G. R. Damodaran College of Science, School of Biotechnology, 641 014, Coimbatore, India
Corresponding author email: sugantham2000@gmail.com
Article Publishing History
Received: 20/07/2020
Accepted After Revision: 28/09/2020
Statins are drugs that lower the level of low-density lipoprotein (LDL) cholesterol levels as statins involve as potent inhibitors of cholesterol synthesis in blood. The use of statins in the medical field has enormous applications. Among few recent applications, Statins used in hospitals are proved to lower the risk of mortality among individuals effected with corona virus, its effective in protection against the neurodegenerative disorder, local application of statin have proved to repair bone.The present study was delineated to isolate the fungal strains and to screen statin production analyzed by growth inhibition of Candida albicans and Aspergillus fumigatus, further applied to evaluate the antioxidant and anticholesterol activity of fungal statin isolated from Aspergillus tamarii. This report depicts the antifungal activity of isolated fungal statin was done by well diffusion method which showed susceptibility of Candida sp. against the fungal statin, antioxidant potential of fungal statin was observed by DPPH (2,2 – diphenyl- 1- picrylhydrazyl) radical scavenging assay that proved to have increasing inhibition percentage with increasing concentration from 25µg/mL to 100µg/mL. Further, the anti-cholesterol analysis was done using male albino rats having high cholesterol diet proved that the high fat diet had adverse effect on the liver, while total bilirubin showed only marginal increase when compared to normal rats. Therefore, this study depicts an unprecedented work of fungal statin from Aspergillus tamarii, showing good potential to be used as antifungal, antioxidant and cholesterol lowering agent. Therefore, these natural statins could be used as a replacement for chemical drugs with no adverse side effects.
Aspergillus Tamarii, Antifungal, Antioxidant, Anti-Cholesterol
Anilkumar N. K, Krishna A. R, Chockalingam J, Balakrishnan L, Janarthanan N. T, Ramachandran P, Palanisami S. D, Dhanaraj S. S, Joseph S, Jayalekshmi S. K, Antony T. M. P, Ramasamy S. Elucidation of Antifungal, Antioxidant and Anticholesterol Activity of Efficiency of Fungal Statin Isolated from Aspergillus tamarii. Biosc.Biotech.Res.Comm. 2020;13(3).
Anilkumar N. K, Krishna A. R, Chockalingam J, Balakrishnan L, Janarthanan N. T, Ramachandran P, Palanisami S. D, Dhanaraj S. S, Joseph S, Jayalekshmi S. K, Antony T. M. P, Ramasamy S. Elucidation of Antifungal, Antioxidant and Anticholesterol Activity of Efficiency of Fungal Statin Isolated from Aspergillus tamarii. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2GmvSOo
Copyright © Anilkumar et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Secondary metabolite production, a hallmark of filamentous fungi, is an inflating area of research for the Aspergilli. A variety of biologically active compounds are produced by fungi, mainly by the polyketide biosynthetic pathway. Fungal polyketides comprise a very large and structurally diverse group and many display important biological properties such as antibiotic activity and other related pharmacological properties (Bedford et al., 1995).Among the fungal metabolites, statins (anticholesterol compounds) are considered as the most important class of secondary metabolites produced by the polyketide pathway. Statins shows anti-fungal effects (Tavakkoli et al., 2020)
Statins are a class of molecules with a polyketide structure, obtainable by secondary fungal metabolism, which can inhibit HMG- CoA (hydroxyl methyl glutaryl – coenzyme A) reductase activity. Thus the mechanism involved in the control of endogenous cholesterol levels by Statins ; makes the molecules suitable for therapeutic use (Alberts et al., 1980; Endo, 1985a; Alberts, 1988; Farnier and Davignon, 1998; Stein et al., 1998; Furberg, 1999; Maron et al., 2000; Chong et al., 2001; Tobert, 2003). Initially statins were discovered in fungi and for many years fungi were the sole source for the statins (Subhan et al., 2016).
Lovastatin is a naturally occurring statin obtained from different genera and species of filamentous fungi. Fungal strains including Aspergillus, Penicillium, Monascus, Paecilomyces, Trichoderma, Scopolariopsis, Doratomyces, Phoma, Phythium, Gymnoascus, Hypomyces and Pleurotus have been reported as lovastatin producers. Of many statin molecules, lovastatin and mevastatin are produced by the fungal species, while other statins like rosuvastatin, simvastatin, pravastatin, fluvastatin, atrovastin, cerivastatin are produced semi- synthetically from lovastatin (Tobert, 2003, Chakravarti and Sahai, 2004).Pleurotus sp. and its related strains produce higher lovastatin (Srinu et al., 2010). Recent research has focussed on the metabolic regulation of MK/lovastatin synthesis and its evidence shows that the combination of extracellular and intracellular factors is an important significance essential for MK/lovastatin metabolism (Zhang et al., 2020).
Research with fungal metabolites identified a series of compounds with potent inhibiting properties for this target enzyme HMG- CoA reductase, from which lovastatin was selected for clinical development. Cholesterol synthesis reduction by lovastatin was confirmed in cell culture, animal studies and in humans. The subsequent reduction in circulating total and low-density lipoprotein (LDL) cholesterol has also been demonstrated in animals and humans. Major mechanism of LDL clearance from the circulation were caused by hepatic LDL receptors, which proves to be the major mechanism, further research in animals has confirmed that these declines in cholesterol are accompanied by an increase in hepatic LDL receptor activity. Statin effectively diminishes endogeneous cholesterol synthesis providing useful therapeutic properties for patients with hypercholesterolemia (Morris et al., 1993; Jenkins et al., 2005).Another interesting property of statins is that they have an effective antifungal potential against both yeast and filamentous fungi; furthermore they can be combined with clinically used antifungal agents (Galgoczy et al., 2011).
Several in vitro and in vivo studies have been carried out targeting the antioxidant potential of various types of natural and synthetic statins such as atorvastatin, (Wassman et al., 2002), pravastatin (Alanazi, 2010), fluvastatin and simvastatin (Franzoni et al., 2003).Fungi have been identified as the new sources of antioxidants due to wide production of secondary metabolites (Arora and Chandra, 2010). Recent studies reported that the preparation and characterization of nano statins using oyster mushroom (Pleurotussajorcaju), reduces toxicity and enhance efficacy for treatment of cardiovascular disease (Mehra et al., 2020).
MATERIAL AND METHODS
Isolation of fungal strains: The fungal isolates to be used in the study were collected from different environment sources which included sources like coffee powder, corn, coconut and cotton seeds . Coconut and corn previously infected with fungus were taken. All the samples which were incubated were further inoculated onto potato dextrose agar and incubated at room temperature until growth was observed. Cultures were subcultured to obtain pure cultures.
Screening of statin production analyzed by growth inhibition of Candida albicans and Aspergillus fumigatus: Candida albicans and Aspergillus fumigatus strains were grown on potato dextrose broth containing various concentrations of 50mg/mL,100mg/mL and 250mg/mL of cholesterol and incubated at 35˚C for 48hours for 5 days. After 5 days of growth, the cultures were then inoculated with YEPD agar to which 100µl of aqueous extract of fungal statin from Aspergillus tamarii was added.
Application of the isolated fungal statin: Antifungal activity of isolated fungal statin: The anti-fungal activity of the isolated statin was compared with commercially available statins which include simvastatin, rosuvastatin, atorvastatin and lovastatin. The cultures include Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Fusarium, Rhizopus, Excerohilumsp.,Candidaalbicans, Candida albicans clinical strains, Candida krusei,Candidaglabrata, Candida tropicalis. The fungal cultures were maintained on potato dextrose agar at 28˚C.
Anti-oxidant analysis of isolated fungal statin: The purified fungal statin and commercial statin were further evaluated for their antioxidant potential. Commercial statin and the isolated compound was dissolved in ethyl acetate and stored at 4˚C. 25µg/ml, 50µg/ml, 75µg/ml, 100µg/ml of the purified statin were subjected to standard antioxidant assays such as DPPH radical scavenging assay. The standard antioxidant assays were checked for percentage inhibition or scavenging activity by the formula, DPPH inhibition percentage had been calculated using the formula.DPPH inhibition (%) = [ Control absorbance – Test absorbance ] x 100
Anticholesterol analysis: Male albino rats study was performed using high cholesterol diet, where high cholesterol products were supplemented with the commercial feed (Table:1). 2mg of commercial statin tablet and 6mg of lyophilized purified fungal extract was dissolved in 1ml sterile water, was administered on all days mixing 1ml with feed. Feeding was continued for about 45days. After one week of adaptation, the animals were divided into 5 groups with 3 animals in each group. The control animals were fed with normal diet (Table:2). At the interval of 15days the body weight was observed. The weight at after 7days of the experiment was taken as the initial weight. After 45days, the rats were deprived with food overnight and the blood was collected by cardiac puncture. The serum was collected from blood, screened for cholesterol levels and liver marker enzymes.
Table 1. Components of the high cholesterol diet administered to albino rats
| Component | Measured amount (g) |
| Wheat flour | 15 |
| Roasted Bengal Flour | 58 |
| Groundnut Flour | 10 |
| Milk Powder | 5 |
| Powdered cashew nut | 4 |
| Salt | 4 |
| Casein | 4 |
Table 2. Grouping of animals for experimental study in vivo
| Groups | Experimental Design |
| Group I / Normal | Served as normal control rats where the diet administered was the normal diet without the cholesterol products. |
| Group II | Rats fed with 10g of high cholesterol diet per kg Body Weight (BW) of the rat for 45 days. |
| Group III | Rats fed with 10g of high cholesterol diet containing per kg of BW of the rat for 45 days along with 2mg of commercial statin per kg BW |
| Group IV | Rats fed with 6mg purified fungal statin from solid state fermentation in addition to 10g of high cholesterol diet per kg BW of the rat for 45 days |
| GroupV | Rats fed with 6 mg purified fungal statin from submerged fermentation in addition to 10 g of high cholesterol diet per kg BW of the rat for 45 days |
Statistical analysis: The data was analysed using statistical package for social sciences (SPSS) 17.0. Mann Whitney U-test was performed to find the significance of the test results obtained as the variables were independent and the sample size was less than 10 (n=3).The significance value was considered at two measure of 95% (p < 0.05) and 99% (p<0.01) were determined to be statistically significant.
RESULTS AND DISCUSSION
Aspergillus section and Flavicontains a number of different fungal species which are well known for their production of secondary metabolites . Most of these secondary metabolites are promising candidates for human use as the natural replacements for artificial chemical compounds. The objective of the study was to isolate and purify fungal statin from Aspergillus tamarii and determine its potency as an anticholesterol agent, antioxidant, antifungal agent and anticancer agent. In this study, we have isolated 15 fungi from various environmental sources, out of which 14 were found to be Aspergillus flavus and one was found to be Aspergillus tamarii. The aflatoxin analysis revealed that the isolated strain of Aspergillus tamarii was non- aflatoxigenic. Osman et al.,(2011) screened twenty three fungal isolates and tested for their ability to produced lovastatin.
Screening of effects of statins on the growth of Aspergillus fumigatus were investigated on solidified minimal media. Aspergillus fumigatus exhibited robust growth with the production of conidia after 4days at 31˚C. In the presence of statins, there was growth inhibition in regular Aspergillus fumigatus strains. Strains were initially grown on various concentrations of cholesterol with PDA and the spores were plated with statin incorporated solidified PDA. The cultures exposed to higher concentrations of cholesterol were shown higher growth in the presence of statin. (Table.3).
Table 3. Bioassay using Candida albicans (MTCC183) showing diameter of inhibition zone using fungal extract on comparison with commercial statin
| S.No. | Concentration of commercial statin (µg/ml) | Diameter of inhibition zone using commercial statin (cm) | Concentration of fungal statin (µg/ml) | Diameter of inhibition zone using fungal statin (cm) |
| 1 | 50 | 1.8 | 50 | 1.2 |
| 2 | 75 | 2.1 | 75 | 1.6 |
| 3 | 100 | 2.6 | 100 | 1.9 |
| 4 | 125 | 3.2 | 125 | 2.8 |
| C | Control (ethyl acetate) | – | Control (ethyl acetate) | – |
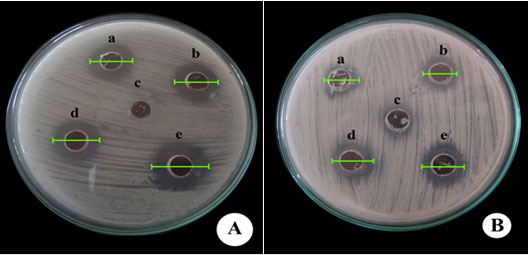
Plate 1: Diameter of zone of inhibition using Candida albicans(MTCC183) against(A) commercial statin and (B) fungal extract
a-50 µg/ml; b-75 µg/ml; c- control (ethyl acetate); d- 100 µg/ml; e-125 µg
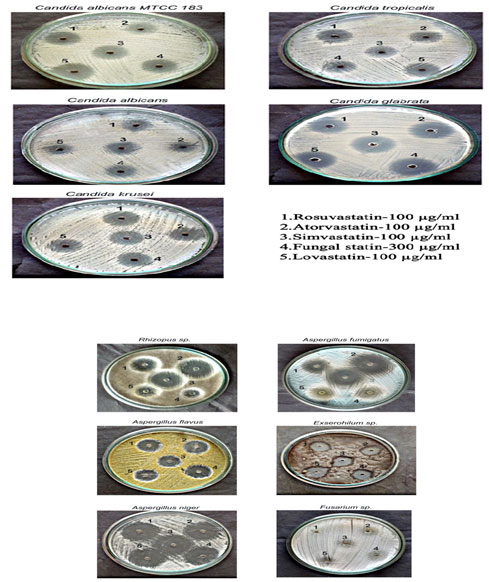
Antifungal activity of isolated fungal statin: The antifungal activity of the isolated fungal statin was analysed against different fungi along with commercially available statin. Antifungal susceptibility of Candida sp. was confirmed against the isolated fungal statin (Table : 4). Galgoczy et al., (2011) studied the in vitro antifungal activity of statins against yeast and filamentous fungal isolates including Candida albicans, Candida glabrata, Candida krusei,Candidaparapsilosis, Candida tropicalis, Rhizopus sp., Aspergillus flavus and Aspergillus fumigatus. The inhibitory potentials of statins were studied in the range 0.25 – 128 µg/ml by broth microdilution. In study simvastatin ( 8µg/ml) displayed the strongest antifungal activity followed by fluvastatin (25- 128 µg/ml), atorvastatin (128 µg/ml), rosuvastatin (128 µg/ml) and lovastatin (5-64 µg/ml) against yeast while antifungal activity of statins against filamentous fungi showed fluvastatin (2µg/ml) to be most potent followed by rosuvastatin (8µ/ml), simvastatin (6.25 µg/ml), lovastatin (25 µg/ml) and atorvastatin (>128 µg/ml). In our study rosuvastatin, atorvastatin , simvastatin, lovastatin, showed potency at (>100 µg/ml), while fungal statin showed inhibition at (300µg/ml) against Candida sp. and filamentous fungi including Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Rhizophus sp., Excerohilum sp. and Fusarium sp. did not show any inhibition against fungal statin and commercial statin.
Table 4. Antifungal activity of different types of statin against various fungal species
| S.No | Fungal cultures | Fungal statin (300µg/ml) | Lovastatin (100µg/ml) | Simvastatin (100µg/ml) | Atorvastatin (100µg/ml) | Rouvastatin (100µg/ml) |
| 1 | Candida albicansMTCC 183 | + | + | + | + | + |
| 2 | Candida albicans | + | + | + | + | + |
| 3 | Candida krusei | + | + | + | + | + |
| 4 | Candida glabrata | + | + | + | + | + |
| 5 | Candida tropicalis | + | + | + | + | + |
| 6 | Aspergillus niger | + | + | + | + | + |
| 7 | Aspergillus flavus | + | + | + | + | + |
| 8 | Aspergillus fumigatus | + | + | + | + | + |
| 9 | Rhizopus | + | + | + | + | + |
| 10 | Exserohilum | + | + | + | + | + |
| 11 | Fusarium | _ | _ | _ | _ | – |
‘+’-Inhibitory effect ‘–‘ – No inhibition
Plate 2: Antifungal activity of lovastatin and fungal statin
1 – Rosuvastatin (100 µg/ml); 2 – Atorvastatin (100 µg/ml); 3 – Simvastatin (100 µg/ml); 4 – Fungal statin (300 µg/ml); 5 – Lovastatin (100 µg/ml)
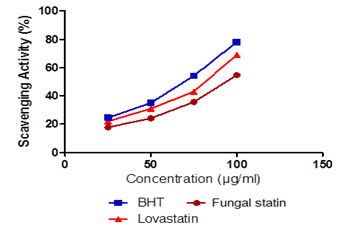
Antioxidant activity of isolated statin: The DPPH activity of the statin compound when compared to the commercial antioxidant BHT .The concentration dependent comparison confirmed extracted statin to possess antioxidant activity (Figure 3). Butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) as commercial antioxidants now play a role in screening of antioxidant potential in metabolites and biopharmaceuticals extracted or isolated from plants or microbes (Ozusaglam and Karakoca, 2013). In our study, DPPH radical scavenging activity of fungal statin showed similar activity when compared to standard statin tablet although there was a significant increase in the antioxidant activity by BHT (the commercial antioxidant). The variation of the fungal statin with respect to enzymatic antioxidants is largely dose dependent. An increase in the antioxidant potential of fungal statin was observed with increase in the concentration from 25 µg/ml to 100 µg/ml. The lower yet statistically significant antioxidant potential confirmed by standard DPPH and enzymatic assays reveal the efficacy of fungal statins as an antioxidant agent.
Figure 1 : Antioxidant activity of isoalted statin
Anticholesterol analysis: At the end of 45days of treatment, thebody weight of all groups treated and normal were compared and represented as mean ± standard error mean (SEM). A gradual increase in the body weight of the normal population was seen. On comparison, there was a spiked increase in the body weight in group II, group III, group IV and group V where the diet was treated with additional fat products such as casein and cashew nuts as shown. (Table 4). The body weight of the animals treated with high fat diet (p<0.05) showed a significant increase in the body weight was seen after 30days and 45days of feeding when compared to normal animals. The levels of serum lipid profile, total cholesterol (TC), triglycerides (TG) , LDL-C, VLDL-C and HDL-C in normal and drug treated rats are presented in (Table 5). Statin induced rats showed significant decrease in serum HDL-C profiles when compared with normal rats. There was also decrease in the levels of cholesterol and triglycerides in both the drug treated and fungal statin treated rats when compared to high fat diet group.
LDL levels were found to be significantly higher in the groups treated with commercial statin and fungal statin when compared to normal controls. The specific liver markers such as bilirubin, SGOT, SGPT, and alkaline phosphatase in serum in all five groups studied. (Table 6) Significant increase in the levels of SGOT, SGPT and alkaline phosphatase in group II when compared with group I. On comparison with group II, there was a significant decrease in SGOT, SGPT, and alkaline phosphatase values in group III, group IV and group V. This showed that high fat diet had negative effect on the liver, while the total bilirubin showed only a marginal increase when compared to normal rats. Tanideh and Badiei, (2013) studied the effect of simvastatin and garlic on lipid profile and liver marker enzymes in rats fed with normal and fat rich diet. Simvastatin significantly reduced the total cholesterol, triglycerides and LDL broth on normal diet and fat rich diet. HDL increases significantly in simvastatin treated rat.
No significant changes were detected in ALT and AST levels in different groups. Rajasekaran and Kalaivani, (2011) studied the hypolipidemic and antioxidant activity of aqueous extract of Monascus purpureus fermented Indian rice in high cholestrol diet fed rats. On in vivo evaluation of anticholestrolemic activity, the plasma total cholestrol, triglycerides, LDL and VLDL significant declined when compared to the high cholesterol fed rats. Recently Yuliana et.al (2020) have reported the fermentation and determination of Anticholesterol Monakolin K from different isolates of Monascus purpureus.
Table 4. Comparison of body weight through the period of 45 days at the intervals of 15 days
| Treatment (mg/kg of BW) | Changes in Body Weight (mg) | |||||||
| 0day (Initial) | 15 days | 30 days | 45 days | |||||
| Normal | 155±1.73 | 159.33 ± 0.57 | 163.33 ± 0.57 | 165.66± 0.57 | ||||
| Group II (High Fat Diet) | 156 ± 3.46b | 179 ± 4.58a | 186.33 ± 2.51a | 190.33 ± 1.52a | ||||
| Group III (High Fat Diet + Commercial Statin) | 156.66 ± 2.08b | 184 ± 4.58a | 174.66 ± 1.52a | 171.33 ± 1.52a | ||||
| Group IV (High Fat diet + Purified fungal statin from solid state) | 158.33 ± 2.08b | 185.33 ± 2.51a | 170.33 ± 4.5a | 165.33 ± 2.08b | ||||
| Group V (High Fat diet + Purified fungal statin from submerged) | 156.66 ± 1.5a | 183 ± 1a | 167 ± 2.64b | 163.66 ± 3.2b | ||||
All values have been mentioned in mean ± SD; a – n=3 observations at p<0.01 on comparison with normal group, b – n=3 observations at p<0.05 on comparison with normal group
Table 5. Effect of purified statin and commercial statin on serum lipid profile of normal and induced adult male albino rats
| Group | TC | TG | HDL-C | LDL-C | VLDL-C | CHO/HDL | LDL/HDL |
| I | 62.3±1.1 | 56.8±3.2 | 26.6±1.52 | 24.9±1.4 | 11.2±0.64 | 2.3±0.644 | 0.9±0.1 |
| II | 85.8±4.7a | 116.6±1.2a | 32.6± 1.5b | 29.5±1.44b | 23.5±2.6a | 2.62±0.03a | 0.9±0.5b |
| III | 68.7±1.5d | 46±8.7e | 22.6±2.51e | 36.8±3.40e | 9.2±1.74d | 3.05±0.38d | 1.6±0.3e |
| IV | 63.5±3.04ec | 34±2.64ec | 12.3±2.5dc | 39.7±1.16dc | 6.8±5.2dc | 5.26±0.87dc | 3.7±0.7dc |
| V | 65.01±2d | 41.66±4.5bd | 15.66±3.8ad | 41.01±3.8bd | 8.33±0.9ad | 4.31 ±1 | 2.74±0.86 |
Each value is given in Mean±SEM; a – n=3 observations at p<0.01 on comparison with group l, b – n=3 observations at p<0.05 on comparison with group I, c – observations with p<0.05 on comparison with group III, d – n=3 observations at p<0.01 on comparison with group II, e –, n=3 observations at p<0.05 on comparison with group II
Table 6. Effect of purified statin obtained on submerged and solid state fermentation and commercial statin on liver marker enzymes in vivo
| Groups | Total Bilirubin | SGOT | SGPT | Alkaline Phosphatase |
| I | 0.3±0.1 | 75.4±5.1 | 24.8±2.5 | 120.25±1.93 |
| II | 1.1±0.1 | 86.6±7.63a | 61.3±3.5b | 175±5a |
| III | 0.4±0.1 | 72.4±2.25c | 23.5±2.21c | 120.3±3.06c |
| IV | 0.4±0.1 | 74.9±1.5c | 30.4±2.4c | 121.8±1.2c |
| V | 0.6±0.1 | 75.2±2.1c | 28.8±1.48c | 122.7±2.5c |
Each value is given in Mean±SEM; a – n=3 observations at p<0.01 on comparison with group l, b – n=3 observations at p<0.05 on comparison with group I, c– n=3 observations at p<0.01 on comparison with group II.
CONCLUSION
This is the first report on the isolation of fungal statin from Aspergillus tamarii. Statin is produced as a part of the polyketide pathway during fungal metabolism. Statins have a variety of biological effects which include anticholestrolemic, antimicrobial and antioxidant properties. This study was designed to isolate the fungal statin from Aspergillus tamarii, that has showed good potential to be used as an antifungal agent, anticancer agent, antioxidant agent and a cholesterol lowering agent. These natural statins could be used as a replacement for the chemical drugs that come along with various side effects.
ACKOWLEGMENTS
We acknowledge the management of GRD institution for providing the research funds and high class infrastructure to complete the research.
Conflict of interest: The authors declare no conflicts of interest.
REFERENCES
Alanazi, H., Zaidan, B.B., Zaidan, A.A., Jalab, H.A., Shabbir, M. and Al-Nabhani, Y., (2010). New comparative study between DES, 3DES and AES within nine factors. arXiv preprint arXiv:1003.4085.
Alberts, A.W., (1988). Discovery, biochemistry and biology of lovastatin. The American journal of cardiology, 62(15), pp.J10-J15.
Alberts, A.W., Chen, J., Kuron, G., Hunt, V., Huff, J., Hoffman, C., Rothrock, J., Lopez, M., Joshua, H., Harris, E. and Patchett, A., (1980). Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proceedings of the National Academy of Sciences, 77(7), pp.3957-3961.
Arora, D.S. and Chandra, P., (2010). Assay of antioxidant potential of two Aspergillus isolates by different methods under various physio-chemical conditions. Brazilian Journal of Microbiology, 41(3), pp.765-777.
Bedford, M.R., (1995). Mechanism of action and potential environmental benefits from the use of feed enzymes. Animal feed science and technology, 53(2), pp.145-155.
Chakravarti, R. and Sahai, V., (2004). Compactin—a review. Applied microbiology and biotechnology, 64(5), pp.618-624.
Chong, R.L., Sunrise Telecom Inc, (2001). Detection of bridge tap using frequency domain analysis. U.S. Patent 6,177,801.
Endo, T., Kobayashi, S. and Onaya, T., (1985). Parvalbumin in rat cerebrum, cerebellum and retina during postnatal development. Neuroscience letters, 60(3), pp.279-282.
Farnier, M. and Davignon, J., (1998). Current and future treatment of hyperlipidemia: the role of statins. The American journal of cardiology, 82(4), pp.3J-10J.
Franzoni, F., Quiñones-Galvan, A., Regoli, F., Ferrannini, E. and Galetta, F., (2003). A comparative study of the in vitro antioxidant activity of statins. International journal of cardiology, 90(2-3), pp.317-321.
Furberg, C.D., Herrington, D.M. and Psaty, B.M., (1999). Are drugs within a class interchangeable?. The Lancet, 354(9185), pp.1202-1204.
Galgóczy, L.N., Nyilasi, I., Papp, T. and Vágvölgyi, C., (2011). Statins as antifungal agents. World Journal of Clinical Infectious Diseases, 1(1), pp.4-10.
Galgóczy, L.N., Nyilasi, I., Papp, T. and Vágvölgyi, C., (2011). Statins as antifungal agents. World Journal of Clinical Infectious Diseases, 1(1), pp.4-10.
Jenkins, S.P., (2005). Survival analysis. Unpublished manuscript, Institute for Social and Economic Research, University of Essex, Colchester, UK, 42, pp.54-56.
Kalaivani, T., Rajasekaran, C., & Mathew, L. (2011). Free radical scavenging, cytotoxic, and hemolytic activities of an active antioxidant compound ethyl gallate from leaves of Acacia nilotica (L.) wild. Ex. Delile subsp. Indica (Benth.) Brenan. Journal of food science, 76(6), T144-T149.
Karakoca, K., Ozusaglam, M.A., Cakmak, Y.S. and Erkul, S.K., (2013). Antioxidative, antimicrobial and cytotoxic properties of Isatis floribunda Boiss. exBornm. extracts. EXCLI journal, 12, p.150.
Maron, D.J., Fazio, S. and Linton, M.F., (2000). Current perspectives on statins. Circulation, 101(2), pp.207-213.
Mehra, A., Narang, R., Jain, V.K. and Nagpal, S., (2020). Preparation and characterization of nano statins using oyster mushroom (Pleurotuss ajorcaju): a new strategy to reduce toxicity and enhance efficacy for the treatment of cardiovascular disease. European Journal of Integrative Medicine, 33, p.101014.
Morris, J.C., Eastman Kodak Co, (1993). Polyester/polycarbonate blends. U.S. Patent 5,239,020.
Osman, S.O., Moustafa, F.M.A., Abd El-Galil, H.A. and Ahmed, A.Y.M., (2011). Effect of yeast and Effective Microorganisms (Em1) application on the yield and fruit characteristics of Bartamuda date palm under Aswan conditions. Assiut J. of Agric. Sci, 42(5), pp.332-349.
Srinu, S.L.S.S. and Sabat, S.L., (2010), October. FPGA implementation of spectrum sensing based on energy detection for cognitive radio. In 2010 International Conference on Communication Control And Computing Technologies (pp. 126-131). IEEE.
Stein, L.H., Stefferud, E.A., Borenstein, N.S. and Rose, M.T., First Virtual Holdings Inc, (1998). Computerized system for making payments and authenticating transactions over the internet. U.S. Patent 5,826,241.
Subhan, M., Faryal, R. and Macreadie, I., (2016). Exploitation of Aspergillus terreus for the Production of Natural Statins. Journal of Fungi, 2(2), p.13.
Tanideh, N. and Badiei, R., (2013). Evaluation of the effects of simvastatin alone and in combination with garlic on lipid profile and liver enzymes in rats fed normal and fat rich diet. MiddLe-East Journal of Scientific Research, 15(9), pp.1237-41.
Tavakkoli, A., Johnston, T.P. and Sahebkar, A., (2020). Antifungal effects of statins. Pharmacology & Therapeutics, 208, p.107483.
Tobert, J.A., (2003). Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nature reviews Drug discovery, 2(7), pp.517-526.
Varga, S.M. and Spaulding, C.J., Procter and Gamble Co, (2011). Systems And Methods For Monitoring On-Line Webs Using Line Scan Cameras. U.S. Patent Application 12/639,266.
Wassman, F., (2002). Green Solutions. Jakob AG, Trubschachen, Switzerland.
Yuliana, A., Zannah, S.U., Rahmiyani, I. and Amin, S., (2020), June. Fermentation and Determination of Anti-Cholesterol Monakolin K from Different Monascus purpureus Isolates. In 2nd Bakti Tunas Husada-Health Science International Conference (BTH-HSIC 2019) (pp. 225-227). Atlantis Press.
Zhang, Y., Chen, Z., Wen, Q., Xiong, Z., Cao, X., Zheng, Z., Zhang, Y. and Huang, Z., (2020). An overview on the biosynthesis and metabolic regulation of monacolin K/lovastatin. Food & Function.