Department of Animal Biotechnology, Madras Veterinary College, TANUVAS Chennai (Tamil Nadu), India
Corresponding author email: sumedha_bobade@rediffmail.com
Article Publishing History
Received: 13/10/2019
Accepted After Revision: 30/11/2019
Exosomes are nanovesicles and is a newly emerging field as a novel form of information exchange in various fields of research. Exosomes are a next-generation therapeutic platform and their role in diagnostics acts as a potential vehicle to deliver therapies to cells of the body, as they are rich in cargos like proteins and nucleic acids. Cargo trafficking provides an opportunity to become effective therapeutic reagents for various diseases, like cancer and are also used as biomarkers for the diagnosis. Exosomes are released in biological fluids, such as plasma, serum, urine, and saliva. The present review article provides a comprehensive account on exosome biogenesis, extraction, and their potential use in diagnostics, therapeutic, neurodegenerative disease diagnosis as well as highly precious biomedical tools and their roles in ttherapeutic vehicles for drug delivery. In future as the field rapidly expands, the different exosome cargos can be used as potential biomarkers which can revolutionize the area of personalized medicine.
Biogenesis, Cargos, ESCRT, MVBs, Nanocarrier
Bobade S, Vijayarani K, Tirumurugaan K. G, Thangavelu A, Vairamuthu S, Kalaiselvi G. Exosome as an Exciting Class of a Next-Generation Therapeutic Platform and its Role in Diagnostics. Biosc.Biotech.Res.Comm. 2019;12(4).
Bobade S, Vijayarani K, Tirumurugaan K. G, Thangavelu A, Vairamuthu S, Kalaiselvi G. Exosome as an Exciting Class of a Next-Generation Therapeutic Platform and its Role in Diagnostics. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/32LmxoI
Copyright © Bobade et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Exosomes were first reported by Pan and Johnstone in 1983 at McGill University while culturing sheep reticulocytes during the process of elimination of transferrin (Tfr) receptors, which occur during the maturation process of reticulocytes and erythrocytes. The term exosomes was coined by Trams et al. (1981) who described the release of EVs with 5′-nucleotidase activity from various normal and neoplastic cell lines. Exosomes are disk-shaped extracellular membranous, nano-size vesicles (50–100 nm) that originated from multivesicular bodies (MVBs) (Janas et al., 2016). Exosomes are rich in cargos, which contain proteins, nucleic acids, miRNA, and lipids that directly reflect the metabolic state of the cells from which it originates. Exosomes are efficient messengers of intercellular communication and isolated from body fluids including urine, blood , malignant ascites and cultured supernatants of cell lines, breast milk, cerebrospinal fluid, saliva, amniotic fluid, lymph, bile under healthy and morbid condition. Exosomes are small membrane vesicles that are released by many cell types, including lymphocytes, dendritic cells (DCs) , macrophages, epithelial cells, platelets and tumorigenic cells ( Tang et al.,2015,Rashed et al., 2017, Liu et al., 2019).
Exosomes formed by membrane invagination of late endosomes, resulting in vesicles that contain cytosolic components and expose the extracellular domain of some plasma membrane receptors at their surface (Huotari and Helenius , 2011). Exosomes remained little studied for the next decade because earlier they were considered as garbage cans which discard unwanted cellular components (Rashed et al., 2017).
Exosomes are biomembrane-like vesicles containing specific protein markers of the endosomal pathway. The first classes of nucleic acids identified in exosomes are miR and mRNA, non-coding RNAs (ncRNAs) also the presence of small fragments of single-stranded DNA. The other identified RNA species in exosomes included transfer RNAs (tRNAs), long noncoding RNAs (lncRNAs), and viral RNA. The mRNA and noncoding RNAs such as miRs and lncRNAs in exosomes are functional and can impact the transcriptome of recipient cells. Exosomal contents are depends on their cellular origin and enriched with targeting molecules, membrane trafficking proteins (e.g. Rab proteins, GTPases, annexins and ARF), proteins involved in MVB formation (e.g., ALIX, clathrin and TSG101), cytoskeletal proteins (e.g., actin and tubulin), signal transduction proteins (e.g., protein kinases and heterotrimeric G proteins), chaperones (e.g. small heat shock proteins HSPs), Hsp60, Hsp70, Hsp90, fusion proteins (e.g., tetraspanins, , intergrins and lactadherin), cytoplasmic enzymes (e.g. peroxidases, GAPDH, pyruvate kinases, and lactate dehydrogenase), MHC class I and II proteins , epithelial cell adhesion molecules (EpCAM), and members of the human epidermal receptor (HER) family (Balaj et al.,2011, Kalluri, 2016).
Exosomes can be released by all eukaryotic cells and their cargos may greatly differ from each other for function of the originated cell types and their current state (e.g. transformed, differentiated, stimulated, and stressed). Exosome-specific protein conformation based on cell type or tissue birthplace from which it originates may differ according to the physiological changes and stimulation that the cell underwent. For example, antigen-presenting cell-derived exosomes are enriched in antigen presenting molecules, major histocompatibility class (MHC)-I and -II complexes, as well as co-stimulatory molecules. Tumor-derived exosomes usually contain tumor antigens in addition to certain immunosuppressive proteins such as FasL, TRAIL, or TGF-β. Exosomes also contain proteins involved in cell signaling pathways, such as the Notch ligand Δ- like 4, Wnt-β-catenin signaling proteins and intercellular cell signaling, such as interleukins (Rashed et al., 2017 Zhang et al., 2019a).
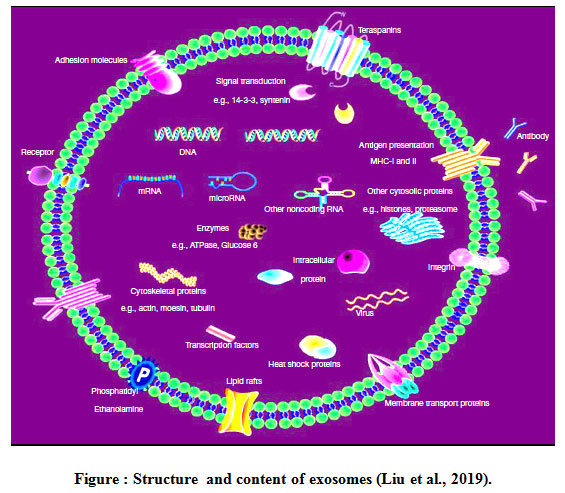 |
Figure 1: Structure and content of exosomes (Liu et al., 2019). |
Exosome biogenesis
Biogenesis of exosomes starts in the endosomal system. Exosomes are a class of EVs that originate from the endosome and released from cells when MVBs containing ILVs (Intraluminal vesicles) fuse with the plasma membrane of the cell and release ILVs (which referred as exosomes) into the extracellular environment. The synthesis of exosomes is carried out by two major pathways .This process is highly regulated by multiple signal transduction cascades. Exosomes release from the cell follows the normal exocytosis mechanism characterized with the vesicular docking and fusion with the aid of SNARE complexes. Both ESCRT-dependent and independent signals have been suggested as determining the sorting of exosomes (Bunggulawa et al., 2018).
ESCRT-dependent mechanisms
ESCRT consists of five distinct protein complexes, namely, ESCRT 0, I, II, and III, AAA ATPase, and Vps4. These complexes drive the inward budding and fission of the membrane to form ILVs sequentially to the cytosolic surface of the endosomal membrane (Rajagopal and Harikumar, 2018). ESCRT-0 is responsible for cargo clustering in a ubiquitin-dependent manner, ESCRT-I and ESCRT-II induce bud formation, ESCRT-III drives vesicle scission, and the accessory proteins (especially the VPS4 ATPase) allow dissociation and recycling of the ESCRT machinery. The ESCRT-III-associated protein ALIX which promote intraluminal budding of vesicles in endosomes and hence exosome biogenesis, upon inter-action with syntenin (Kowal et al., 2014).
The ESCRT mechanism is initiated by recognition and sequestration of ubiquitinated proteins to specific domains of the endosomal membrane via ubiquitin binding subunits of ESCRT-0 (Zhang et al., 2019a). ESCRT-0 is recruited to pre-MVB endosomes . ESCRT-I and -II also have ubiquitin-interaction domains and sort ubiquitinated cargos at ILV with ESCRT-0. ESCRT-I and ESCRT-II recruit ESCRT-III, which drives the invagination and constriction of the membrane (Mc Gough and Vincent, 2016). The ESCRT complexes accumulate ubiquitinated cargos in exosomes containing both membrane and cytoplasmic proteins. The ESCRT-1 complexes subunit TSG101 is essential for exosomal secretion (Tamai et al., 2010). A study with RNA interference screen targeting, twenty three ESCRT components and associated proteins in HeLa cells reported, ESCRT0 (Hrs, STAM1), ESCRT1 (TSG 101), and the late-acting VPS4 as the main genes responsible for exosomal biogenesis. Some of the components of ESCRT, such as vacuolar protein sorting protein 31 (VPS31), vacuolar protein sorting protein 4B (VPS4B), and TSG101, have been found in endosome-like plasma membrane domains that generate exosomes (Colombo et al., 2013).
ESCRT-independent mechanisms
The second pathway is independent of the ESCRT machinery and is based on the specific lipid composition of the endosomal membrane. Ceramide was proposed to induce inward curvature of the limiting membrane of MVBs to form ILVs. Raft-based micro domains are present on the limiting plasma membrane of endosomal compartments and contain high amounts of sphingolipids which represent substrates for the neutral sphingomylinase2 (nSMase2). The nSMase2 convert sphingolipids to ceramide at endosomal membrane which induces coalescence of micro domains into larger structures thereby promoting domain induced budding and formation of ILVs. Following the formation of MVBs, they are either destined for the degradative or the secretory pathways, which are governed by Rab GTPases. While Rab7 can mediate the degradation through the fusion of MVBs with lysosomal compartments while other Rab proteins like Rab5b, Rab9a, RAB27a, RAB27b, and Rab35 were reported to be crucial for intracellular MVB trafficking and secretion . The final release of ILVs occurs upon MVB fusion with the cellular plasma membrane, a process which probably mediated, at least in part, by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), like the vesicle-associated membrane protein (VAMP) TI-VAMP/VAMP7 . Once the ILVs are secreted, they are termed exosomes. In tumor cells genotoxic stress leads to an increased activation of p53 transcription factors and to an enhanced p53 and tumor suppressor-activated pathway 6 (TSAP6) expression, which mediates augmented exosome secretion. Release of exosomes can also be induced by diverse signaling pathways including the activation of the Wnt pathway (Dreyer and Baur ,2016).
Cargo sorting
ESCRT proteins, including ESCRT-I, ESCRT-II, and ESCRT-III, are need for cargo selection and the inward budding process (away from the cytoplasm). Some of the components of ESCRT, such as vacuolar protein sorting protein 31 (VPS31), vacuolar protein sorting protein 4B (VPS4B), and TSG101, have been found in endosome-like plasma membrane domains that generate exosomes. Tumor cell exosomes have been shown to contain syndecan, syntenin, and ALIX. Down-modulation of these proteins reduced exosomal release, and production of syndecan, syntenin, and ALIX-containing exosomes was dependent on the normal functioning of the ESCRT machinery proteins. The secretion of syntenin into exosomes is driven by syndecan, and this process induces heparin sulfate clustering. Over expression of the enzyme heparinase cleaves the heparin sulfate, causing a noticeable increase in the secretion of exosomes (Rashed et al., 2017).
Exosomal heterogeneity
The molecular composition of exosomes is not only cell-type dependent but can differ even when the exosomes originate from the same parental cells. The subcellular origin of exosomes and donor cell activation status can contribute to their molecular heterogeneity (Ferguson and Nguyen , 2016).
Exosomes web domains
EVpedia and ExoCarta, Vesiclepedia are the web domains in which researchers are able to upload proteomic lists of identified proteins to the database of the exosomes they are working with. This gives an opportunity to create a listing of the most frequently identified proteins generally found in exosomes (Ronquist 2019).
Isolation of Exosomes
The major hurdle in the clinical utilization of exosomes has been the lack of consistent and dependable methods to isolate a pure exosome population (Thery et al., 2006). The differential ultracentrifugation (DC) has been widely used as conventional isolation techniques that separate exosomes and other EVs based on their sizes and buoyant density. Ultracentrifugation is used to pellet crude exosomes. Exosomes were successfully isolated from serum by one-step precipitation method by using commercially available reagents such as ExoQuick (System Biosciences, Palo Alto, CA, USA) as an alternative approach to ultracentrifugation (Rekker et al., 2014). The affinity capture (AC) methods is used to target established exosomal markers such as CD63, CD81, TSG 101, HSP 70 and Alix, which allows selective capturing of exosome population followed by detection of cancer specific markers. The NTA (Nanoparticle tracking analysis)-based instruments called Nanosight (LM10, LM20, NS200 and NS500) allow exosomes to be counted and sized by combining light microscopy and the software that tracks Brownian motion of exosomes. NTA method becomes a gold standard to measure the concentration of exosomes (Soung et al., 2017).
Function of Exosomes
The key mechanism by which exosome exert biological function on cell by direct contact between surface molecules of cells and vesicles, endocytosis ,vesicle cell membrane fusion and horizontal transfer of mRNA and miRNA which alters specific gene expression patterns in recipient cells. Biological functions and effects of exosomes promote intercellular communication and are involved in various physiological as well as pathological processes, such as antigen presentation, immune regulation, and tissue development. The nucleic acids in exosomes is important for embryonic and organ development, normal physiology, and various pathologies. Tumor cell-derived exosomes play key role in tumorigenesis, metastasis, and response to therapy through the transfer of oncogenes and onco-miRNAs between cancer cells and the tumor stroma. Exosomes from stimulated blood cells and the vascular endothelium is involved in neurological disorders such as multiple sclerosis, transient ischemic attacks, and antiphospholipid syndrome (Lin et al., 2015).
The molecular composition of exosomes has significant potential as biomarkers for disease diagnosis. Exosomes derived from antigen presenting cells have ability to express major histocompatibility complex (MHC) class I and II molecules on the cell surface, which helps inactivating CD8+ and CD4+ T-cells to induce specific immune responses by carrying prostaglandins, exosomes secreted by platelets are involved in the inflammatory response. Glioblastoma cells also secret exosomes containing mRNA, miRNA and angio genetic proteins to microvascular endothelial cells, stimulating angiogenesis. Exosomes are involved in many biological processes, including the maturation of erythrocytes, the elimination of unnecessary proteins and RNA (Ha et al., 2016). Exosomes biobanking activities and its role in clinical personalized applications rely on discrimination and precise diagnosis, targeted therapies of choice for each patient, dose adjustment methods to optimize the benefit-risk ratio of treatment and biomarkers of efficacy, toxicity, treatment discontinuation, relapse will be beneficial if coordinated with pathologists and clinicians at hospital and health care centers (Mora et al., 2016).
Exosomes in Cancer diagnostics and therapeutics
Cancer cells derived exosomes have a great potential to serve as a liquid biopsy tool for various diseases. The cargo reflective of genetic or signaling alterations in cancer cells of origin are carry through cancer derived exosomes served as biomarker for early detection of cancer. Exosome based liquid biopsy merits over conventional tissue biopsy because it provides the convenient and non-invasive way of diagnosis over tissue biopsy that requires surgery (Soung et al., 2017).
Exosomes can be loaded with different therapeutic drugs, antibodies or RNAi for gene expression, manipulation in order to treat cancer cells in a more efficient manner. Doxorubicin, a chemotherapeutic agent, loaded in breast cancer-derived exosomes (ExoDOX) is more efficient than free doxorubicin in the treatment of breast cancer and ovarian cancer mouse models . Therapeutic approaches that take advantage of exosomes and their characteristics have shown to improve the efficacy of chemotherapy (Bastos et al., 2018).
Exosomal Surface Proteins as Cancer Biomarkers
Exosomes contain a variety of proteins that reflect their origin and alteration of the parental cells. Based on endosome-based biogenesis pathway, exosome specific protein markers include endosome associated proteins (e.g., small Rab family GTPases, annexins and flotillin), proteins involved in exosome biogenesis (e.g., Alix, Tsg101 and ESCRT complex), tetraspanins (e.g., CD9, CD37, CD53, CD63, CD81 and CD82), heat shock proteins (Hsp70, Hsp90) and epithelial cell adhesion molecules (EpCam) . The proteomic analyses of cancer-derived exosomes led to the identification of potential exosomal markers to serve as a liquid biopsy in breast, prostate, pancreatic, ovarian, colorectal cancers and glioblastoma (Soung et al., 2017). Tumor-derived exosomes (TEX) are important intercellular messengers that contribute to tumorigenesis and metastasis through a variety of mechanisms such as immunosuppression and metabolic reprogramming that generate a pre-metastatic niche favorable to tumor progression (Tung et al., 2019).
The Role of Exosomes in Infectious Diseases
Pathogen-released exosomes apart from the widely studied immunomodulatory effects are known to carry specific virulence factors, such as proteins, mRNA, and miRNA, which contribute to spread the infection. It is known that even parasitic trematodes and nematodes release exosomes as an immunomodulatory mechanism (De Toro et al., 2015). Exosomes play an important role not only in the process of infection by pathogens but also in anti-infection. Exosomes mediate infection through transferring pathogen-related molecules (pathogenic genes and proteins) or even the entire pathogens. Exosomes can be either directly infectious, alter nuclear gene expression, or mediate toxic reactions, participating in pathogen immune escape mechanisms; inhibiting immune responses by inducing immune cell apoptosis. Exosomes can play anti-infective roles by inhibiting pathogen proliferation and infection directly inducing immune responses (Zhang et al., 2018).
Exosomes in drug delivery
The exosome-based drug delivery gained momentum after successful demonstration of nerve cell-derived exosomes for increasing immunity in cancer patients and for targeting cancer genes. Other cell types that have been used as exosome factories include stem cells, human and mouse cancer cells, etc. Exosomes from these cells were shown to deliver small RNAs for gene silencing and small drugs (e.g., doxorubicin and curcumin). The concern about safety and cancer-stimulating properties of exosomes derived from the stem cells and cancer cells are much more. Exosome-like particles and lipids derived from fruits (e.g., grapes and grapefruit) were recently examined as an alternative drug carrier. Vesicles isolated from the bovine milk are mostly used for drug delivery works. It is considered as a good exosomal source for the drug delivery purposes because of no adverse immune and inflammatory effects. The milk exosomes represent the most useful drug-carriers that can be exploited to deliver all kinds of agents ranging from small molecule drugs to nucleic acids to proteins (Munagala et al., 2016). Exosomes are used in packaging of drugs instead of using synthetic nanoparticles. Exosomes are promising agents for drug delivery because of low immunogenicity, innate stability, and high delivery efficiency. Peptide-conjugated exosomes loaded with curcumin as a drug has been proved as a good system for the effective drug delivery for brain ischemia (Tian et al., 2018).
Exosomes in degenerative disease
Neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, dementia with Lewy bodies or fronto-temporal dementia, show clinical and pathological overlaps and difficulty in specific and differential diagnosis. Now a days biomarkers for each of these types of dementia have been investigated in the cerebrospinal fluid or blood (Gamez-Valero et al., 2019).
A common pathological feature of many neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, ALS or prion diseases, is the abnormal deposition of proteins in the brain with implicated exosome function. Increasing evidence implicates exosomes in synaptic function, exosomal transfer of proteins, mRNAs, and miRNAs plays a role in synaptopathies an important mechanism by which NPCs may propagate some of their immune modulatory functions (Janas et al., 2016).
Exosomes are proposed to be involved in the spread of ‘toxic’ proteins in neurodegenerative disorders. Mutated or misfolded proteins serve as template for the formation of oligomers, leading to neuronal toxicity. Removal of these accumulated proteins is thought to occur by processing them through endosomal pathway, which either leads to lysosomal degradation or incorporation into MVBs and release into the extracellular space as exosomes. This phenomenon was reported in the case of Aβ peptide in Alzheimer’s disease. Exosomal release of Aβ is likely a mechanism to remove excess intracellular Aβ Exosomes appear to be a major vehicle that shuttles amyloids out of the cell and act as seeds for plaque formation. Neuron-derived exosomes containing normal Aβ levels and neuroprotective factors may act as scavengers for synaptotoxin Aβ species, thereby mediating neuroprotection (Janas et al., 2016).
Hamlett et al. (2018) reported neuron-derived exosomes contain elevated levels of amyloid-beta peptides and phosphorylated-Tau that could indicate a preclinical AD (Alzheimer’s disease) phase in people with DS (Dejerine-sottas disease) starting in childhood. The inhibition of exosome secretion is associated with reduced AD(Alzheimer’s disease)-like pathology in a transgenic mouse model of AD. Dinkins et al. (2014) found exosomal proteins accumulating in the plaques of AD patient brains, and able to reduce amyloid plaque load in AD brains in vivo by decreased secretion of exosomes, achieved by inhibition of nSMase2. Recently, exosomes emerged as novel biological source with increasing interest for age-related neurodegenerative disease biomarkers (D’Anca et al., 2019).
Targeted exosome – drug addiction
Cell derived exosomes have been demonstrated to be efficient carriers of small RNAs to neighbouring or distant cells as carriers for gene therapy over other artificial delivery tools. Modified exosomes expressing the neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver opioid receptor mu (MOR) siRNA into the brain to treat morphine addiction. MOR siRNA delivered by the RVG exosomes strongly result in inhibition of morphine relapse via the down-regulation of MOR expression levels. This provides a brand new strategy to treat drug relapse and diseases of the central nervous system (Liu et al., 2015).
Regenerative and Anti-inflammatory Therapies
Mesenchymal stem cells (MSCs) which are well known to possess anti-inflammatory and regenerative effects are the most commonly used source of exosomes. It showed that exosomes secreted from iPS cells, embryonic stem cells, and cardiac progenitor cells have therapeutic effects similar to MSC-derived exosomes (Khan et al., 2015, Vrijsen et al., 2016).
Exosomes in diagnostics
Exosomal contents, nucleic acids, proteins and lipids, are altered during disease conditions. Therefore these are an attractive target for clinical diagnostics and biomarker discovery. Exosomes can be isolated non-invasively from easily accessible biological fluids including urine, blood and saliva. Non-invasive approach allows for early diagnosis of disease, which is importance in diseases of the CNS. The exosomal contents are protected inside a membranous structure, which gives an advantage over conventional specimens as the potential biomarkers are protected from degradation. These are highly stable, making their clinical use feasible as samples can be stored for prolonged periods before analysis. Exosomes can be traced to their origin as they express surface markers related to their cellular origin (Kanninen et al., 2016). Exosomes from human serum are employed to detect HIV-positive patients. At present this test is commercialized (Vlassov et al., 2012). Thirty three unique proteins of Mycobacterium tuberculosis were identified from exosomes isolated from human serum that may serve as biomarkers for persistent active and latent tuberculosis (Kruh-Garcia et al., 2014).
Exosomes in Angiogenesis
Angiogenesis is a major process which regulates nutrient availability of fast growing solid tumors. Tetraspanins are key players in the process of angiogenesis . Exosomal interaction and uptake of endothelial cells (ECs) will induce angiogenesis with the incorporation of vesicular cargos such as tetraspanin 8 and CD106 and 49d, which can activate vascular endothelial growth factors (VEGFs). Exosomes released from the endothelial progenitor cells interact with mature endothelial cells and its cargo integration triggers AKT signaling, resulting in angiogenesis (Rajagopal and Harikumar, 2018). Neoangiogenesis is as entry point for metastatic cells into systemic circulation. Neoangiogenesis has to be stimulated to avoid cancer necrosis. Cancer-associated fibroblasts stimulate neoangiogenesis via secretion of exosomal SDF-1 (stromal cell derived factor-1). The released SDF-1 is responsible for the recruitment of endothelial progenitor cells by chemotaxis .The same result was found after treatment of umbilical cord mesenchymal stem cells with gastric-cancer cells derived exosomes or with exosomes from hepatic cancer cells (Steinbichler et al., 2017).
Theragnosis is a concept in next-generation medicine that simultaneously combines accurate diagnostics with therapeutic effects. Molecular components in exosomes have been found to be related to certain diseases and treatment responses, indicating that they may have applications in diagnosis via molecular imaging and biomarker detection (Kim et al., 2018).
The role of exosomes in pregnancy
Exosomes are produced and secreted by syncytiotrophoblast of the human placenta continuously and constitutively in the maternal bloodstream. This exhibits a redundant number of mechanisms that inhibit the function of the maternal immune system during pregnancy and promote the survival of the fetus. The role of immunosuppressive placental exosomes during normal pregnancy is clear, the contribution of exosomes in pathological pregnancies and related diseases, such as recurrent abortions and infertility, need a more profound evaluation. The knowledge derived in these areas will set up possibilities for novel, exosome based treatments of infertility and pregnancy failure (De Toro et al., 2015).
Cancer Vaccines
Exosomes in immunotherapy could form a viable basis for the development of novel cancer vaccines, via antigen-presenting cell technology, to prime the immune system to recognize and kill cancer cells (Tan et al., 2010). Exosomes derived from professional antigen presenting cells enable them to activate directly CD8+ and CD4+ T-cells inducing a strong immunogenic response. Exosomes can activate T-cells either by a direct antigen presentation or by an indirect presentation through transfer of antigenic peptides to APCs. Tumor-derived exosomes express tumor antigens that can activate DCs, thereby priming the immune system to recognize and promote a specific cytotoxic response with a higher immunogenicity than that accomplished by tumor cell lysates or soluble antigens when used as vaccines (De Toro et al., 2015). Serum-derived exosomes from pigs are revealed for its use for the development of the vaccine against porcine reproductive and respiratory virus (PRRSV) ( Montaner-Tarbes et al., 2016).
Exosomes as immune modulators, may be either immune activating or immunosuppressive agents. Exosomes were proposed as acellular antigens for the development of vaccines against either infectious diseases or tumors. Ascetic cell- derived exosomes (AEX) taken from peritoneal cavity fluid in cancer patients have been shown to cause tumor cell lysis by inducing dendritic cells to prime T lymphocytes via an MHC I dependent pathway to kill cancer cells. This also triggers the release of IFN-γ by peripheral blood lymphocytes in vitro experimental models,(Andre et al., 2002).Exosome-based cancer immunotherapy is an attractive approach against cancer as tumor derived exosomes carrying tumor associated antigens are reported to recruit the immune responses (Shao et al., 2016). Exosomes derived from dendritic cells (DCs) may be useful as anticancer vaccines because of the nature of DCs as antigen-presenting cells (APCs). Major histocompatibility complex (MHC)-I, MHC-II, and co-stimulating factor such as CD86 are expressed on the surface of DC-derived exosomes (Yamashita et al., 2018).
Future Perspectives
Exosomes offer a promising tool for the invasive diagnosis, monitoring cancer, diagnosis in tumero genic studies and neuro generative disorders. The great potential of this ‘platform’ technology has several advantages over other nano carrier based therapies and offers simplicity and versatility approach for delivery of drugs. The future prospects for exosomes as therapeutic agents have lots of potential, as new biomarkers can be identified and use them in diagnostic applications. The available information on exosome biogenesis and functions can be used as significant opportunities to manipulate their properties, composition and cell interactions to further advance improves their therapeutic platform and drug delivery potential. Recent advances in using exosomes as biomarkers for disease detection and as drug/gene delivery systems have been stimulating (Li et al., 2019). The combination of exosomes with different therapeutic cargoes often makes them immunogenic based on the nature of parental donor cells, and therefore, further studies are needed to describe immunogenic reactions after administration. Nanotechnology coupled with engineered exosomes is emerging as new and novel approach for cancer vaccine development (Tan et al., 2010). The exosomal miRNA has potential to explore as non invasive biomarker or to indicate disease status of patient on the basis of difference in amount and composition of exosomal miRNA from healthy and diseased patient (Zhang et al., 2015). Further exploration about the structure and function of exosomes to find ways of developing and standardizing appropriate methods to modify exosome contents in a loading process and drug delivery will contribute to the therapeutic, and clinical application of exosomes. The progress achieved in the evolving field of exosomes in recent years has been exciting and quick (Wang et al., 2019). The role of tumor-derived exosomes in cancer progression and use of exosomes for cancer diagnosis and treatment may change effectiveness of cancer treatment. This can lead to personalized medicine and influence the direction of future research on exosomes. Exosomes have the inherent ability to cross the BBB, the major obstacle for brain targeting. Exosomes can be excellent vehicles for drugs, including small molecules as well as bio macromolecules to treat CNS diseases (Zhang et al., 2019 b).
CONCLUSION
The area of research on utilization of exosomes and their cargos is a novel and rapidly growing field with interest. Exosomes are cell-derived membrane vesicles secreted by different cell types and harbor verity of molecular constituents and proteins which are present in various body fluids. The interest in exosomes has increased exponentially mainly because of the important potential application in biological, pathological and physiological function. This holds a great promise as novel biomarker, in clinical diagnosis ,prognostic indicators, development of therapeutics, drug delivery and vaccines. In future a more thorough understanding and analysis of exosomes and their components will enable the development of safer and more effective exosomes with defined structures and functions.
REFERENCES
Andre F., Schartz N.E., Movassagh M., Flament C., Pautier P., Morice P., Pomel C., Lhomme C., Escudier B., Le Chevalier T., Tursz T., Amigorena S., Raposo G., Angevin E. and Zitvogel L. (2002) Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 360(9329): 295–305.
Balaj L, Lessard R., Dai L., Cho Y.J., Pomeroy S. L., Breakefield X.O. and Skog J. (2011) Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun.1,2:180.
Bastos N., Ruivo C.F. , da Silva S. and Melo S.A (2018). Exosomes in cancer: Use them or target them? Semin Cell Dev Biol. 78:13-21.
Bunggulawa E,J., Wang W., Yin T., Wang N., Durkan C., Wang Y. and Wang G. (2018) Recent advancements in the use of exosomes as drug delivery systems. Nanobiotechnol 16(1):81.
Colombo M., Moita C. , van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L.F., Thery C. and Raposo G. (2013) Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci . 126:5553–5565.
D’Anca M., Fenoglio C., Serpente M., Arosio B., Cesari M., Scarpini E.A. and Galimberti D. (2019) Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 11:232.
De Toro J., Herschlik L., Waldner C. and Mongini C. (2015) Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front. Immunol. 4(6):203.
Dinkins M.B., Dasgupta S., Wang G., Zhu G. and Bieberich E. (2014) Exosome reduction invivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 35 (8) 35(8):1792-1800.
Dreyer F. and Baur A. (2016) Biogenesis and Functions of Exosomes and Extracellular Vesicles. In: Federico M. (eds) Lentiviral Vectors and Exosomes as Gene and Protein Delivery Tools. Methods in Molecular Biology, vol 1448. Humana Press, New York, NY
Ferguson S.W. and Nguyen J. (2016) Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. Journal of Controlled Release . 228 : 179–190.
Gamez-Valero A., Beyer K. and Borras F. E. ( 2019) Extracellular vesicles, new actors in the search for biomarkers of dementias. Neurobiology of Aging.74:15-20
Ha D., Yang N. and Nadithe V. (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica. 6(4):287–296.
Hamlett E.D., Ledreux A., Potter H., Chial H.J., Patterson D., Espinosa J.M., Bettcher B.M., Granholm A.C. (2018) Exosomal biomarkers in Down syndrome and Alzheimer’s disease Free Radical Biology and Medicine 114 : 110–121.
Huotari J. and Helenius A. (2011) Endosome maturation, EMBO J. 30(17) : 3481–3500.
Janas A. M., Sapon K., Janas T., Stowell M.H. and Janas T. (2016) Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta. 1858:1139–1151.
Kalluri R. (2016) The biology and function of exosomes in cancer. J Clin Invest. 126(4):1208-1215.
Kanninen K. M. , Bister N. , Koistinaho J. and T. Malm (2016) Exosomes as new diagnostic tools in CNS diseases. Biochimica et Biophysica Acta 1862(3) : 403–410.
Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., Mackie A.R., Vaughan E., Garikipati V.N., Benedict C., Ramirez V., Lambers E., Ito A., Gao E., Misener S., Luongo T., Elrod J., Qin G., Houser S.R., Koch W.J., Kishore R. (2015) Embryonic stem cell derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 117(1): 52–64 .
Kim Y., Ahn J., Kim S., Kim H., Kim S. and Kang J. (2018) The potential theragnostic (diagnostic-therapeutic) application of exosomes in diverse biomedical fields. Korean J Physiol Pharmacol. 22(2):113-125.
Kowal J., Tkach M. and Thery C. (2014) Biogenesis and secretion of exosomes. Current Opinion in Cell Biolog. 29:116-125.
Kruh-Garcia N.A., Wolfe L.M., Chaisson L.H., Worodria W.O., Nahid P., Schorey J.S., Davis J.L. and Dobos K.M. (2014) Detection of Mycobacterium tuberculosis peptides in the exosomes of patients with active and latent M. tuberculosis infection using MRM-MS. PLoS One .9(7):e103811.
Li X., Corbett A.L., Taatizadeh E., Tasnim N., Little J.P., Garnis C., Daugaard M., Guns E., Hoorfar M. and Li I. T. S. (2019) Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 3, 011503 .
Lin J. , Li J., Huang B., Liu J., Chen X., Chen X.M., Xu Y., Huang L. and Wang X. (2015) Exosomes: Novel Biomarkers for Clinical Diagnosis. The Scientific World Journal. http://dx.doi.org/10.1155/2015/657086
Liu S., Sun P., Li Y., Liu S. and Lu Y. (2019). Exosomes as critical mediators of cell-to-cell communication in cancer pathogenesis and their potential clinical application. Transl Cancer Res. 8(1):298-311.
Liu Y., Li D., Liu Z., Zhou Y., Chu D., Li X., Jiang X., Hou D., Chen X., Chen Y., Yang Z., Jin L., Jiang W., Tian C., Zhou G., Zen K., Zhang J., Zhang Y., Li J. and Zhang C. (2015) Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 5:17543.
Mc Gough I.J and Vincent J.P. (2016) Exosomes in developmental signalling. Development . 143(14):2482-2493.
Montaner-Tarbes S., Borras F.E., Montoya M. , Fraile L. and Del Portillo H.A. (2016) Serum- derived exosomes from non-viremic animals previously exposed to the porcine respiratory and reproductive virus contain antigenic viral proteins. Vet Res.47(1): 59.
Mora E.M., Alvarez-Cubela S. and Oltra E.( 2016) Biobanking of Exosomes in the Era of Precision Medicine: Are We There Yet? Int. J. Mol. Sci. 17:1-13.
Munagala R., Aqil F., Jeyabalan J. and Gupta R.C. (2016) Bovine milk-derived exosomes for drug delivery. Cancer Lett . 371(1) :48–61.
Pan, B.T. and Johnstone, R.M. (1983). Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell., 33:967–978.
Rajagopal C. and Harikumar K. B. (2018) The Origin and Functions of Exosomes in Cancer. Front. Oncol. 8:66.
Rashed M.H. , Bayraktar E., Helal G. K. , Abd-Ellah M. F., Amero P., Chavez-Reyes A. and Rodriguez-Aguayo C.(2017) Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 18: 538.
Rekker K., Saare M., Roost A.M., Kubo A.L., Zarovni N., Chiesi A., Salumets A. and Peters M.. (2014) Comparison of serum exosome isolation methods for microRNA profiling. Clin. Biochem. 47(1-2): 135–138.
Ronquist K. (2019) Extracellular vesicles and energy metabolism. Clinica Chimica Acta .488:116-121.
Shao Y., Shen Y. , Chen T., Xu F., Chen X. and Zheng S. (2016) The functions and clinical applications of tumor-derived exosomes. Oncotarget 7(37): 60736–60751.
Soung Y.H., Ford S., Zhang V.and Chung J. (2017) Exosomes in Cancer Diagnostics. Cancers .9(1):8; doi:10.3390/cancers9010008.
Steinbichler T.B., Dudasa J., Riechelmanna H. and Skvortsova I. (2017) The role of exosomes in cancer metastasis. Seminars in Cancer Biology , 44:170–181.
Tamai K., Tanaka N., Nakano T., Kakazu E., Kondo Y., Inoue J., Shiina M., Fukushima K., Hoshino T., Sano K., Ueno Y., Shimosegawa T. and Sugamura K. ( 2010) Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun . 399(3):384–390.
Tan A., De La Pena H. and Seifalian A.M. (2010) The application of exosomes as a nanoscale cancer vaccine. Int. J. of Nanomed. 5: 889–900.
Tang M.K. and Wong A. S. (2015) Exosomes: Emerging biomarkers and targets for ovarian cancer. Cancer Letters. 367 : 26–33.
Thery C., Amigorena S. , Raposo G. and Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Pages 3.22.1-3.22.29.
Tian T., Zhang H.X., He C.P., Fan S., Zhu Y.L., Qi C., Huang N.P., Xiao Z.D., Lu Z.H., Tannous B.A. and Gao J. (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 150:137–149.
Trams E.G., Lauter C.J., Salem N. and Heine J.U.(1981). Exfoliation of membrane ecto-enzymes in the form of microvesicles. Biochim Biophys Acta., 645:63–70.
Tung K. H., Ernstoff M. S. , Allen C., and Shu S. L.(2019) A Review of Exosomes and their Role in The Tumor Microenvironment and Host–Tumor “Macroenvironment” J Immunol Sci. 3(1): 4–8.
Vlassov A.V., Magdaleno S., Setterquist R. and Conrad R. (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta .1820(7) :940–948.
Vrijsen K.R., Maring J.A., Chamuleau S.A., Verhage V., Mol E.A., Deddens J.C., Metz C.H., Lodder K., van Eeuwijk E.C., van Dommelen S.M., Doevendans P.A., Smits A.M., Goumans M.J. and Sluijter J.P. (2016) Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Advanced Healthcare Materials, 5(19):2555-2565.
Wang B., Xing D., Zhu Y. , Dong S. and Zhao B. (2019) The State of Exosomes Research: A Global Visualized Analysis. BioMed Research International Vol.2019, Article ID 1495130,1- 10
Yamashita T., Takahashi Y. and Takakura Y. (2018) Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 41(6):835-842.
Zhang J., Li S., Li L., Li M., Guo C., Yao J. and Mi S. (2015) Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genomics, Proteomics & Bioinformatics. 13(1)-17-24.
Zhang M., Zanga X. , Wang M. , Lid Z. , Qiaoa M. , Hua H. and Chen D.(2019b) Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: Recent advances and challenges. J. Mater. Chem. B, 00, 1-3.
Zhang W.C., Jiang X.F., Bao J.H., Wang Y., Liu H.X. and Tang L.J. (2018) Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions. Front. Immunol. 9:90.
Zhang Y., Liu Y., Haiying L. and Tang W. H. (2019a). Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci . 9:19.


