 180 | 83.68±0.19 | 30.17±0.78 |
| 210 | 92.26±0.37 | 33.61±0.71 |
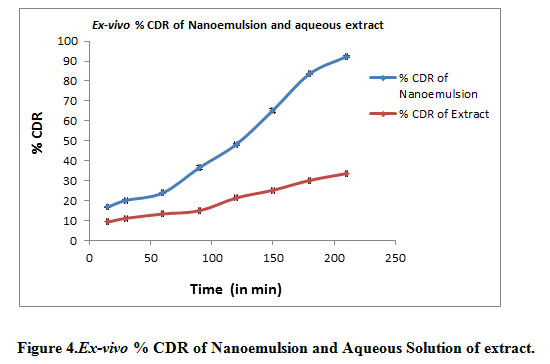 |
Figure 4: Ex-vivo % CDR of Nanoemulsion and Aqueous Solution of extract |
Ex vivo permeation study results shows that drug permeation through skin in 210 min (3.5 hr) is 92.26% and through aqueous solution of extract it was only 33.61%. This proved that the nanoemulsion enhance the ability of drug to permeation through the skin.
Nano emulsion incorporated gel found to be homogeneous with pH5.65±0.02 visosity118.66±0.30 and spreadabilty5.36±0.11 with good extrudability property.Physical parameters of gel evaluation are given in Table7.
Table 7: Evaluation parametersof nanoemulsion incorporated gel
| Formulation | Homogenecity | pH | Viscosity | Spreadability | Extrudability |
| NEIG | Homogeneous | 5.65±
0.02 |
118.66±
0.30 |
5.36±0.11 | Good |
Skin irritation studies :It was observed that nanoemulsionincorporatedgel (NEIG) application on Swiss albino mice no signs of erythema and skin irritation even on applicationfor 21 days. Thus, the developed formulation is non-sensitizing and safe for use.In vitro diffusion through the dialysis membrane of formulation seen in Table 8, Figure 5.
Table 8: In vitro Drug Diffusion data of NEIG
| Time (Minutes) | % Cumulative Drug Release (Mean ± SD) of NEIG | % Cumulative Drug Release (Mean ± SD) of Extract in Gel base |
| 15 | 17.30±0.10 | 9.75±0.08 |
| 30 | 21.32±0.42 | 12.75±0.09 |
| 60 | 24.88±0.26 | 16.31±0.31 |
| 90 | 38.43±1.02 | 18.48±0.13 |
| 120 | 49.10±0.15 | 22.31+0.43 |
| 150 | 67.22±0.19 | 25.97+0.42 |
| 180 | 85.24±0.21 | 31.92+1.07 |
| 210 | 98.19±0.22 | 36.54+1.25 |
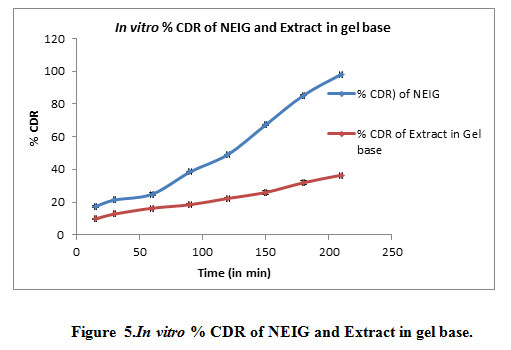 |
Figure 5: In vitro % CDR of NEIG and Extract in gel base |
After 3.5 hours drug release from the NEIG 98.19% while drug release from extract in gel base is 36.54% which is much less as compared to NEIG. It was observed that there was increase permeation of GG extract from NEIG.It was observed that the when nanoemulsion incorporated in Carbopol gel it will enhance its permeation than nanoemulsion as such.
The nanoemulsion shows the positive susceptibility test for all the six bacterial and two fungal cultures so subjected forMIC determination.MIC of nanoemulsion and extract given in Table 9.
Table 9: Minimum inhibitory concentration (MIC) of Extract and nanoemulsion for different micro-organisms
| Micro-organism | MIC of Extract
mg/ml |
MIC of nanoemulsion
mg/ml |
| Escherichia coli | 5+0.3 | 2.5 |
| Staphylococcus aureus, | 10+1.00 | 2.5 |
| Pseudomonas aeruginosa, | 15+1.74 | 2.0 |
| BascillusSubstalis | 15+1.52 | 2.5 |
| Proteous species | 20+1.98 | 1.25 |
| Shigellasoni | 15+1.02 | 3.75 |
| Aspergillus niger | 5+0.25 | 3.75 |
| Candida Albicans | 20+1.06 | 3.75 |
As the MIC of Nanoemulsion was less than Extract for all the bacterial and fungal cultures we can say that nanosized droplets have more energy and surfactant to destabilize the targeted microbes. The nanoemulsion particles are thermodynamically driven to fuse with lipid-containing organisms. When sufficient nanoparticles fuse with the pathogens, they release part of the energy trapped within the emulsion. Both the active ingredient and the energy released destabilize the pathogen lipid membrane, resulting in cell lysis and death.
The results of anti-inflammatory activity i.e. increase in paw volume at different time interval and % inhibition after topical administration of Diclofenac gel,NEIG,Extract in gel are given in Table 10 and Figure 6
Table 10: Mean paw volume and % inhibition at different time interval
| Paw volume at different time after carrageenan injection | |||||||
| Treatment | Initial | 1h | 2h | 3h | 4h | 5h | 6h |
| Group I
(Control) |
1.19 ±
0.020** |
1.43 ±
0.021*** |
1.62 ±
0.018*** |
1.67 ±
0.017*** |
1.71 ±
0.015***
|
1.63 ±
0.013*** |
1.61 ±
0.010*** |
| GroupII Standard
(Diclofenac) |
1.17 ±
0.003** — |
1.23 ±
0.009*** (13.80) |
1.29 ±
0.008*** (20.43) |
1.31 ±
0.002*** (21.28) |
1.39 ±
0.01*** (18.74) |
1.36 ±
0.013*** (16.55) |
1.32 ±
0.019*** (17.67) |
| Group III
Test1 (NEIG) |
1.08 ±
0.021** — |
1.18 ±
0.02*** (16.85) |
1.26 ±
0.01*** (22.55) |
1.286 ±
0.01*** (23.04) |
1.345 ±
0.01*** (21.52) |
1.33 ±
0.01*** (18.26) |
1.32 ±
0.001*** (17.88) |
| Group IV
Test2 (Extract gel) |
1.12 ±
0.025** — |
1.29 ±
0.039*** (9.42) |
1.36 ±
0.038*** (15.90) |
1.44 ±
0.04*** (13.78) |
1.50 ±
0.04*** (11.92) |
1.48 ±
0.04*** (8.60) |
1.48 ±
0.038*** (8.01) |
All the values expressed mean±S.E.M. (percent inhibition) n=6 .Data was analyzed byone way ANOVA followedby Dunnet test ** P<0.05 ,*** P< 0.001
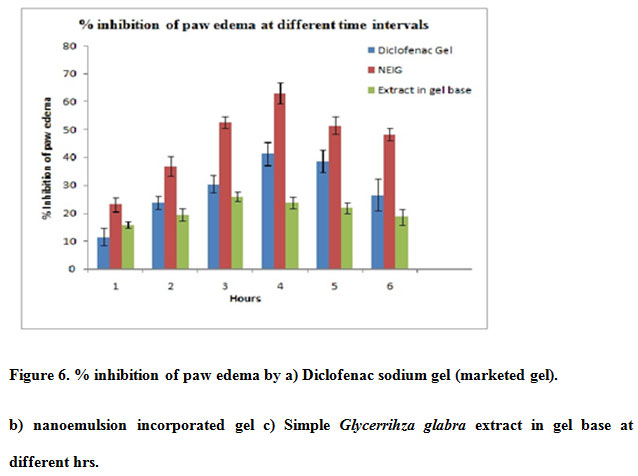 |
Figure 6: % inhibition of paw edema by a) Diclofenac sodium gel (marketed gel). b) nanoemulsion incorporated gel c) Simple Glycerrihza glabra extract in gel base at different hrs. |
Statistical analysis showed that the topical preparation has significant inhibition of carrageenan induced rat paw edema when compared with control group. It was also observed that the % inhibition of edema by NEIG was greater than standard diclofenac gel and Extract in gel base. Glycyrrhizaglabra extract has anti-inflammatory activity so it reduces the paw edema but when we use NEIG activity increases two fold which could be due to enhanced permeation of MAG through skinSo it was concluded that the nanosize of extract in NEIG responsible for better absorption so as to increase its bioavailability and therapeutic effect (Mahboobian 2017., Rai et.al 2018)
Conclusion
In-vitro drug diffusion and Ex-vivo permeation study was concluded that permeation rate was faster in nanoemulsion as compared to solution of extract; also MIC value of nanoemulsion was very less as compared to extract it means better antimicrobial activity. Nanoemulsion further incorporated in Carbopol 934 (NEIG) with good homogenisity.In-vitro diffusion study revealed that the nanoemulsion incorporated in Carbopol gel enhances its permeation than nanoemulsion alone.Drug delivery through the skin to the systemic circulation is convenient for a number of clinical conditions due to which there has been a considerable interest in this area.NEIG has significant anti-inflammatory and antimicrobial effect attributed to nanosize of extract in responsible for rapid and complete absorption improving its therapeutic effect. Use of nanoemulsion in transdermal drug delivery represents an important area of research in drug delivery, which enhances the therapeutic efficacy and also the bioavailability of the drugs without any adverse effects. It is also regarded as a promising technique with many advantages including, high storage stability, low preparation cost, thermodynamic stability, absence of organic solvents, and good production feasibility.
Acknowledgments
Authors are thankful to Amsar Pvt Ltd Indore, India for providing gift samples of Glycyrrhiza glabra extract. The authors are also thankful to the Department of Pharmaceutics, (R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, for providing necessary facilities to carry out this work.
Disclosure: The authors report no conflict of interest in this work
References
Alam M. S, Ali M. S, Alam N, Alam M. I, Anwer T, Imam F, &ShamimM. (2012). Design and characterization of nanostructure topical gel of betamethasone dipropionate for psoriasis. Journal of Applied Pharmaceutical Science, 2(10) : Pages 148.
Alam M. S, Ali M. S, Alam N, Alam M. I, Anwer T, Imam F, &ShamimM. (2012). Design and characterization of nanostructure topical gel of betamethasone dipropionate for psoriasis. Journal of Applied Pharmaceutical Science, 2(10) : Pages 148.2.
Barakat N, Fouad E, &Elmedany A. (2011). Formulation design of indomethacin-loaded nanoemulsion for transdermal delivery. Pharm Anal Acta S, 2: Pages 1-8.3.
Chang R. K., Raw A., Lionberger R., &Yu L, (2013). Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products. The AAPS journal, 15(1) : Pages 41-52.4.
Das K, Tiwari R. K. S, &Shrivastava D. K, (2010). Techniques for evaluation of medicinal plant products as antimicrobial agents: current methods and future trends. Journal of medicinal plants research, 4(2) : Pages 104-111.5.
Dasari S, Shouri, R. N. D, Wudayagiri R, &Valluru L. (2014). Antimicrobial activity of Lactobacillus against microbial flora of cervicovaginal infections. AsianPacific Journal of Tropical Disease, 4(1) : Pages 18.6.
Devarajan V & RavichandranV. (2011). Nanoemulsions: as modified drug delivery tool. International journal of comprehensive pharmacy, 4(01) : Pages 1-6.7.
Eid A. M, Elmarzugi, N. A, & El-Enshasy H. A. (2013). Development of avocado oil nanoemulsion hydrogel using sucrose ester stearate. Journal of Applied Pharmaceutical Science, 3(12) : Pages 145.8.
Ghosh V, Mukherjee A, &Chandrasekaran N. (2013). Formulation and characterization of plant essential oil based nanoemulsion: evaluation of its larvicidal activity against Aedes aegypti. Asian Journal of Chemistry, 25(Supplementary Issue) : Pages S321.9.
Gopalasathees kumar, K., Komala, S., Soundarya, R., Parthiban, S., Bharathi, B. D., & Elango, S. (2017). Review on emulgel formulations with non-steroidal anti-inflammatory drugs for topical administration. Pharma Science Monitor, 8(1).10.
Gurpreet, K., & Singh, S. K. (2018). Review of Nanoemulsion Formulation and Characterization Techniques. Indian Journal of Pharmaceutical Sciences, 80(5): Pages 781-789.
Krishna, S. P., & Gejjalagere, H. C. (2018). Advancement of Biodegradable Polysaccharide Nanocarriers for Delivery of Herbal Extracts and Bio-Actives. In Polysaccharide based Nano-Biocarrier in Drug Delivery: Page. 191-205.
Liao, W., Liu, Z., Zhang, T., Sun, S., Ye, J., Li, Z. & Ren, J. (2017). Enhancement of anti-inflammatory properties of nobiletin in macrophages by a nano-emulsion preparation. Journal of agricultural and food chemistry, 66(1):Page 91-98.
Mahboobian, M. M., Seyfoddin, A., Rupenthal, I. D., Aboofazeli, R., & Foroutan, S. M. (2017). Formulation development and evaluation of the therapeutic efficacy of brinzolamide containing nanoemulsions. Iranian journal of pharmaceutical research: IJPR, 16(3): page 847.14.
Osanloo, M., Sereshti, H., Sedaghat, M. M., & Amani, A. (2018). Nanoemulsion of Dill essential oil as a green and potent larvicide against Anopheles stephensi. Environmental Science and Pollution Research, 25(7), 6466-6473.
Pastore, M. N., Kalia, Y. N., Horstmann, M., & Roberts, M. S. (2015). Transdermal patches: history, development and pharmacology. British journal of pharmacology, 172(9), 2179-2209.
Rai, V. K., Mishra, N., Yadav, K. S., & Yadav, N. P. (2018). Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. Journal of controlled release, 270, 203-22517. Sonneville-Aubrun, O., Yukuyama, M. N., & Pizzino, A. (2018). Application of Nanoemulsions in Cosmetics. Nanoemulsions page: 435-475.
Yen, C. C., Chen, Y. C., Wu, M. T., Wang, C. C., & Wu, Y. T. (2018). Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. International journal of nanomedicine, Pages : 13, 669.
Gur M, Verep D, Guney K, Guder A, &AltunerE. M. (2017). Determination of Some Flavonoids and Antimicrobial Behaviour of Some Plants’ Extracts. Indian Journal Of Pharmaceutical Education And Research, 51(3) : Pages S225-S229.20.
Hani U, &Shivakumar H. G. (2014). olubility enhancement and delivery systems of curcumin a herbal medicine: a review. Current drug delivery, 11(6) : Pages 792-804.21.
Hasan H. A, Raauf A. M. R, Razik B. M. A, & Hassan B. A. R. (2012). Chemical composition and antimicrobial activity of the crude extracts isolated from Zingiber officinale by different solvents. Pharmaceut Anal Acta, 3(9) : Pages 1-5.22.
Karri V. N. R, Raman S. K, Kuppusamy G, Mulukutla S, Ramaswamy S, &Malayandi R. (2015). Terbinafine hydrochloride loaded nanoemulsion based gel for topical application. Journal of Pharmaceutical Investigation, 45(1) : Pages 79-89.23.
Kharat N, Shylaja H, Viswanatha G. L, &Lakshman K. (2010). Anti-inflammatory and analgesic activity of topical preparation of root extracts of Ichnocarpus frutescens(L.) R. Br. Int J Appl Biol Pharmac Tech, 1(3).24.
Meshram G. G, Kumar A, Rizvi W, Tripathi C. D, &Khan R. A. (2016). Evaluation of the anti-inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. Journal of traditional and complementary medicine, 6(2) : Pages 172-175.25.
Modi J. D, & Patel J. K. (2011). Nanoemulsion-based gel formulation of aceclofenac for topical delivery. International Journal of Pharmacy and Pharmaceutical Science Research, 1(1) : Pages 6-12.26.
Prakash, P. R, Rao N. R, & Chowdary S. (2010). Formulation, evaluation and anti-inflammatory activity of topical etoricoxib gel. Asian J. of Pharm. and ClinicalRes, 3: Pages 126.27. Rachmawati H, Budiputra D. K, & Mauludin R. (2015). Curcumin nanoemulsion for transdermal application: formulation and evaluation. Drug development and industrial pharmacy, 41(4) : Pages 560-566.28.
Seema A. (2014). Recent development of herbal formulation—a novel drug delivery system. International Ayurvedic Medical Journal, 2(6): Pages 952-958.29.
Shivhare U. D, Jain K. B, Mathur V. B, Bhusari K. P, & Roy, A. A. (2009). Formulation Development And Evaluation Of Diclofenac Sodium Gel Using Water Soluble Polyacrylamide Polymer. Digest Journal of Nanomaterials & Biostructures(DJNB),4(2).30.
Sonia K, &Anupama D. (2011). Microemulsion based transdermal drug delivery of tea tree oil. International Journal of Drug Development & Research, 3(1) : Pages 191-198.31.
Srilatha R, Aparna C, Srinivas P, &Sadanandam M. (2013). Formulation, evaluation and characterization of glipizide nanoemulsion. Asian J Pharm ClinResearch, 6(2) : Pages 66-71.32.
Thakur A, Walia M. K, & Kumar S. (2013). Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: a review. Pharmacophore, 4(1) : Pages 15-25.33.
Wagner H, &Bladt S. (1996). Plant drug analysis: a thin layer chromatography atlas. Springer Science & Business Media.34.
Zhou H, Yue Y, Liu G, Li Y, Zhang J, Gong Q &DuanM. (2010). Preparation and characterization of a lecithin nanoemulsion as a topical delivery system. Nanoscale research letters, 5(1) : Page 224.


