1Department of Zoology, Shri R.L.T. College of Science, Akola, India.
2 B.S. Patel College, Pimplegaonkale, India.
Corresponding author email: rashmisawalkar75@gmail.com
Article Publishing History
Received: 11/03/2021
Accepted After Revision: 28/06/2021
The silkworm Bombyx mori is a domesticated tiny insect having a remarkable economic significance. Occurrence of diseases in silkworm Bombyx mori is fairly common and inflict serious losses. The major disease affecting mulberry silkworms is Grasserie, which is a viral infection causing reduced production of silk in India and other countries primarily by the Bombyx mori, Nuclear Polyhedrosis Virus (BmNPV). In India, >50 % of silk cocoon crop losses are attributed to BmNPV infections. Presently, there are no specific preventive measures to treat the spread of BmNPV infection other than sanitized rearing methods, where the only commercial practice today is to discard large stocks of worms during the infection.
Once infected, the disease pathogen in silkworm becomes highly efficient at manipulating the physiological and endocrinological responses of the body. In order to provide references for further study on the infection and pathogenesis of BmNPV, this research was explored for the changes in the haemolymph enzyme activities. For the study, healthy and Grasserie infected mulberry silkworms were collected from the local sericulture units and reared in the laboratory to analyse the intensity of enzyme activity. The enzyme profile of both healthy and infected worms was recorded by using Clinical Analyser. The study revealed, significant decrease in enzyme activity of alkaline phosphatase, acid phosphatase, alanine amino transferase, aspartate amino transferase, in the infected larvae as compared with control healthy silkworms. Enzyme alterations reported in the present study, can be used as a marker, for indication of Grasserie disease in local mulberry silkworm colonies.
Acid Phosphatase, Alanine Amino Transferase, Alkaline Phosphatase, Aspartate Amino Transferase, Bombyx Mori.
Joshi R. P, Raja I. A. Enzyme Alterations in Haemolymph of the Silkworm, Bombyx mori During Grasserie Infection. Biosc.Biotech.Res.Comm. 2021;14(2).
Joshi R. P, Raja I. A. Enzyme Alterations in Haemolymph of the Silkworm, Bombyx mori During Grasserie Infection. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a
href=”https://bit.ly/3o7Q4Fr“>https://bit.ly/3o7Q4Fr</a>
Copyright © Joshi and Raja This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The silkworm Bombyx mori is a domesticated tiny insect having considerable economic significance. It is a well-known and economically important insect as it is a producer of valuable silk. Over 85% of the global silk production comes from the mulberry silkworm, B. mori. In India, the silk industry has made significant progress during the past two decades where it occupies the second position in global silk production next to China. As per the statistics, India has 82 lakh farmers in 62,000 villages engaged in sericulture and Indian raw silk production is 35,000 metric tonnes, (Statista Research Department, 2021).
Diseases in silkworm Bombyx mori are fairly common in occurrence and are serious in inflicting losses. The country has produced 35,820 MT of silk against the target of 38,530 MT during 2019-20 achieving 93.0% of the target. This is the progress during the past decades of silkworm industry but, this progress stops or reduces production when disease occurs. Grasserie is a viral infection, in silkworms which is the major cause for damage and reduced production of silk in India and other countries. The disease is caused primarily by Bombyx mori Nuclear Polyhedrosis Virus (BmNPV). The disease occurrence is a common phenomenon during all stages in silkworm rearing (Joshi and Raja 2013, Annual Report 2020).
The investigation revealed by Joshi (2015), these pathogenic diseases form major factors that greatly contribute to the crop failure and instability in cocoon production in all the rearing seasons. Once infected the disease pathogen in silkworm becomes highly efficient at manipulating the physiological and endocrinological responses of the body. In the present study, by doing investigation the oxidizing enzymes activity in the hemolymph of healthy or control and infected or diseased silkworms was recorded.
Aspartate amino Transferase (AST), Alanine amino Transferase (ALT), Alkaline Phosphatase (ALP), Acid Phosphatase (ACP) are the parameters widely used to understand the effect of diseases or infection, as these could give indications of progressive toxic effects long before the actual manifestation of the disease. The mode of viral infection and the activity of enzyme metabolic alterations involvement in silkworm immunity was studied by Babu et al. (2009). This study reveals, to understand the molecular and metabolic changes in diseased and control silkworms, it may form the potential basis and can be used in other fields such as medicine and virology (Babu et al. 2009; Joshi 2015 Gau et al 2020).
Rajitha and Savitri (2014) reviewed that among the many constraints that influence the success of cocoon production, the menace of disease is the prime one. The major disease affecting mulberry silkworm is Grasserie. The Grasserie disease occurs when the silkworms feed on mulberry leaves, contaminated with BmNPV (Rajitha and Savitri 2014; Jiang and Xia 2014 Gao et al 2020). All these biomolecules play very important role in many pathways that operate within the living cells as they are also the intermediates of many physiological reactions.
The enzyme levels in haemolymph especially Alkaline phosphatase (ALP), Acid phosphatase (ACP), Aspartate amino Transferase (AST) and Alanine amino Transferase (ALT) are the parameters broadly used in the disease diagnosis in Silkworms and it can be used as a model organism in virological research (Jiang and Xia 2014, Gao et al 2020).
The present study reveals that the enzyme alterations observed could prove a good model for studying the interaction between insect and virus. Alterations in the enzyme parameters, on exposure to disease causing pathogens, in the hemolymph could be used as an indicator for the health status of Silkworm Bombyx mori, as well as can be taken as target system to measure physiological and biomolecular stress induced alterations in the body during the occurrence of diseases.
MATERIAL AND METHODS
In the present study healthy and Grasserie infected mulberry silkworm larvae were collected from the local sericulture units, carried and reared in the laboratory. The haemolymph of reared silkworms were collected at early (Day 3) and late (Day 6) stages of Vth instar larva. Haemolymph was collected everyday into pre-chilled centrifuge tubes with a pinch of phenyl thiourea by clipping third pair of abdominal legs of silkworm larvae and the haemolymph was taken for the enzymatic studies. At the end the statistical analysis of the recorded data was done and recorded in the Table I and graphically given in Fig I. The analysis for the intensity of enzyme activity in both controlled and Grasserie infected larval samples were done by using Clinical Analyser. The Chemical kits used for enzyme assay were from Excel Diagnostics Ltd. The following method was used to estimate the enzyme alterations.

Enzyme Activity (U/L) = Change in (Absorbance /min) x 1746 factor
1 KA units/dl =7.1 U/Lit
RESULTS AND DISCUSSION
In the present study, the enzyme profile of healthy and Grasserie infected silkworms was recorded by the method described above. All the recorded data has been summarized in the Table I
Table 1. The Enzyme Profile of the silkworm haemolymph during Grasserie infection
| Enzyme | Healthy Control | Grasserie Infection | ||
| Early | Late | Early | Late | |
| Alanine Amino
Transferase (ALT) U/Lit |
375.31 ±2.00 | 645.33 ±3.00 | 445.67 ±5.00 a | 241.36 ±2.90 b |
| Aspartate Amino Transferase (AST) U/Lit | 240.21 ±8.2 | 281.8 ±4.00 | 283.7 ±8.00 a | 242.1 ±3.30 a |
| Alkaline Phosphatase (ALP) U/Lit | 0.037 ±0.06 | 0.125 ±0.08 | 0.286 ±0.13b | 0.609 ±0.39 b |
| Acid Phosphatase (ACP) U/Lit | 0.162 ±0.10 | 0.195 ±0.133 | 0.183 ±0.06b | 0.354 ±0.21a |
Conc.: Concentration, mean ± SE followed with the same letter (a): is not significantly different (P>0.05), (b): significantly different (P<0.05), (c): highly significantly different (P<0.01), (d): very highly significantly different (P<0.001).
Figure 1:Showing significant differences in enzyme activities in haemolymph in the silkworm during infection with Grasserie
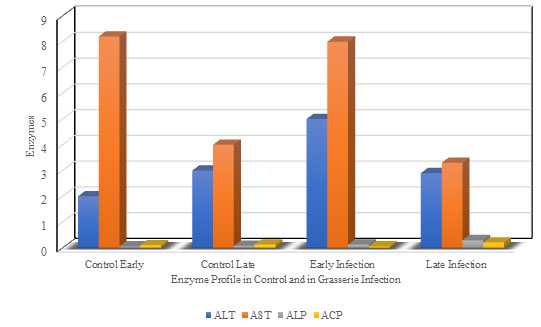
The levels of Alanine aminotransferase (ALT) in the haemolymph of healthy and Grasserie infected Silkworms are recorded in the Table I and Fig I. On the basis of unit volume of haemolymph, the ALT levels were low at the beginning of the larval development both in the healthy and Grasserie infected silkworms.
At early stage it was recorded 445.67 U/Lit. as compared to the healthy control 375.31 U/Lit. At late stage of infection, the changes in the levels of the healthy and infected larvae were 645.37 U/Lit and 241.36 U/Lit respectively, which showed decreased trend in infected larvae as compare to non-infected healthy worms. Accordingly, Aspartate aminotransferase (AST) activity in the haemolymph during both early and late infection with Grasserie were disturbed a little. At early infection the enzyme activity in the haemolymph showed significantly reducing effect 283.70 U/L in infection than the control level of the enzyme in haemolymph 240.21 U/Lit.
On the other hand, the enhancing effect on AST activity in the haemolymph was exhibited in late infection was 242.10 U/Lit as compared to the healthy control 281.80 U/Lit. From the values depicted in Table I and Fig. I, the Alkaline phosphatase (ALP) activity enhanced initially 0.286 U/L during early infection with Grasserie in comparison to the control non infected larva 0.037 U/Lit at early stage. However, in late stage the haemolymph in the diseased worm undergoes rapid increase in Alkaline phosphatase (ALP) activity 0.609 U/L, when compared to the control 0.125 U/Lit. Results.
recorded in Table I and Fig. I, explored the effect of Grasserie on the Acid phosphatase (ACP) activity in haemolymph of silkworm at early and late infection. Acid phosphatase activity (0.183 U/L) enhanced initially during early infection with Grasserie in comparison to the control non infected larva 0.162 U/Lit, on day two of 5th instar larva. However, on 5th day the haemolymph in the diseased worm undergoes a rapid rise in Acid phosphatase activity 0.354 U/L, when compared with the control 0.195 U/Lit.
Enzymes provide the energy needed for metabolic reactions essential to immune health. Enzymes have been shown to stimulate the body’s natural defenses while breaking down offending pathogenic immune complexes. This helps relieve stress on the body and strengthen the immune system, which can speed the healing process. Changes in enzymatic action along with metabolic modulations during the progress of pathogen play an important role in under-standing the interaction between the host and pathogen as a part of a survival strategy.
In view of this, the present study has been carried out to understand the dynamics of enzyme during the progress of BmNPV in silkworm Bombyx mori. Many workers through their studies have emphasized the importance of diseases to explain the effects on bimolecular and physiological mechanisms of the infected silkworm especially as they relate to, the composition of body fluids, enzyme systems, development of immunity, and predisposition to diseases and parasites (Gillespie et al. 1997; Doreswamy et al. 2004).
In the present study we found the significant fluctuations in enzyme activity levels in the haemolymph. In insects, ALPs are involved in several biological processes and respond to stress, including pathogenesis, or infection Miao (2002). Senthil et al., (2005) reported a decrease in the activity level of the Acid phosphatase in Spodoptera litura after exposure to pathogenic infections with virus. The effects of NPV on midgut enzymes of Spodoptera litura were investigated by Nathan et al. (2005) and it was demonstrated that alkaline phosphatase is decreased after infection by virus.
Conversely, Matindoost (2006) showed that BmNPV had caused a considerable decrease in activity of this enzyme in silkworm after infection of a cell line established from silkworm embryo (Bm-EK1). It was noted that infection with microbial pathogens is capable of activating, inactivating or neutralizing enzyme production and subsequent release to the system. These types of alterations in enzyme profile could possibly be resulting due to mobilization and detoxification of microbial toxins and explains the sudden alterations in enzyme activity as observed in the present study (Doreswamy 2004; Etebari 2007; Nirupama 2015).
Many workers, reported the significant fluctuations in enzyme activity levels in the in haemolymph of healthy and Grasserie infected silkworms. Nirupama (2015) reported the same alterations in enzymes at healthy and infected silkworms as we recorded in the present study. Enzymes are important biological molecules responsible for the thousands of biochemical interconversions that sustain life. Enzymes serve a wide variety of functions, act as catalysts and help in complex reactions occur everywhere in life. Without enzymes, metabolism would neither progress through the same steps, nor be fast enough to serve the needs of the cell. In the absence of enzymes, this occurs so slowly or insignificantly (Reddy 2004; Mahesha et al. 2009; Mahalingam et al. 2010; Mahesha et al. 2013; Nirupama 2015).
The present investigation reveals that the haemolymph levels of Acid Phosphatase non-significantly changes during Grasserie. The present study reported similar alterations during infection (Gao et al. 2020). He reviewed that, the expression levels of (ALP) alkaline phosphatase activity were all increases first and then decreases sharply in the midgut, haemolymph and fat body of silkworm after infection with BmNPV. The gene expression was basically consistent with the change of enzyme activity, which was closely related to the physiological metabolic process of silkworm after infection with BmNPV. It suggested that the alkaline phosphatase played an important role in the antiviral process of silkworm (Gao et al. 2020).
CONCLUSION
Enzyme alterations in the studied parameters, on exposure to disease causing pathogens, in the hemolymph, suggest that these parameters could be used as indicators of the health status of the silkworm, Bombyx mori, as well as can be taken as target system to measure physiological and biomolecular stress induced alterations in the body during the occurrence of diseases. This can assist in better monitoring and effective health management of silkworms which is an economically important silk producing bio-machine. The present information may provide the underlying mechanisms in altering the metabolic modulations in pre-spinning silkworm larvae during Grasserie infection and to protect the commercial characteristics of cocoon yield in addition to suggest suitable measures in regulating the disease.
ACKNOWLEDGEMENTS
The authors are grateful for the cooperation and help by the regional farmers and District Sericulture Office for providing the healthy as well Grasserie infected larvae and also the suggestions and guidance during the preparation of this article. Cooperation and guidance in research are expected in future.
Conflict of Interests: There is no conflict among the interests of the participating authors.
REFERENCES
Annual Report, (2015), Central Silk Board, Ministry of Textiles, Government of India. http://www.csb.gov.in/assets/Uploads/pdf-files/CSBAR-1415 English.pdf
Annual Report, (2020), Central Silk Board, Ministry of Textiles, Government of India. CSB-ANNUAL-REPORT-2019-20-compressed-97-196-eng.pdf
Babu, K.R., Ramakrishna, S., Reddy, Y.H.K., Lakshmi, G., Naidu, N.V., Basha, S.S. and Bhaskar, M., (2009). Metabolic alterations and molecular mechanism in silkworm larvae during viral infection: A review. African Journal of Biotechnology, 8(6).
Doreswamy C, Govindan R, Devaiah M. C., and Muniswamappa M. V. (2004). Deterioration of cocoon traits of silkworm, Bombyx mori L. by the synergistic infection with late larval flacherie pathogens. Karnataka J. Agric. Sci. 17: 345-348.
Etebari K L, Matindoost S. Z. and Mirhoseini M. W. (2007). The effect of BmNPV infection on protein metabolism in silkworm (Bombyx mori) larva. ISJ 4: 13 -17.
Etebari K., Bizhannia A. R., Sorati R. and Matindoost L. (2007). Biochemical Changes in Hemolymph of Silkworm Larva Due to Admiral Residue. Pesticide Biochem. Physiol. 88 No 1: 14 – 19.
Gao, J., Da-Ping, Y., Yang, W. and Ding, Z., (2020). Effects of BmNPV infection on alkaline phosphatase activity and its gene expression in different tissues of Bombyx mori. Southwest China Journal of Agricultural Sciences, 33(5), pp.1101-1104.
Gillespie J. P., Kanost M. R, and Trenczek T. (1997). Biological mediators of insect immunity. Annu Rev Entomology 42: 611-643.
Jiang L, and Xia QY. (2014). The progress and future of enhancing antiviral capacity by transgenic technology in the silkworm Bombyx mori. Insect Biochem Mol Biol. 48: 1–7. Crossref
Joshi R. P. and Raja I. A. (2013) A preliminary survey of incidence of seasonal diseases in commercial crops of silkworm in Akola and Washim districts of Maharashtra, J. Biosci. Biotech. Res. Comm.6 (2) 123-28
Joshi R.P. (2015) Some Physiological and Molecular Aspects of Silkworm Bombyx mori during the Seasonal Incidence of Diseases In Vidarbha Region. PhD Thesis SGBAU, Amravati University Amravati, Maharashtra. Pp 170.
Mahalingam C. A., Murugesh K. A. and Shanmugam R. (2010). Grasserie Disease Incidence on Silkworm and Development of Botanical Based Management Strategy. Trends in Biosciences 3 No 2: 212-215.
Mahesha H. B., Rahamathulla G. and Thejaswini P. (2013). Studies on Induction of Tolerance against Nuclear Polyhedrosis in Silkworm Bombyx mori L. And Its Biochemical Aspects. IJBPAS 2 No 7: 1501 – 1512.
Mahesha H.B. and Thejaswini P.H. (2009). Studies on induction of resistance against nuclear polyhedrosis in silkworm Bombyx mori L and its biochemical aspects. In: National Conference on Recent Trends in Animal Physiology, University of Mysore, Mysore 29- 30 October
Reddy B.M. (2004). Metabolic modulations in various tissues of pre spinning silkworm, Bombyx mori (L) Larvae during Grassarie (BmNPV) disease, Sri Venkateswara University, Tirupathi, India.
Matindoost L. (2006). Establishment and characterization of a new cell line from embryonic tissue of Bombyx mori and its susceptibility to baculovirus (BmNPV). M.Sc. Thesis on Entomology, the Univ. Guilan, Iran.
Miao Y. G. (2002). Studies on the activity of the alkaline phosphatase in the midgut of infected silkworm, Bombyx mori L. J Appl Entomol., 126: 138 – 42
Nataraju, B., (2007). The effect of infection with Bombyx mori densovirus BmDNV1 on the biochemical parameters of the resistant and sensitive varieties of Bombyx mori. Acta Entomologica Sinica, 50 (1), pp.74-78.
Nirupama, R. (2015) Biochemical studies on total protein, carbohydrate and lipids content level during the infection by fungi white muscardine disease of silkworm, Bombyx mori L. Munis Entomology & Zoology, 10 (2): 446-454
Rajitha K and Savithri G. (2014) Day to Day Analysis of Amylase and Trehalase Activity in the Haemolymph of Silkworm Bombyx mori L. Infected with Fungal Pathogen Beauveria bassiana (BALS.) Vuill., International Journal of Life Sciences Biotechnology and Pharma Research, Vol. 3, No. 1, pp. 225-230,
Senthil Nathan S., Kalaivani, K. and Murugan, K. (2006). Effect of biopesticides on the lactate dehydrogenase (LDH) of the rice leaf folder, Cnaphalo crocis (Insecta: Lepidoptera: Pyralidae). Ecotoxicol. Environ. Safety 65 102 – 107.
Statista Research Department Report, (2021) https://www.statista.com/statistics/622914/silk-production-india/


