Department of Genetics, OsmaniaUniversity, Hyderabad-500007, Telangana, Hyderabad, India.
Corresponding author email: anupallirr@ gmail.com Email: rams.nenavath@gmail.com
Article Publishing History
Received: 14/06/2023
Accepted After Revision: 30/09/2023
The present investigation is intended to prepare Acalypha indica leaf extract and assess the antioxidant and glucose-lowering effects utilizing in vitro models. The plant methanolic extract of Acalypha indica leaves was exposed to phytochemical examination to identify the bioactive factors by GC-MS. We assessed the antioxidant properties of the extract by using the DPPH scavenging method, ABTS assay, TBARS assay and NO assay. Likewise, the anti-diabetic activity was assessed by ɑ-amylase and α-glucosidase enzyme inhibition and glucose diffusion inhibitory techniques. We found that methanolic extract of Acalypha indica leaves contains a high measure of phenolics, flavonoids and tannins. The GC-MS assay identified the bioactive components. We also found that methanolic extract of Acalypha indica leaves had significant higher antioxidant and glucose-lowering effects. In conclusion, it could be reasoned that due to the nearness of antioxidant components, the methanolic leaf extract of Acalypha indica has good potential in the control of hyperglycemia, diabetes and the related state of oxidative stress.
Antioxidants, DPPH, GC-MS, diabetes mellitus and Flavonoids.
Kumar N. R, Srikrishna P, Rani S, Swathi, Rani A. R. Evaluation of Antioxidant and Antidiabetic Potential of Acalypha indica leaf extract: Identification of Bioactive Factors. Biosc.Biotech.Res.Comm. 2023;16(3).
Kumar N. R, Srikrishna P, Rani S, Swathi, Rani A. R. Evaluation of Antioxidant and Antidiabetic Potential of Acalypha indica leaf extract: Identification of Bioactive Factors. Biosc.Biotech.Res.Comm. 2023;16(3). Available from: <a href=”https://bit.ly/2U8EBeg“>http://surl.li/msqxk</a>
INTRODUCTION
Diabetes mellitus (DM) is a glucose or insulin associated metabolic disorder that affects the digestion of carbohydrates, fats and proteins. It is a combinatorial disorder accompanied by high blood glucose level (hyperglycemia) and glucose intolerance due to the relative inadequate hypoinsulinemia or growing insulin tolerance in peripheral tissues. This is accompanied by several complications like hyperlipidemia (abnormal state of lipid in the blood), oxidative stress, and advanced enzymatic glycation of proteins.
Approximately 450 million adults (20-79 years) are living with DM; by the year 2045 (Galicia-Garcia et al., 2020). this estimate will ascend to 629 million. 79% of adults with DM are living in low and center salary nations, 1 of every 2 (212 million) individuals with DM are undetected, and 352 million individuals are in danger of acquiring type 2 DM. DM is prevalent in most developing nations like India. According to ICMR-INDIAB reports, India has the highest number of diabetic patients in the world and has been notoriously named as the “diabetic capital of the world” (Anjana et al., 2011, Parim 2019, Banday & Nissar 2020 & Sun 2021).
There is substantial evidence that the biochemical and molecular pathways enacted by hyperglycemia are related to the increase in reactive oxygen species (ROS), resulting in increased oxidative stress. Excessive ROS production prompts the initiation of stress sensitive intracellular signaling pathways that, in turn, promote cellular damage and contribute to several complications and disease progression. In recent times, several plant species have been widely characterized for their antioxidant activity. Antioxidants from fragrant, stimulating, therapeutic, and other plants are concentrated to create natural antioxidant formulations for nourishment, anti-diabetic properties, and other applications ( Seebaluck 2014, Zahidin et al., 2017, Parim, 2019, Sun 2021).
On the other hand, the number and classes of oral glucose lowering agents in use for the treatment of DM have increased in last couple of years. At present, viable and safe medications are not adequately available, and they have significant adverse side effects. A large number of these drugs have even been pulled back from the market due to undesirable impacts. As a result of these adverse reactions and limitations, there is rising popularity of plant-based formulations. Dietary considerations and nature-based cures can be used as a supplement to local systems of prescription for managing DM more efficiently (Uddandrao et al., 2020).
Acalypha indica Linn is a herb belonging to family Euphorbiaceae. The twigs and leaves of A. indica posses flavonoids, catachols, alkaloids, saponins, volatile oil, fatty acids, phenolic compounds and steroids (Ravi et al., 2017, Ninave & Patil 2022). It plays an important role in diverse folk medicines including the Ayurveda system and is supposed to boost defense against various diseases. Hence, in this study we made an endeavor to evaluate the ameliorative potentials of A.indica and assess their antioxidant and glucose lowering potential (Brahma et al., 2015).
MATERIALS AND METHODS
Sample Collection: Dried Acalypha indica Linn leaves were obtained from the botanical garden, Osmania University, Hyderabad in the month of June 2018, their identity was authenticated by a taxonomist Dr L Rasingam, Scientist-E, Botanical Survey of India, Hyderabab, India, voucher number BSI/DRC/22-23 Identification/552 dated 16-10-2022, and a specimen has been preserved at the departmental herbarium. Leaves were shade dried and made into course powder. The powder (2.5kg) was soaked sequentially with hexane, ethylacetate, methanol, and double distilled water each 6 d at room temperature in a 10 L aspirator jar to collect the extracts. These extracts were evaporated by using a rotavapor to obtain respective dry extracts to be used for further studies. The extracts were dissolved in Tween 80 for pharmacological studies (Erum Iqbal, 2015) (Erum et al., 2015).
Preliminary Phytochemical Screening: The methanolic extract of Acalypha indica Linn leaves was screened for the presence of various phytoconstituents by the methods described by Mopuri et al (Shafodino et al., 2023). Quantitative Estimation of Phytochemicals: In light of the fundamental phytochemical examination results, further, we exploited the Acalypha indica Linn leaves extracts to quantitative estimation of secondary metabolites, for example, total phenolic content (Ninave & Patil 2022), total flavonoids [13] and total tannins (Erum et al., 2015) were measured according to the respective protocols.
GC-MS (Gas Chromatography-Mass Spectrophotometry) Analysis: The Clarus 680 GC was used in the analysis employed a fused silica column, packed with Elite-5MS (5% biphenyl 95% dimethylpolysiloxane, 30 m × 0.25 mm ID × 250μm df) and the components were separated using Helium as carrier gas at a constant flow of 1 mL/min. The injector temperature was set at 260°C during the chromatographic run. The 1 μL of extract sample injected into the instrument the oven temperature was as follows: 60°C (2 min); followed by 300°C at the rate of 10°C min−1; and 300°C, where it was held for 6 min. The mass detector conditions were: transfer line tem- perature 240°C; ion source temperature 240°C; and ionization mode electron impact at 70 eV, scan time 0.2 sec and scan interval of 0.1 sec, the fragments from 40 to 600 Da. Interpretation of mass spectrum GC-MS was conducted using the database of National Institute Standard and Technology (NIST) and the compounds were identified (Mopuri et al., 2017).
Evaluation of Antioxidant Activity: The antioxidant ability of Acalypha indica leaves extract was measured through 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, ABTS assay, TBARS assay and NO assay activity as per phosphomolybdate assay proposed by (Mopuri et al., 2017) & (Oyebode et al., 2021).
Assessment of in vitro Glucose-lowering Effects
Inhibition of ɑ-amylase Enzyme Assay: A total of 500 μL of test samples and standard drug (100- 1000μg/mL) were added to 500 μL of 0.20 mM phosphate buffer (pH 6.9) containing α-amylase (0.5mg/mL) solution and were incubated at 25°C for 10 min. After these, 500 μL of a 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9) was added to each tube. The reaction mixtures were then incubated at 25°C for 10 min. The reaction was stopped with 1.0 mL of 3, 5 dinitrosalicylic acid color reagent.
The test tubes were then incubated in a boiling water bath for 5 min, cooled to room temperature. The reaction mixture was then diluted after adding 10 mL distilled water and absorbance was measured at 540 nm. Control represented 100% enzyme activity and was conducted in a similar way by replacing extract with vehicle [19]. All assays were carried out in triplicate and values were presented in percentage of inhibition. The activity of α-amylase was determined as pursues (Chekuri et al., 2023):
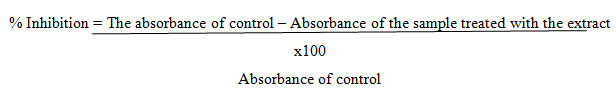
Inhibition of α-glucosidase Enzyme Assay: The inhibitory activity on α-glucosidase was determined by incubating a solution of the starch substrate (2% w/v maltose or sucrose) with 1mL 0.2 M Tris buffer (pH 8.0) containing concentrations of Acalypha indica leaves extracts for 5 min at 37°C. The reaction was initiated by adding 1mL of the α-glucosidase enzyme to it, followed by incubation at 37°C for 10 min. Then, the reaction mixture was heated for 2 min in a boiling water bath to stop the reaction. The amount of liberated glucose is measured by the glucose oxidase peroxidase method (Pallapothu et al., 2021). All assays were carried out in triplicate and values were presented in percentage of inhibition.
Glucose Diffusion Inhibitory Study: Glucose diffusion inhibitory study was carried out by using in vitro model consisted of a dialysis tube (6 cm X 15 mm) (Spectra/Por®, MWCO:2000) into which 6 mL of Acalypha indica leaves extracts and 2 mL of 0.15 M NaCl containing 1.65 mM D-glucose were added (Penumala et al., 2017). The dialysis tube was sealed at each end and placed in a centrifuge tube containing 45 mL 0.15 M NaCl. The tubes were shaken occasionally and incubated at 37°C for 3 h. The concentration of glucose within the dialysis tube was measured and control tests were conducted in the absence of test samples. Glucose concentrations were analysed by the DNS method. All tests were carried out in triplicate and the results were presented as means ± SD (Penumala et al., 2017).
Statistical analysis: The experimental results were expressed as mean ± standard deviation (SD) of three replicates. EC50 values (concentration at which 50% inhibition was achieved) were obtained from the regression plots. Where applicable, the results were treated to a one-way analysis of variance (ANOVA) and the significant difference (p<0.05) between means was determined by the least significant difference (LSD) using Statistical Package for Social Sciences (SPSS) version 15.0 for Windows.
RESULTS AND DISCUSSION
Preliminary Phytochemical Screening: Phytochemical screening was performed on all the studied fractions, and the results are given in Table 1. The methanol extract of Acalypha indica leaves contained almost all classes of secondary metabolites such as alkaloids, flavonoids, steroids, phenols, carbohydrates, amino acids, proteins, terpenoids, diterpenes, triterpenes, polyphenols, and lipids. However, saponins were absent in methanol extract of Acalypha indica leaves [Table-1].
Table-1. Phytochemical analysis of Acalypha india extracts
| S. No | Phytoconstituents | Hexane | Ethyl acetate | Methanol | Ethanol |
| 1 | Alkaloids | + | + | +++ | + |
| 2 | Carbohydrate | + | + | ++ | + |
| 3 | Glycosides | + | + | ++ | + |
| 4 | Phenols | + | + | +++ | + |
| 5 | Flavanoids | + | + | +++ | – |
| 6 | Proteins&amino acids | + | + | ++ | + |
| 7 | Steroids | + | + | ++ | + |
| 8 | Tannins | + | + | +++ | – |
| 9 | Saponins | + | ‒ | +++ | – |
| 10 | Terpenoids | + | + | +++ | + |
| 11 | Diterpenes | + | + | ++ | + |
| 12 | Triterpenes | + | + | ++ | + |
| 13 | Polysterols | + | + | ++ | + |
| 14 | Lipids | + | + | + | + |
(+ : Present, ‒ : Absent of the particular compound).
Quantitative Estimation of Phytochemicals: Table-2 depicts the quantitation of secondary metabolites such as phenolics, flavonoids, and tannins present in the methanol extract of Acalypha indica leaves. Compared to other extracts, methanolic extract contains the highest amount of phenolics, flavonoids and tannins. These estimations revealed that the amounts of phenolics and flavonoids were higher than tannins. The other extracts had a considerably good amount of these secondary metabolites.
Table-2. Acalypha indica leaf methanolic extract quantitative phytochemical analysis
| S. No | Phytochemical | Quantitative analysis |
| 1 | Alkaloids | 7.20±0.05 |
| 2 | Flavonoids | 2.18±0.03 |
| 3 | Glycosides | 0.05±0.00 |
| 4 | Saponins | 4.30±0.02 |
| 5 | Steroids | 0.50±0.00 |
| 6 | Phenols | 0.080±0.00 |
| 7 | Terpenoids | 0.40 ±0.01 |
| 8 | Anthraquinones | 1.40 ± 0.03 |
| 9 | Taninns | 4.80 ± 0.03 |
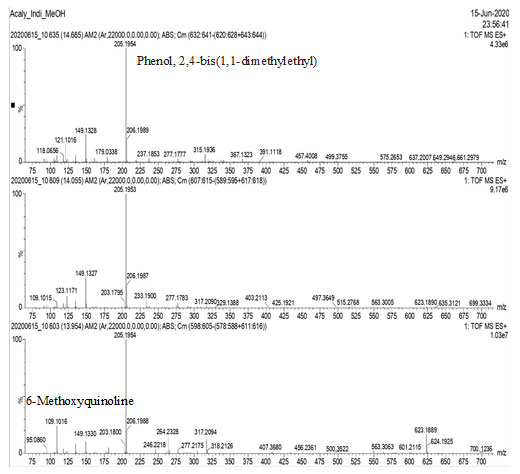
GC-MS Analysis: GC-MS analysis of the methanol extract of Acalypha indica leaves, identified several compounds as shown in Figs. 1, 2, 3 & 4.
Figure-1: GC-MS analysis of the methanol extract of Acalypha indica leaves
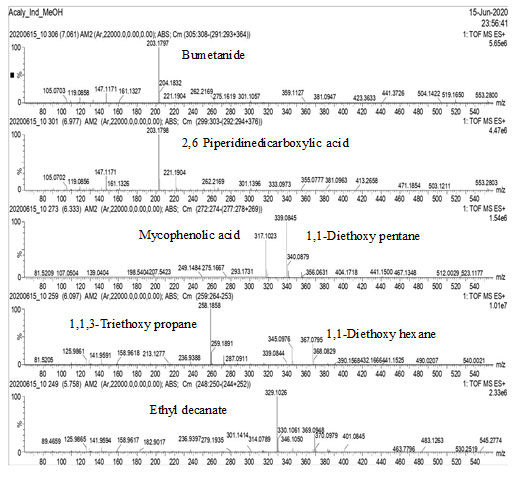
Figure-2: GC-MS analysis of the methanol extract of Acalypha indica leaves
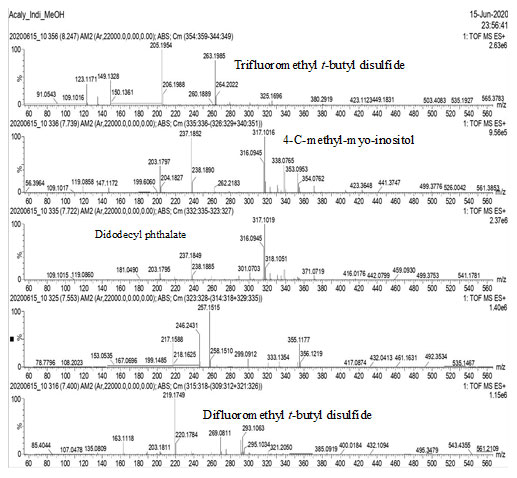
Figure-3: GC-MS analysis of the methanol extract of Acalypha indica leaves

Figure-4: GC-MS analysis of the methanol extract of Acalypha indica leaves
Antioxidant Activity: The DPPH radical scavenging activities of extracts of Acalypha indica leaves at different concentrations are shown in Table-3 and Figure-5, and were compared to ascorbic acid as reference. methanol extract of Acalypha indica leaves demonstrated the highest DPPH radical scavenging activity at concentrations ranging from 5μg/mL to 25μg/mL, when compared to the other extracts, which showed only moderate activity.
Table 3. DPPH activity MEAL
| Concentration
(µg/ml) |
% Inhibition of DPPH free radical | |
| BHA | MEAL | |
| 5 | 72.1± 1.7 | 66.0 ± 2.1 |
| 10 | 79.6 ± 2.4 | 73.7 ± 3.5 |
| 15 | 93.3 ± 2.3 | 78.9 ± 2.6 |
| 20 | 95.6 ± 1.7 | 84.1 ± 2.3 |
| 25 | 96.9 ± 1.5 | 89.8 ± 2.5 |
Figure 5: DPPH activity of MEAL
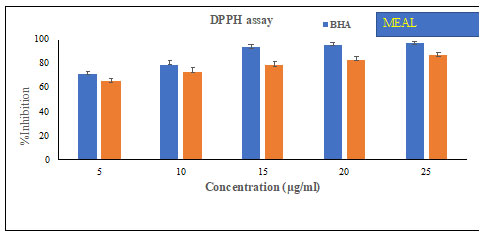
Figure-5 demonstrates the DPPH scavenging activities of extracts of Acalypha indica leaves at different concentrations. methanol extract of Acalypha indica leaves showed DPPH decomposition activity in a dose dependent manner, measured highest at 25μg/mL. Moderate DPPH scavenging activities were noted with other extracts of Acalypha indica leaves but less compared to methanol extract of Acalypha indica leaves or ascorbic acid (Erum et al., 2017).
Table 4. % Inhibition of ABTS radical scavenging activity
| Concentration
(µg/ml) |
% Inhibition of ABTS radical scavenging activity | |
| BHA | MEAL compound | |
| 5 | 70.2 ± 2.9 | 62.0 ± 232 |
| 10 | 81.3 ± 2.4 | 68.3 ± 3.5 |
| 15 | 92.5 ± 2.4 | 72.2 ± 3.7 |
| 20 | 94.7 ± 1.8 | 77.1 ± 1.3 |
| 25 | 96.1 ± 1.5 | 82.5± 1.5 |
Figure 6: % Inhibition of ABTS radical scavenging activity
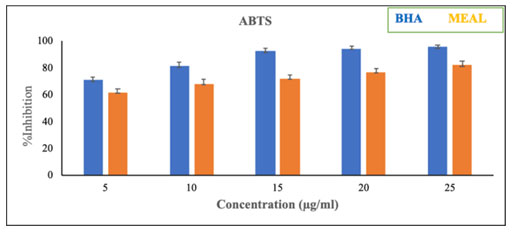
Table-5. % Inhibition of Nitric oxide scavenging activity
| Concentration
(µg/ml) |
% Inhibition of Nitric oxide scavenging activity | |
| BHA | MEAL | |
| 5 | 70.9 ± 9.5 | 65.0 ± 2.1 |
| 10 | 70.5 ± 2.6 | 72.2 ± 3.6 |
| 15 | 93.1 ± 2.2 | 75.9 ± 2.7 |
| 20 | 95.9 ± 1.8 | 82.1 ± 2.2 |
| 25 | 96.3 ± 1.2 | 88.4 ± 2.8 |
Figure 7: % Inhibition of Nitric oxide scavenging activity
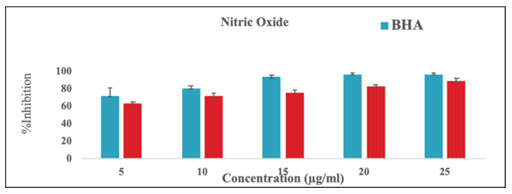
Figure-6, table-4 and Figure-7 & table-5 depicts the ABTS and NO percentage inhibition using the extracts of Acalypha indica leaves at different concentrations. These results clearly indicated that the methanol extract of Acalypha indica leaves can potentially inhibit ABTS and NO in a dose-dependent manner.
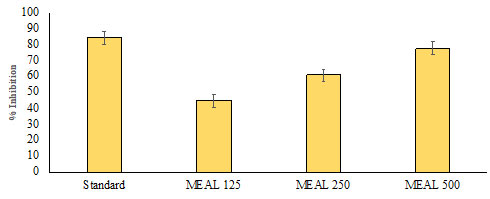
In vitro Alpha-amylase Inhibitory Activity: The results of the current study demonstrated a dose dependent increase in percentage inhibitory activity against the ɑ-amylase enzyme. The methanol extract of Acalypha indica leaves had significant inhibitory activity (p < 0.05) of the ɑ-amylase enzyme that was the highest at a dose of 500μg/mL, in contrast with the standard acarbose. On the other hand, the other extracts of Acalypha indica leaves did not show significant ɑ-amylase inhibitory activities when compared to methanol extract of Acalypha indica leaves and acarbose. A comparison of ɑ-amylase inhibitory activity between the standard drug acarbose and Acalypha indica extracts is depicted in Fig. (8).
Figure 8: In vitro Alpha-amylase Inhibitory Activity of MEAL
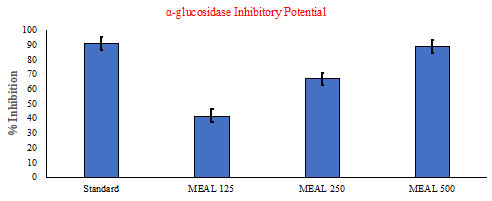
In vitro α-glucosidase Inhibitory Potential: The results of the antidiabetic study using α-glucosidase inhibitory assay of the extracts of Acalypha indica leaves are shown in Fig. (9). Among all the extracts, methanol extract of Acalypha indica leaves established a significant (p<0.05) inhibitory action of α-glucosidase enzyme in a dose-dependent manner when compared to other extracts.
Figure 9: In vitro α-glucosidase Inhibitory Potential Activity of MEAL.
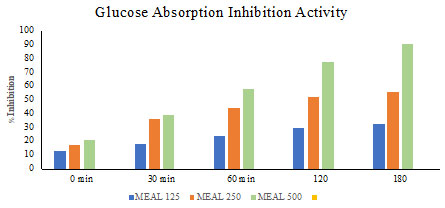
Glucose Absorption Inhibition Activity: A simple model was used to investigate glucose absorption inhibition by extract of Acalypha indica leaves shown in Fig. (10). methanol extract of Acalypha indica leaves exhibited the highest potential in inhibiting the movement of glucose molecules across the dialysis membrane when compared to the control. Other extracts were less effective in preventing the diffusion of glucose molecules when compared to methanol extract of Acalypha indica leaves.
Figure 10: Glucose Absorption Inhibition Activity of MEAL.
Diabetes mellitus (DM) is a metabolic disorder, with an increasing trend in occurrence all over the world. Conventional medication offers great clinical chances and demonstrates a splendid future in the treatment and management of DM and its complications (Parim et al., 2019). In spite of the fact that various types of oral antihyperglycemic drugs along with insulin are used for the treatment of DM, there is a renewed interest in patients to use natural products with antidiabetic action (Montero-Muñoz et al., 2020). A considerable amount of conventional or indigenous drugs are also compelling the cutting edge arrangement of prescription. In the present investigation, we made an endeavor to prepare different solvent extracts of Acalypha indica leaves and assessed their antioxidant and antidiabetic activities (Brahma et al., 2014).
Since DM is a life style and health threat to our population; researchers around the globe are trying to identify natural active agents by screening phytochemicals (Ninave et al., 2022). In a previous study, a phytochemical screening of different plant extracts of the demonstrated the presence of exacerbates, which accounted for its activities (Brahma et al., 2015). We found that our methanolic extracts of Acalypha indica leaves contained very high amounts of total phenolics, total flavonoids, and tannin content compared to individual plant extracts. Phenolics, flavonoids and tannins are one of the most widespread groups of natural constituents found in the plants, which can neutralize the free radicals and showed antioxidant activity through scavenging or chelating, and also reported having antidiabetic activities (Erum et al., 2015) (Shafodino et al., 2023).
Free radicals like reactive oxygen species (ROS) are associated with DM, metabolic disorders, obesity, inflammation, cardiovascular disarranges, atherosclerosis, aging and neoplastic ailments (Shafodino et al., 2023) (Mopuri et al., 2017). The balance between the rate of free radical generation and elimination is essential. Abundance in cell radical generation can be hurtful; in any case, if there is a critical increment in a radical generation or abatement in radical elimination from the cell, oxidative cellular stress follows (Penumala et al., 2017). Experimental evidence and clinical proof suggest that oxidative stress is associated with DM initiation and progression (Oyebode et al., 2021).
In the present investigation, we assessed the antioxidant activities of different solvent extracts of Acalypha indica leaves by using DPPH radical and H2O2 scavenging action, TBARS test and measuring total antioxidant activity. DPPH is a steady, nitrogen focused free radical, which, after receiving hydrogen from the antioxidants present in the polyphenolic extract, is converted to diphenylpicryl hydrazine (Mouli et al., 2012). The noted decrease of DPPH by the methanolic extracts of Acalypha indica leaves was either because of the exchange of a hydrogen atom or the exchange of an electron. Phenolic compounds and flavonoids are additionally thriving hydrogen benefactors, which make them great antioxidants (Priya et al., 2016). H2O2 is a weak oxidizing agent and can directly inactivate a few enzymes, usually by the oxidation of essential thiol groups. H2O2 can cross cell membranes rapidly, and once inside the cell, H2O2 likely reacts with Fe2+ and possibly Cu2+ ions to form hydroxyl radicals, which are detrimental for the cells. The secondary metabolic compounds may go about as free radical scavengers on account of their hydrogen giving capacity and scavenging capacity (Agrawal et al., 2023).
Our investigation showed that the total antioxidant capacity of methanolic extracts of Acalypha indica leaves was much higher compared to other solvent extracts. A few reports have indicated direct correlation between total phenolic contents and antioxidative activity. The chemical composition and chemical structures of active components of the extracts are significant elements contributing to the adequacy of natural antioxidants (Chakraborty et al., 2023) (Thepwiwatjit et al., 2022). In this study, we found high amounts of total phenolic content, flavonoids, tannins, and bioactive factors by GC-MS in methanolic extracts of Acalypha indica leaves. These components may account for the potent antioxidant activity shown by methanolic extracts of Acalypha indica leaves. However, a further detailed study is necessary to confirm the action of individual compounds present in the methanolic extracts of Acalypha indica leaves.
Carbohydrate hydrolyzing enzymes, principally α- amylase and α-glucosidase, contribute to postprandial hyperglycemia. α-amylase initiates the procedure of carbohydrate digestion by hydrolysis of 1, 4-glycosidic linkages of starch and glycogen (polysaccharides) to disaccharides, and α- glucosidase mediates the further hydrolysis of disaccharides to monosaccharides, which leads to postprandial hyperglycemia (Penumala et al., 2017) (Parim et al., 2015. Therefore, inhibition of α-amylase and α- glucosidase are useful in the management of hyperglycemia as they delay carbohydrate digestion, which subsequently diminishes the postprandial plasma glucose level. Ongoing advances in understanding the action of intestinal enzymes (α-amylase and α-glucosidase both are significant in starch assimilation and glucose retention) have led to the improvement of pharmacological agents. High postprandial blood glucose levels are related to micro and macrovascular complications in diabetes and its associated cardiovascular diseases. an α- glucosidase enzyme secreted by the intestinal lumen plays an important role in the digestion of carbohydrates by breaking down starch and oligosaccharides to monosaccharides before they can be absorbed.
It is recommended that concealment of the movement of such digestive enzymes would defer the debasement of starch and oligosaccharides, which would reduce glucose absorption and subsequent decrease in postprandial blood glucose levels (Hanuma et al., 2021). α-glucosidase inhibitors like acarbose and miglitol impede the processing of carbo- hydrates and hinder the assimilation (Meriga et al., 2012). Consequently, one of the restorative methodologies for decreasing postprandial blood glucose levels in patients with hyperglycemia is to counteract the assimilation of carbohydrates after meals. Restraining these enzymes (α-amylase and α-glucosidases) diminished the high postprandial blood glucose peaks in diabetes (Sahukari et al., 2021). Acarbose and miglitol also inhibit α-glucosidases and reduce the assimilation of starch and disaccharides (Sivasankari et al., 2014), (Perez et al., 2006) & (Penumala et al., 2017).
The α-amylase inhibitors are used as aide supplements that deter the assimilation and ingestion of carbohydrates. Acarbose, a perplexing oligosaccharide, restrains the activity of pancreatic amylase in breaking down starch. At the same time, synthetic inhibitors cause several side effects, such as abdominal pain, diarrhea, and soft feces in the colon (Oyebode et al., 2017). On the other hand, plant-based compounds and products derived from natural sources have been documented for their effective inhibitory activities of the enzymes, but have no or fewer side effects (Penumala et al., 2017). Our finding uncovers that different solvent extracts of Acalypha indica leaves productively holds back α-amylase enzyme activity in vitro, although the mechanism of inhibition requires detailed investigation. However, a previous report suggests that the plant secondary metabolites may cause conformational changes in structure (Montero-Muñoz et al., 2020).
Methanolic extracts of Acalypha indica leaves also showed noteworthy inhibition of glucose movement into the external solutions across the dialysis membrane. These outcomes suggest that the glucose lowering effects of methanolic extracts of Acalypha indica leaves might be due to reduced glucose absorption. The main mechanism behind the role of dietary fiber in bringing down postprandial serum glucose is the viscosity of various dietary strands, which hamper diffusion of glucose, thus, interfering with absorption and digestion of carbohydrates (Brahma et al., 2014). Gallagher et al. examined the capacity of various plants to repress glucose dispersion.
The various phytochemical compounds such as polyphenolic compounds, triterpenoids and other bioactive compounds present in the plant may contribute to the observed glucose lowering impacts of the extracts (Mopuri et al., 2017). Tannins, flavonoids, saponins, alkaloids and other bioactive mixes present in the plant are theorized to contribute to the hypoglycemic impact (Oyebode et al., 2018) (Penumala et al., 2017). Hence, the observed α-amylase and α-glucosidase inhibitory effects might be due to the presence of these phytoconstituents.
In the current study, we found that the glucose lowering effect of methanolic extracts of Acalypha indica leaves is higher than that of any of the different solvent extracts, which might be due to the less number of phytochemicals.
CONCLUSION
In conclusion, our results reveal that the methanolic extracts of Acalypha indica leaves contains a high amount of secondary metabolites and bioactive components, which have therapeutic potential. We found that the methanolic extracts of Acalypha indica leaves has free radical scavenging activity and inhibitory action against α-amylase and α-glucosidase. These properties could be explored for the management of postprandial glycemia in individuals with type 2 DM. Although the effects have been set up in vitro, these results suggest that methanolic extracts of Acalypha indica leaves may be a potential dietary health supplement. Further detailed examinations are in progress to elucidate whether the methanolic extracts of Acalypha indica leaves has antidiabetic potential by in vivo for corroborating the traditional claim of Acalypha indica.
Ethics Approval and Consent to Participate: Not applicable.
Human and Animal Rights: No Animals/Humans were used for studies that are base of this research.
Consent for Publication: Not applicable.
Availability of Data and Materials: Not applicable.
Funding: University Grant Commission (UGC), Rajiv Gandhi National Fellowship Scheme (RGNF) Government of India, JRF Research Fellowship (RGNF-SRF) (Grant Ref No: 202021-NFST-Tel-00696 dated: 20-09-2021).
Conflict of Interest: The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank the University Grant Commission (UGC), Rajiv Gandhi National Fellowship Scheme (RGNF) & DST-FIST, Government of India, and financial assistance for this work. The authors also thank the Department of Genetics, Osmania University, Hyderabad, India for providing all the facilities for studies.
REFERENCES
Agrawal A, Kulkarni GT, Lakshmayya (2023). Topical application of aerial portion of Acalypha indica Linn ameliorates psoriasis in rodents: Evidences from in vivo and in silico studies. J Ethnopharmacol. 28;315:116685. doi: 10.1016/j.jep.2023.116685. Epub 2023 May 25. PMID: 37236382.
Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, Nath LM, Das AK, Madhu SV, Rao PV, Shukla DK, Kaur T, Ali MK, Mohan V (2011). The Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study: methodological details. J Diabetes Sci Technol. 1;5(4):906-14. doi: 10.1177/193229681100500413. PMID: 21880233; PMCID: PMC3192597.
Banday MZ, Sameer AS, Nissar S (2020). Pathophysiology of diabetes: An overview. Avicenna J Med. 13;10(4):174-188. doi: 10.4103/ajm.ajm_53_20. PMID: 33437689; PMCID: PMC7791288.
Brahma Naidu P, Nemani H, Meriga B, Mehar SK, Potana S, Ramgopalrao S (2014). Mitigating efficacy of piperine in the physiological derangements of high fat diet induced obesity in Sprague Dawley rats. Chem Biol Interact. 2014 Sep 25;221:42-51. doi: 10.1016/j.cbi.2014.07.008. PMID: 25087745.
Brahma Naidu P, Uddandrao VV, Ravindar Naik R, Suresh P, Meriga B, Begum MS, Pandiyan R, Saravanan G (2015). Ameliorative potential of gingerol: Promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol. 2016 Jan 5;419:139-47. doi: 10.1016/j.mce.2015.10.007. PMID: 26493465.
Chakraborty B, Bhat MP, Basavarajappa DS, Rudrappa M, Nayaka S, Kumar RS, Almansour AI, Perumal K (2023). Biosynthesis and characterization of polysaccharide-capped silver nanoparticles from Acalypha indica L. and evaluation of their biological activities. Environ Res. 15;225:115614. doi: 10.1016/j.envres.2023.115614. Epub 2023 Mar 6. PMID: 36889569.
Chekuri S, Vyshnava SS, Somisetti SL, Cheniya SBK, Gandu C, Anupalli RR (2023). Isolation and anticancer activity of quercetin from Acalypha indica L. against breast cancer cell lines MCF-7 and MDA-MB-231 ;13(8):289. doi: 10.1007/s13205-023-03705-w. PMID: 37547624; PMCID: PMC10397153.
Erum I, Kamariah AS, Linda B.L. Lim (2015). Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. Journal of King Saud University – Science. 27: 224-232. 10.1016/j.jksus.2015.02.003
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C (2020). Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 30;21(17):6275. doi: 10.3390/ijms21176275. PMID: 32872570; PMCID: PMC7503727.
Hanuma Kumar GEN, Kumar SS, Balaji M, Maurya DK, Kesavulu M (2021). Pterocarpus santalinus L. extract mitigates gamma radiation-inflicted derangements in BALB/c mice by Nrf2 upregulation. Biomed Pharmacother. 141: 111801. doi:10.1016/j.biopha.2021.111801.
Ikewuchi JC, Onyeike EN, Uwakwe AA, Ikewuchi CC (2011). Effect of aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’ Muell Arg (Euphorbiaceae) on the hematology, plasma biochemistry and ocular indices of oxidative stress in alloxan induced diabetic rats. J Ethnopharmacol. 11;137(3):1415-24. doi: 10.1016/j.jep.2011.08.015. Epub 2011 Aug 16. PMID: 21864665.
Meriga B, Mopuri R, MuraliKrishna T (2012). Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac J Trop Med. 5(5):391-395. doi:10.1016/S1995-7645(12)60065-0.
Montero-Muñoz I, Levin GA, Cardiel JM (2020). Four new species of Acalypha L. (Euphorbiaceae, Acalyphoideae) from the West Indian Ocean Region. PhytoKeys. ;140:57-73. doi: 10.3897/phytokeys.140.50229. PMID: 32148432; PMCID: PMC7052020.
Mopuri R, Ganjayi M, Meriga B, Koorbanally NA, Islam MS (2017). The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J Food Drug Anal. 2018 Jan;26(1):201-210. doi: 10.1016/j.jfda.2017.03.001. PMID: 29389556; PMCID: PMC9332642.
Mopuri R, Islam MS (2017). Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed Pharmacother. 2017 May;89:1442-1452. doi: 10.1016/j.biopha.2017.02.108. PMID: 28372259.
Mouli KC, Vijaya T, Dattatreya Rao S (2012). Effectiveness of flavonoid-rich leaf extract of Acalypha indica in reversing experimental myocardial ischemia: biochemical and histopathological evidence. Zhong Xi Yi Jie He Xue Bao. 10(7):784-92. doi: 10.3736/jcim20120709. PMID: 22805085.
Ninave PB, Patil SD (2022). Pharmacological screening of Acalypha indica L Possible role in the treatment of asthma. J Ethnopharmacol. 290:115093. doi: 10.1016/j.jep.2022.115093. Epub 2022 Feb 8. PMID: 35149129.
Oyebode OA, Erukainure OL, Koorbanally NA, Islam MS (2018). Acalypha Wilkesiana ‘Java White’: Identification of Some Bioactive Compounds by Gc-Ms and Their Effects on Key Enzymes Linked to Type 2 Diabete. Acta Pharm. 1;68(4):425-439. doi: 10.2478/acph-2018-0037. PMID: 31259705.
Oyebode OA, Erukainure OL, Koorbanally NA, Islam MS (2018). Acalypha Wilkesiana ‘Java White’: Identification of Some Bioactive Compounds by Gc-Ms and Their Effects on Key Enzymes Linked to Type 2 Diabete. Acta Pharm. 68(4):425-439. doi: 10.2478/acph-2018-0037. PMID: 31259705.
Oyebode OA, Erukainure OL, Mopuri R, Sanni O, Koorbanally NA, Islam MS (2021). Butanol fraction of Alstonia boonei De Wild. leaves ameliorate oxidative stress and modulate key hypoglycaemic processes in diabetic rats. Arch Physiol Biochem. 12:1-14. doi: 10.1080/13813455.2021.1899240. Epub ahead of print. PMID: 33840309.
Pallapothu B, Sankar J (2021). Acalypha indica-Induced Hemolysis and Methemoglobinemia in a Child With G6PD Deficiency. Indian Pediatr 15;58(1):92-93. PMID: 33452792.
Parim B, Harishankar N, Balaji M, Pothana S, Sajjalaguddam RR (2015). Effects of Piper nigrum extracts: Restorative perspectives of high-fat diet-induced changes on lipid profile, body composition, and hormones in Sprague-Dawley rats. Pharm Biol. 5; 3(9):1318-1328. doi:10.3109/13880209.2014.980585.
Parim B, Sathibabu Uddandrao VV, Saravanan G (2019). Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail Rev. 24(2):279-299. doi: 10.1007/s10741-018-9749-1. PMID: 30349977.
Penumala M, Zinka RB, Shaik JB, Amooru Gangaiah D (2017). In Vitro Screening of Three Indian Medicinal Plants for Their Phytochemicals, Anticholinesterase, Antiglucosidase, Antioxidant, and Neuroprotective Effects. Biomed Res Int. 2017;2017:5140506. doi: 10.1155/2017/5140506. PMID: 29204442; PMCID: PMC5674485.
Penumala M, Zinka RB, Shaik JB, Amooru Gangaiah D (2017). In Vitro Screening of Three Indian Medicinal Plants for Their Phytochemicals, Anticholinesterase, Antiglucosidase, Antioxidant, and Neuroprotective Effects. Biomed Res Int. 2017;2017:5140506. doi: 10.1155/2017/5140506 PMID: 29204442; PMCID: PMC5674485.
Penumala M, Zinka RB, Shaik JB, Amooru Gangaiah D (2017). In Vitro Screening of Three Indian Medicinal Plants for Their Phytochemicals, Anticholinesterase, Antiglucosidase, Antioxidant, and Neuroprotective Effects. Biomed Res Int. :5140506. doi: 10.1155/2017/5140506. PMID: 29204442; PMCID: PMC5674485.
Perez Gutierrez RM, Vargas S R (2006). Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia. 77(4):286-9. doi: 10.1016/j.fitote.2006.03.011. Epub 2006 May 19. PMID: 16713129.
Priya CL, Bhaskara Rao KV (2016). Postprandial Antihyperglycemic And Antioxidant Activities of Acalypha indica Linn Stem Extract: An In-vivo Study. Pharmacogn Mag. 12(Suppl 4):S475-S481. doi: 10.4103/0973-1296.191461. PMID: 27761078; PMCID: PMC5068127.
Ravi S, Shanmugam B, Subbaiah GV, Prasad SH, Reddy KS (2017). Identification of food preservative, stress relief compounds by GC-MS and HR-LC/Q-TOF/MS; evaluation of antioxidant activity of Acalypha indica leaves methanolic extract (in vitro) and polyphenolic fraction (in vivo). J Food Sci Technol. 2017 May;54(6):1585-1596. doi: 10.1007/s13197-017-2590-z. PMID: 28559618; PMCID: PMC5430191.
Sahukari R, Punabaka J, Bhasha S, Ganjikunta VS, Kondeti Ramudu S, Kesireddy SR, Ye W, Korivi M (2021). Phytochemical Profile, Free Radical Scavenging and Anti-Inflammatory Properties of Acalypha indica Root Extract: Evidence from In Vitro and In Vivo Studies. Molecules. 26(20):6251. doi: 10.3390/molecules26206251. PMID: 34684831; PMCID: PMC8537703.
Seebaluck R, Gurib-Fakim A, Mahomoodally F (2014). Medicinal plants from the genus Acalypha (Euphorbiaceae)–a review of their ethnopharmacology and phytochemistry. J Ethnopharmacol. 2015 Jan 15;159:137-57. doi: 10.1016/j.jep.2014.10.040. PMID: 25446604.
Shafodino FS, Lusilao JM, Mwapagha LM (2023). Preparation of medicinally active extracts and phytochemical characterisation of phytoconstituents from medicinal plants. Nat Prod Res. 1:1-11. doi: 10.1080/14786419.2023.2252976. Epub ahead of print. PMID: 37655608.
Sivasankari B, Anandharaj M, Gunasekaran P (2014). An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J Ethnopharmacol. 153(2):408-23. doi: 10.1016/j.jep.2014.02.040. Epub 2014 Feb 27. PMID: 24583241.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ (2021). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 183: 109119. doi: 10.1016/j.diabres.2021.109119. PMID: 34879977.
Thepwiwatjit S, Teawtrakul N, Krikeerati T, Mitsungnern T (2022). Acalypha indica-induced transient glucose-6-phosphate dehydrogenase deficiency with acute haemolysis. BMJ Case Rep. 1;15(3):e245447. doi: 10.1136/bcr-2021-245447. PMID: 35232731; PMCID: PMC8889154.
Uddandrao VVS, Brahmanaidu P, Ganapathy S (2020). Evaluation of the Antioxidant and Antidiabetic Potential of the Poly Herbal Formulation: Identification of Bioactive Factors. Cardiovasc Hematol Agents Med Chem. 18(2):111-123. doi: 10.2174/1871525718666200207103238. PMID: 32031078.
Zahidin NS, Saidin S, Zulkifli RM, Muhamad II, Ya’akob H, Nur H. A review of Acalypha indica L (2017). (Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J Ethnopharmacol. 2017 Jul 31;207:146-173. doi: 10.1016/j.jep.2017.06.019. PMID: 28647509.


