Molecular and Cellular Engineering, Jacob Institute of Biotechnology and Bioengineering, SHUATS University, Naini, Prayagraj, India-211007
Corresponding author email: sam.masih@shiats.edu.in
Article Publishing History
Received: 05/01/2020
Accepted After Revision: 25/03/2020
PGPR are potential tools to alleviate plant growth and augment tolerance to abiotic stress tolerance, with reduced level of agro-chemical application, as excessive use of fertilizers poses threat to soil fertility, soil ecology and fertilizer run-off leads to water contamination, water eutrophication. PGPR also elicit ‘induced systemic tolerance’ to salt and drought. The present study indicated that soil inoculation with rhizobacterial strains of Bacillus subtilis and Bacillus cereus (NCBI accession numbers: LC480918 and LC481470 respectively) promotes growth of Capsicum annuum under both non-saline and saline conditions by directly or indirectly regulating plant chlorophyll content, leaf osmotic potential. The potential of the two rhizobacterial strain to produce exopolysaccharide, indole acetic acid, gibberelic acid production confirmed its ability as plant growth promoting isolates. Present study recorded maximum root length of 22.9 cm and total chlorophyll content of 182 µg/g in B.subtilis inoculated plants compared to root length of 22.6 cm and total chlorophyll content of 160 µg/g in control plants, under non saline condition. B. subtilis inoculated plants under salt stress showed root length of 17.2 cm total chlorophyll content of 69 µg/g compared to root length of 16.0 cm total chlorophyll content of 64 µg/g in control plants at 200mM salt concentration. Different paradigms of applicability of the PGPR have been displayed comprehensively under both normal and stress conditions to highlight the recent trends with the aim to develop future insight into the role of PGPRs inoculum as biofertilizers for sustainable agriculture productivity and reclaiming soil fertility unlike chemical fertilizers.
PGPR, Bacillus subtilis, Bacillus cereus, Salt stress tolerance, Capsicum annuum
Maxton A, Jaiswal R, Kaushik I, Mishra R, Masih S. A. Efficiency of Bacillus subtilis and Bacillus cereus to Abate Salinity Stress and Augment Plant Growth. Biosc.Biotech.Res.Comm. 2020;13(1).
Maxton A, Jaiswal R, Kaushik I, Mishra R, Masih S. A. Efficiency of Bacillus subtilis and Bacillus cereus to Abate Salinity Stress and Augment Plant Growth. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/39vIsEG
Copyright © Maxton et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Soil fertilization is required for agricultural production but can also cause nitrate and phosphate accumulation that eventually contaminates surface and ground waters. Fertilizer run-off leads to water contamination and phosphate run-off augments eutrophication of surface waters, resulting in fish mortality (Srivastava et al., 2017). These environmental impacts of fertilization can be attributed, in part, to low uptake nutrient efficiency of crops. Phosphorous is highly reactive with iron, aluminum and calcium in soils, which can result in precipitation of up to 90% of the soil phosphorous, making it largely unavailable to plants (Meller et al., 2019).
Owing to these environmental concerns and the increasing prices of fertilizers, there is an urge from farmers worldwide to reduce use of fertilizers below the recommended amount for optimum yields; however, such reductions would exert abiotic stress on plants. Over the last few decades, the agriculture policy in India has undergone a major change influenced by the root system, referred to as the rhizosphere is a versatile environment of intensive plant microbe interaction for extracting essential micronutrients from a confident nutrient pool (Singh et al., 2012). The plant roots exude a huge diversity of organic nutrients and signals that attracts the microbial populations, especially those able to metabolize plant excluded compounds and proliferate in this habitat (Lareen et al., 2016). PGPR allevate plant growth by various mechanisms that involve soil structure formation, decomposition of organic matter, recycling of essential elements, solubilization of mineral nutrients, producing numerous plant growth regulators, modulating phytohormone level, degrading organic pollutants, stimulation of root growth, vital for soil fertility, biocontrol of soil and seed borne plant pathogens based on their ability to produce antimicrobial or hydrolytic enzymes (Kamilova et al., 2009). Bacillus species with potent plant growth promoting traits such as essential phytohormone production and biocontrol attributes are considered as safe microorganism that holds remarkable abilities for synthesizing vast array of beneficial substances (Hashem et al., 2019).
Recent work by several groups shows that PGPR also elicit so-called ‘induced systemic tolerance’ to salt and drought. Salinity in arid regions is frequently a crucial limiting factor for the productivity of agricultural crops, with adverse effects on germination, plant vigour and crop yield (Singh and Jha, 2016). Soil salinity promotes osmotic stress, water deficit, stomatal closure and reduced leaf expansion (James et al. 2011); moreover, soil salinity causes deficiency of essential nutrients such as K+. Elevated Na+ inside plants can decrease plant photosynthetic rates and biomass accumulation (Zhang and Shi, 2013). Therefore, use of PGPR is a new ways to cope with the threat of global soil salinization to agriculture. A wide range of PGPR produces 1-aminocyclopropane-1-carboxylate (ACC) deaminase, conferred induced systemic tolerance (IST) to salt and drought stress in pepper (Capsicum annuum L.) and tomato (Solanum lycopersicum L.) plants (Mayak et al., 2004). Many current studies are underway that will further define the utility of PGPR in nutrient management strategies aimed at reducing fertilizer application rates and nutrient runoff from agricultural sources. To maintain the growth and development of Capsicum annuum (chilli) in saline condition, the current work was to evaluate the efficiency of Bacillus subtilis and Bacillus cereus for growth promotion and salt tolerance in Capsicum annuum.
MATERIALS AND METHODS
Present study was conducted at Department of Molecular and Cellular Engineering, SHUATS University, Prayagraj whereas Capsicum annuum seeds (Kashi Surkh) variety was obtained from Indian Institute of Vegetable Research, Varanasi, Uttar Pradesh, India.
Isolation of Rhizobacterial Strains: After selecting Dhatura plant a 6”X 2.5”X 5” rhizospheric soil sample was serially diluted and appropriate dilutions (10-3, 10-5, 10-7) were spread plated on nutrient agar plates. The plates were incubated at 37°C and developed colonies were identified on the basis of cultural, morphological characteristics as described in the Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994). Selective colonies were taken and streaked on selective media for individual strains and kept in incubator at 37°C for 24 h to obtain colonies.
Screening of Rhizobacteria for plant growth promoting activities:Production of Indole Acetic Acid (IAA):Nutrient broth amended with 5-mmol tryptophan were inoculated with overnight raised selected bacterial cultures (0.5 OD at 600 nm) and incubated at 37°C for 48 h. One ml of culture was centrifuged at 3,000 rpm for 20 min and supernatant separated. To the supernatant, 4 ml of Salkowasky reagent were added followed by incubation for 1 h at room temperature under dark conditions. Absorbance of the pink colour developed was read at 530 nm (Pandey et al., 2013). Concentration of the proteins in the pellet was determined by Bradford method and the amount of IAA produced was expressed in µg/ml.
Production of Gibberellic Acid (GBA) :Extraction of Gibberellic Acid:For gibberellic acid production bacterial isolate was grown in 100 ml nutrient broth medium at 30°C for 72 h. Following the incubation period, cultures were centrifuged at 3,000 rpm for 10 min and the pH of supernatant was adjusted to 2.5 using 1 N HCl. It was extracted with equal volume of ethyl acetate in a separating funnel, shaked vigorously and excess of ethyl acetate fraction was discarded from funnel and allowed the ethyl acetate to by exposing in air. The extract was transferred to a separating funnel and retreated with equal volume of ethyl acetate and then solvent fraction was separated and allowed to by exposing in air. The process was repeated 2 to 3 times to get a large amount of fine quality of gibberellic acid. The concentrated solution was re-evaporated and the residues were dissolved in water containing 0.5% Tween 20 (Holbrook et al., 1961).
Spectrophotometric method for gibberellic acid:In this method, one ml of gibberellic extract was pipetted into 15 ml of phyosphomolybdic acid reagent. The content was mixed thoroughly and kept in boiling water bath for 1 h. Flask was removed and rapidly immersed in ice cold, After cooling till room temperature, volume was made till 25 ml with double distilled water and optical density was taken at 780 nm.
Ninhydrin assay for ACC deaminase concentration determination :The DF-ACC medium (with an ACC concentration of 3.0 mmol lˉ¹was diluted with the DF medium to respective ACC working concentrations of 0.005, 0.01, 0.015, 0.02, 0.03, 0.04, 0.05, 0.10, 0.15, 0.20, 0.25, 0.30, 0.40 and 0.50 mmol lˉ¹. After the addition of 1 ml of ACC working solution and 2 ml of ninhydrin reagent, glass test tubes were capped and shaken and placed in a boiling water bath. After 15 min, the tubes were moved into a water bath at room temperature for 2 min and then shaken for 30 sec according to Li et al. (2011). After standing at room temperature for 10 min solution turns to purple (Ruhemann’s purple), the solution was transferred into a cuvette and absorbance was measured at 570 nm with spectrophotometer. The DF medium was used as a blank. Each working solution was run in triplicate. In addition, 1 ml of a tenfold diluted supernatant of a bacterial culture was used to determine ACC in bacterial cultures with the standard ninhydrin assay.
Characterization of PGPR strains:After confirming their potential for plant growth promoting efficacy, selected culture(s) were characterized by several biochemical analysis viz., indole, methyl red, Voges-Proskauer, citrate utilization, carbohydrate fermentation, amylase, uerease and nitrate reduction according to Bergey’s Manual of Determinative Bacteriology (Holt et al., 1994) and then molecular characterization using 16s rRNA gene amplification under standard conditions (initial denaturation 94°C for 3 min, 35 cycles of denaturation at 94°C for 30 sec, annealing at 60°C for 30s, extension at 72°C for 60s, and final extension at 72°C for 7 min). The amplified product was sequenced and found the size of 237 and 1247 bp for Bacillus cereus and Bacillus subtillis respectively. The sequence of 16S rRNA genes of both isolates were compared with the existing database using BLAST and submitted to GenBank of NCBI.
In-vivo test: Screening for salt (NaCl) tolerance on Capsicum annuum plant by isolated plant growth promoting B. cereus and B. subtilis rhizobacteria :Pots were filled with of air dried sieved soil and single surface sterilized seeds were placed in each pot. After germination, five ml of inoculum (B. cereus/B. subtilis) with population density of 107-108 cfu/ml were applied into the rhizosphere through a syringe. Capsicum annuum plants were irrigated with 800 ml of sodium chloride (0, 50, 100, and 200 mM) according to water requirement. Each treatment was performed in triplicates. Plants were harvested after 30 days and data regarding root length, shoot length, root and shoot fresh and dry weights were recorded according to Shahzadi et al. (2013).The three replicates taken for each treatment were used to calculate the mean of each measurement. Several measurements like length of the shoot system, primary root legth, number of plant leaves as well as plant’s fresh (immediately) and dry weights (keeping in oven at 70oC until the weight were stable).
Estimation of total chlorophyll: The conc. of chlorophyll was determined by measuring in a 1.0 ml cell suspension by taking 1 gm of fresh leaves. Few drops of liquid nitrogen was added and grounded with mini pestles. 1.0 ml DMSO was added to each eppendorf containing grounded leaf and mix well followed by centrifugation at 4000 rpm for 5 min. Supernatant was removed and 1.0 ml DMSO to pellet and re-extract followed by centrifugation. Supernatant was discarded and 1.0 ml DMSO was added and absorbance was recorded at 645 (Ali et al., 2014).
Statistical analysis: Basic descriptive statistics was calculated for all the growth parameters of all samples. Standard deviation was obtained by using mean values in order to have treatments comparison at P≤0.05 significance level. All of the statistical procedures were performed by using Statistics software i.e., Web Agri Stat Package 1.0 (an ICAR Web based package).
RESULTS AND DISCUSSION
Many colonies of Bacillus spp. were isolated from the soil sample as plates were incubated at 37+2°C and developed colonies selective isolation of bacillus species. Many Colonies of Bacillus spp. were identified on the basis of cultural, morphological (Table 1) as described in the Bergey’s Manual of Determinative Bacteriology and then screening for plant growth promotion was done. Spectrophotometric quantification of isolated genomic DNA was done and found 1.04 μg/ml for B. cereus and 1.04 μg/mL for B. subtilis. Genomic DNA samples were amplified and found size of 237, 1247 bp for B. cereus and B. subtilis respectively and were then sequenced for 16S r RNA gene and submitted to NCBI under accession number of LC481470 and LC480918.
Table 1: Culture, Morphological and biochemical characteristics
| Culture Characteristics | Results | |
| B. subtilis | B.cereus | |
| Colony shape | Irregular | Round |
| Margin | Undulate or lobate | Lobate |
| Colony elevation | Umbonate | Slightly convex |
| Color | White or dull | Opaque |
| Texture | Dry | Dry |
| Gram staining | Positive | Positive |
| Indole | Positive | Negative |
| Methyl red | Positive | Negative |
| Voges-Proskauer | Positive | Positive |
| Citrate utilization | Positive | Positive |
| Amylase production | Positive | Positive |
| Gelatin hydrolysis | Positive | Positive |
| Urease | Positive | Negative |
| Ammonia production | Positive | Positive |
| Nitrate reduction | Positive | Positive |
| Carbohydrate fermentation (galactose, fructose, Inositol, rafinose, riboflavin, manitol) | (+,+,-,+,+,+,+) | (+,+,-,+,+,+,+) |
The results obtained from both qualitative and quantitative assays of Indole Acetic Acid (IAA) reflected the ability of tested bacteria to produce indole compounds. The bacillus sp. exhibited a purple colour with a little variation in intensity. In the quantitative measurements, the highest value of IAA production was 0.398 and 0.26 µg/ml by B. subtilis by B.cereus species.Isolate screened for EPS production under both no stressed conditions as well as under minimum water potential (−0.30 MPa). The strain B. subtilis produced maximum amount of EPS (2.9mg/mg) whereas B. cereus could produce 2.1 mg/mg protein. Ali et al. (2014) conducted study on Pseudomonas isolate and found maximum 3.22 mg/mg protein EPS production (from Rdgp10 strain) while another strain (BriP15). Razack et al. (2013) conducted their study to understand the influence of various parameters on elevation of EPS yield by B. subtilis in agro waste containing medium and observed positive results.
In Ninhydrin assay, selected isolate were found to positive for ACC deaminase activity a as sample colour turned from yellow to red. The ACC deaminase activity of rhizobacteria was quantified. The ACC deaminase activity of B. subtilis and B. cereus was 0.13 and 0.05 µg/ml respectively. ACC deaminase-containing rhizobacteria were evaluated for their potential to promote growth (Maxton et al., 2018). Studies of Zhang et al. (2008) showed that ethylene content in tomato seedlings when exposed to high salt was reduced due to Achromobacter piechaudii (PGPR), indicating that bacterial ACC deaminase was functional and its significant role under stress conditions. A. piechaudii, which produces ACC, increased the growth of tomato seedlings by as much as 66% in the presence of high salt contents.
Bacillus subtilis was tested for gibberellic acid production. Gibberellic acid concentration by B. subtilis was recorded as 0.42μg/ml whereas B. cereus showed 0.32μg/ml gibberellin production. Our results are in accordance with another study which states that the growth promotion in plants induced by Azospirillum infection, may occur by a combination of both gibberellin production and gibberellin glucoside or glucosyl ester de-conjugation by the bacterium (Piccoli et al., 1997) Similar concentration of gibberellins was recorded in cultures of Bacillus subtilis; A. brasiliense (Janzen et al., 1992).
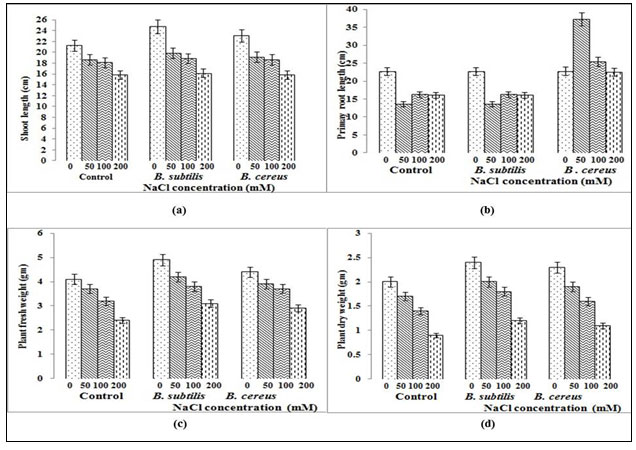
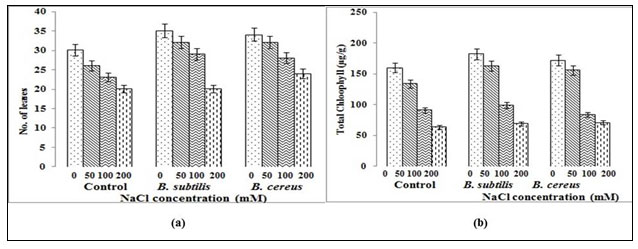
Plants of Capsicum annuum were treated with B. cereus/B. subtilis inoculum under varying salt concentration (0,50,100 and 200mM) and physical parameters like shoo length, primary root length, number of leaves, plant fresh, dry weight (fig. 1) and chlorophyll content was recorded (fig. 2,3). Inoculation of B. cereus/B. subtilis significantly increased the plant growth compare to control plant (fig. 2,3). However plant growth started to decrease as salinity stress increases but significant increase compares to control plant was noticed almost in all plant growth parameters (fig. 1).
Compared to uninoculated plant, plant with Bacillus cereus inoculum shows maximum shoot length 23cm without any salt stress and maximum primary root length 37.2 cm at 50mM salt conc. (fig. 2a) but decreased with further increases in salinity levels. Our results were confirmed by Ghorbani et al. (2014) with Nitraria schoberi and Panahi et al. (2015) with Salsola orientalis, showing moderate salinity levels may improve several growth parameters and the plant will be injured as increasing salinity level increases. Inoculated plant at 50mM salt conc. shows maximum number of leaves (32) in comparison to uninoculated plant which is 26 in number (fig. 3a). Studies of Zhang et al. (2008) showed that in Arabidopsis, B. subtilis strain regulates cell expansion and auxin homeostasis, augments photosynthesis by lowering glucose sensing levels that utlimately promotes salt tolerance as well as reduces total Na+ by regulating tissue specific expression.
Figure 1: Effect of B. cereus/ B. subtilis on growth measurements, for the plant exposed to saline treatments (a) and (b) without rhizobacteria (c) and (d) Bacillus cereus treatment.
Whereas 24.7 cm maximum shoot length was recorded without salt stress compare to 21.2 cm without inoculated plant and sustained till 16.1 cm even at 200mM salt conc. while B. subtilis inoculum was applied (fig. 2a). Primary root length was also showed sustainable improvement in the presence of inoculated plant and recorded 17.2cm compare to 16 cm (fig. 2b) in uninoculated plant even at 200mM salinity stress whereas study conducted by Hussein and Joo (2014) on raddish using Azotobacter chrococcum showed 9cm root length at 150mM NaCl conc. However in some treatments elongated root development was noticed compare to noticeable increase in shoot/number of leaves present (fig. 1) as some PGPR promote root development (Kloepper et al., 2007) and alter root architecture by the production of phytohormones such as indole acetic acid (IAA), resulting in increased root surface area and numbers of root tips. Such stimulation of roots can aid plant defense against pathogens and can also relate to induced systemic resistance (IST).
Similar increase pattern was noticed in plant fresh (3.1 gm with B.cereus while 2.9 gm with B. subtilis at 200mM compare to 2.4 gm in contol) and dry weight as well (fig. 2c,d). The notable differences recorded in all plant growth parameters may be due to the bacterial inoculation efficiency and their quorum sensing ability with another microbes present in soil. When plant growth suppression is the result of ethylene stress, PGPR with ACCD can be exploited (Saleem et al., 2007). ACCD has ability to metabolize ACC, a precursor of ethylene in biosynthesis pathway and thus controlling total amount of ethylene stress that may be produced (Arora et al., 2012).
Figure 2: Comparison of (a) Shoot length (b) Root length (c) Fresh plant weight and (d) Dry plant weight for B. cereus/B.subtilis in treated/untreated Capsicum annuum plant under salt stress where error bars indicate standard deviation and represents significance at p≤0.05 level.
During our study significant development in number of leaves at almost inoculum stage was notices (fig. 3a) that is in accordance with studies of Han et al. (2014) using B. subtilis strain where around 80% more leaves were observed PGPR have been demonstrated to activate the synthesis of antioxidants and indole acetic acid, which can stimulate root growth (Jha and Subramanian, 2014). Total chlorophyll content (µg/g) was also recorded (fig. 3b) in both B. cereus/B. subtilis inoculam and recorded as 160/172 µg/g in uninoculated whereas 182µg/g in inoculated plant under zero salinity stress. However, 71 µg/g was recorded with B. cereus inoculated and 69 µg/g with B. subtilis inoculated plant at 200mM salinity stress compare to 64 µg/g (fig. 3b) in uninoculated plant that showed another significant effect of these inoculums to as plant growth promotion. Studies of Han et al. (2014) using B. subtilis also observed promotion of leaf growth, as well as leaf chlorophyll content under both non-saline and salinity stress.
Figure 3: Comparison of (a) Number of leaves (b) Chlorophyll content for B. cereus/B.subtilis in treated/untreated Capsicum annuum plant under salt stress where error bars indicate standard deviation and represents significance at p≤0.05 level.
Several polyamines secreted through PGPR have also been exposed to mitigate stress ethylene levels as well as alleviate osmotic stress (Xie et al., 2014). Recently, PGPR were shown to alter mineral uptake, which results in a favorable increase in the cellular ratio of K+/Na+; and elevated generation of quorum-sensing molecules, which can lead to modifications.
CONCLUSION
Different paradigms of the PGPRs have been displayed comprehensively under both normal and salinity stress conditions, with the aim to develop future insight into the role of PGPR inoculum as biofertilizers for sustainable agriculture productivity and reclaiming soil fertility. The present study established that the inoculation of the rhizobacteria Bacillus subtilis and Bacillus cereus are capable of significantly increasing plant growth and biomass of Capsicum annuum under both non-saline and saline conditions, by the production of specific rhizobacterial determinants like IAA, GBA, exopolysaccharides, ACC deaminase. Plant root and shoot length, fresh and dry weight, number of leaves, chlorophyll content was recorded to be higher in inoculated plants as compared to control plants, under both non-saline and saline conditions. Therefore PGPR elicited stress tolerance can aid the growth of crops in environmentally unfavourable conditions.
Conflict of interest: There is no conflict of interest in the present study
ACKNOWLEDGMENT
Authors are thankful to SHUATS University, Prayagraj for granting In-house project (DR/Cir/JIBB/IRPs/2018/23) and providing required lab facility.
REFERENCES
Ali S, Charles TC and Glick BR (2014) Amelioration of high salinity stress damage by plant growthpromoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 80:160-67.
Arora NK, Tewari S, Singh S, Lal N and Maheshwari DK (2012) PGPR for protection of plant health under saline conditions, pp. 239-258. In Maheshwari DK (ed.). Bacteria in Agrobiology: Stress Management. Springer, Berlin.
Ghorbani M, Ranjbar FA, Panahi F, Attarha J, Marzbani N and Moases M (2014) Salinity and Nitraria schoberi: growth parameters, chlorophyll content and ion accumulation. Int. J. Agri. Crop Sci. 11:853-62.
Han QQ, Lü PX, Bai JP, Qiao Y, Pare PW, Wang SM, Zhang JL, Wu YN, Pang XP , Xu WB and Wang ZL (2014) Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 5:1-8.
Hashem A, Tabassum B. and Allah E. (2019). Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 26: 1291-1297.
Holbrook A, Edge W and Bailey F (1961) Spectrophotometric method for determination of gibberellin acid. Adv. Chem. Serv. 28: 159-167.
Holt JG, Krieng N, Sneath P, Staley P and Williams J (1994) S.T. Bergey’s Manual of Determinative Bacteriology. (9nd ed.). Baltimore.
Hussein KA and Joo JH (2018) Plant Growth-Promoting Rhizobacteria Improved Salinity Tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 28:938–45.
James RA, Blake C, Byrt CS and Munns R (2011) Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accu- mulation in bread wheat leaves under saline and water logged conditions. J. Exp. Bot. 62: 2939–47.
Janzen RA, Rood SB, Dormaar JF and McGill WB (1992) Azospirillum brasilense produces gibberellin in pure culture on chemically-defined medium and in co-culture on straw. Soil Biol. Biochem. 24:1061-64.
Jha Y and Subramanian RB (2014) PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plant. 20: 201-7.
Kloepper JW, Gutiérrez EA and McInroy JA (2007) Photoperiod regulates elicitation of growth promotion but not induced resistance by plant growth-promoting rhizobacteria. Can. J. Microbiol. 53: 159-67.
Lareen A, Burton F, Schäfer P (2016) Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol. 90(6):575–587.
Li Z, Chang C, Lin L, Li Y and An Q (2011) A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett. Appl. Microbiol. 53: 178-85.
Lugtenberg B and Kamilova F (2009) Plant-Growth-Promoting Rhizobacteria. Ann. Rev. Microbiol. 63 :541-56.
Maxton A, Singh P, Aruna A, Prasad SM and Masih SA (2018) PGPR: A Boon in Stress Tolerance and Biocontrol. Res. J. Biotechnol. 13: 105-11.
Mayak S, Tirosh T and Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 166: 525-30.
Meller S, Frossard E and Luster J (2019) Phosphorus Allocation to Leaves of Beech Saplings Reacts to Soil Phosphorus Availability. Front. Plant Sci. 10:744.
Panahi F, Asareh MH, Jafari M, Givar A, Tavili A, Arzani H and Ghorbani M (2015) The responses of Salsola orientalis to salt stress. Int. J. Advanced Biol. Biomed. Res. 3(2):163-71.
Pandey S, Ghosh PK, Ghosh S, De TK and Maiti TK (2013) Role of heavy metal resistant Ochrobactrum spp. and Bacillus Spp. Strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 51: 11-17.
Piccoli P, Masciarelli O and Bottini R (1997) Metabolism of 17,17-[2H2]-gibberellin A4, A9 and A20 by Azospirillum lipoferum in chemically defined culture medium. Symbiosis. 21: 263–274.
Razack AS, Velayutham V and Thangavelu V (2013) Medium optimization for production of Exopolysaccharide by Bacillus subtilis using synthetic sources and agro wastes. Turkish J. Biol. 37: 280-288.
Saleem M, Arshad M, Hussain S and Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Indust. Microbiol. Biotechnol. 34: 635-48.
Shahzadi IA, Khalid S, Mahmood MA, Mahmood T and Aziz I (2013) Effect of bacteria containing ACC deaminase on growth of wheat seedlings grown with chromium contaminated water. Pak. J. Bot. 45: 487–94.
Singh RP and Jha PN (2016) A Halotolerant Bacterium Bacillus licheniformis HSW-16 Augments Induced Systemic Tolerance to Salt Stress in Wheat Plant (Triticum aestivum). Front. Plant Sci. 7:1890
Singh, S., Najar, G., and Singh, U. (2012). Phosphorus management in field pea (Pisum sativum)-rice (Oryza sativa) cropping system under temperate conditions. Ind. J. Agric. Sci. 82:494.
Srivastava V, Sarkar A, Singh S, Singh P, Araujo A and Singh R (2017) Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 5:64.
Xie SS, Wu HJ, Zang HY, Wu LM, Zhu QQ and Gao XW (2014) Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol. Plant Microbe Int. 27:655-63.
Zhang H, Kim MS, Sun Y, Dowd SE, Shi H and Paré PW (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe. Int. 21: 737-44.
Zhang JL and Shi H (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosynthesis Research 115:1-22.