Laboratory of Mycopathology and Microbial Technology, Centre of Advanced Study (CAS) in Botany,
Institute of Science, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
Corresponding author email: kumaridivyanshubhu@gmail.com
Article Publishing History
Received:
Accepted After Revision:
The study was conducted to examine the effect of plant growth promoting rhizobacteria; Pseudomonas punonensis LMT03 (R1), Pseudomonas plecoglossicida (R4), Pseudomonas aeruginosa DSM 50071 (R2), Alcaligenes faecalis (DBHU5) and their consortium on yield and lignin deposition of the barley crop. Consortium treated plants had the highest plant height, leaf surface area, number of fertile tillers, spike length, grains per spike, 1000 grain weight, grain yield, straw yield, total biomass, and harvest index percent and the lowest values were found in control plots. The consortium treated plant produced the highest grain yield 7976 kg/ha, while control plants produced 3200 kg/ha. In comparison to the control plant, the PGPR-treated barley plant showed dense lignin deposition in the vascular bundles of the stem section.This is the first report on the effect of P.punonensis and P. plecoglossicida on barley crop yield parameters under field conditions, also the first report on lignin deposition in barley plant treated with P. punonensis and P. plecoglossicida strains.
Barley, Grain Yield, Inoculation, Lignin, Plant Growth Promoting Rhizobacteria
Divyanshu K, Yadav M, Upadhyay R. S. Effectiveness of Pseudomonas and Alcaligenes sp. on the Yield and Lignin Deposition of Barley Hordeum vulgare L. Crop. Biosc.Biotech.Res.Comm. 2022;15(3).
Divyanshu K, Yadav M, Upadhyay R. S. Effectiveness of Pseudomonas and Alcaligenes sp. on the Yield and Lignin Deposition of Barley Hordeum vulgare L. Crop. Biosc.Biotech.Res.Comm. 2022;15(3). Available from: <a href=”https://bit.ly/3ShLD9r“>https://bit.ly/3ShLD9r</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Barley (Hordeum vulgare L.) is a member of the Poaceae grass family, ranking fourth among cereals behind maize, wheat and rice with respect to its worldwide production. It is the fast growing annual crop, grows in winter season (Ghanbari et al. 2012). Barley is a diploid species with a high degree of inbreeding (Self pollinating) capability. It has a low chromosome number (2n=14) with a large genome size, can be easily cultivated and adapt to different climatic and environmental conditions; and easily cross-bredable. Because of its adaptability and robustness, barley is grown in over 100 countries worldwide (Harwood 2019). Barley crops were used in the brewing industry, as animal feed, and as healthy food options for human consumption. They were also used as a cover crop to increase soil fertility (Ghanbari et al. 2012).
The inclusion of barley in the diet provides several health benefits, including lowers blood cholesterol levels, increased fibre intake, and a good source of beta-glucan, the highest amount of beta-glucan was found in barley (Behall et al. 2004; Harwood 2019).
The amount of grain produced is insufficient to meet the food demand of India’s uncontrolled growing population; thus, to increase crop productivity and yield to feed the growing population, synthetic fertilizers and pesticides were incorporated into farming without regard for the negative and hazardous impact on the environment, food chain, and human health. The uncontrolled use of chemical fertilizers in agriculture is presently the topic of debate due to environmental concern and fear for living being health (Turan et al. 2010). The synthetic chemical fertilizers are the inorganic fertilizers rich in major nutrients NPK (nitrogen, phosphorus, potassium) in huge amount and do not incorporate organic manures consequently results in deterioration of soil quality and its fertility (Choudhry 2005; Harwood 2019).
Plant growth promoting rhizobacteria (PGPR) were the healthy and cost effective strategy to enhance the crop productivity. Application of PGPR as biofertilizer are the most effective approach to enhance the sustainable agricultural systems (Sharma 2003). PGPR are soilborne bacteria that aggressively colonize the rhizospheric region of plants or when applied to the seeds or crops enhances the growth and yield of plants (Kaymak 2011). Incorporation of PGPR instead of chemical fertilizers are known to improve grain yield through supply of plant nutrients may help to sustain and protect environmental health (O’Connell 1992). Inoculation of phytomicrobiome members in agriculture for crop productivity is a sustainable and cost-effective approach to disease control and artificial, chemical, synthetic supplements could reduce the negative effects associated with the excessive use of chemical fertilisers and pesticides (Antar et al. 2021b). These phytmicrobiomes have been used as an effective strategy to reduce biotic and abiotic stresses that could improve crop productivity (Khan et al. 2020; Antar et al. 2021b).
Lignin is the second richest biopolymer of high molecular weight having complex phenolic structure and called as major structural component of plant cell (Nayak et al. 2020). Lignin deposition in the cell wall is the important step during stem development, provides strength to stem which is interlinked to barley growth, agronomic traits and hence affects yield (Jones et al. 2001; Begović et al. 2015). Lignin deposition also play crucial role in water and mineral transport, activates defence mechanism against biotic and abiotic stresses, provides rigidity and mechanical strength to the tissues through thickening of plant cell wall and development of secondary growth, helps in plant tissue/organ growth and development, imparts culm lodging resistance and many more in favour of plant metabolism (Jayamohan and Kumudini 2011; Liu et al. 2018).
It was found that lignin deposition in monocot plant such as barley is not very much intensive and thus have not that much intense secondary growth in comparision to dicot plants, in which the secondary cell wall is made up of 20% of lignin (Vogel 2008). Lignin deposition protects the monocot plants such as barley from poor culm strength which is also termed as culm lodging (Hai et al. 2005; Ma 2009). Moreover, lignin deposition generate resistance against culm or stalk bending (reduction in culm height), imparts strength to the barley culm which have direct impact on grain yield as bending of culm or reduction in culm height leads to shorter plant height which consequently reduces the grain yield (Lalić et al. 2005; Bonawitz and Chapple 2013).
It was reported that PGPR treated plants can enhances the lignification in plants (Jha 2019). Increased lignin deposition were found in Azospirillum brasilense treated strawberry plant which also provide resistance against charcoal rot disease (Viejobueno et al. 2021) Maximum lignin deposition was found in vascular bundle of chickpea plant inoculated by fluorescent Pseudomonas and Rhizobium PGPR strains (Singh et al. 2013; Viejobueno et al. 2021).
PGPR application as biofertilizer in plant is a sustainable approach to improving crop production. Employment of PGPR can be introduced to achieve the pupose of achieving the sustainable and resilient agricultural production system without application of additional chemical fertilization. In the view of above background information the present investigation was aimed to evaluate the efficacy of PGPR isolates; Pseudomonas punonensis (R1), Pseudomonas plecoglossicida (R4), Pseudomonas aeruginosa (R2), Alcaligenes faecalis (DBHU5) and their consortium on yield and yield attributing agronomic parameters such as plant height, leaf surface area, number of fertile tillers, spike length, grains per spike, 1000 grain weight, grain yield, straw yield, total biomass and harvest index % of barley plant, further to investigate the effect of these PGPR strains on lignin deposition at the vascular bundle, cell wall of stem section of barley.
MATERIAL AND METHODS
In order to investigate the impact of PGPR on yield and yield components of barley (Hordeum vulgare L.) in the field condition, the barley variety PL- 426 were sown during the Rabi season of 2018-19 at Botanical Garden of Banaras Hindu University, Uttar Pradesh, India. The soil of experimental plot was fertile, alluvial loam and is characterized as type of Indo-Gangatic plains. Rabi season is the winter season in the northern India where crop is shown in the month of November-December and harvested in March-April of the subsequent year.
The experimental design was laid out in split randomise block design plot with date of sowing on 1st December 2018, having 2 conditions that are PGPR treated and control (without any treatment of PGPR and chemical fertilizer) having 6 treatments, with 3 replications of each treatment. Seeds were sown in 6m by 3m total area (1m by 1m each plot) having 6 rows and 3 columns,a total of 18 plots. Row to row distance were 20 cm. Seeds were inoculated by PGPR strains by dipping the seeds for 5 hours into the bacterial broth prior to sowing in the field. Maximum precautions were taken to avoid any contamination and mixing of bacterial inoculations during sowing.The field was plowed twice prior to sowing the seed also weeds, unwanted materials were removed and cleaned manually. Plots were irrigated regularly with raw water without any mixture of chemical fertilizers.The crops were harvested during first week of April. The experiment had 6 treatments which are described below
R1- Seeds inoculated with PGPR Pseudomonas punonensis LMT03(Accession no. MT677939)
R4- Seeds inoculated with PGPR Pseudomonas plecoglossicida (Accession no. MT883433)
R2- Seeds inoculated with PGPR Pseudomonas aeruginosa DSM 5007(Accession no. MT845116)
DBHU5- Seeds inoculated with PGPR Alcaligenes faecalis (Accession no. MT872514)
Consortium- Combined treatment of all the 4 PGPRs (R1, R4, R2 and DBHU5)
Control- Without any PGPR treatment and any fertilizer (Non inoculated). Irrigation with raw water only
For evaluation of yield and yield attributing agronomic parameters ten randomly crop plants were selected from each of three replicates, all the parameters were recorded from selected plants.Yield was estimated through harvesting all the crop plants of each plot (three replication of each treatment). Mean value of all the three replications of each treatments were considerd for calculation of all agronomic traits. The agronomic parameters studied was: Leaf surface area (cm2) – Leaf surface area were taken by measuring the length and width of a leaf using scale.Plant height (cm)- At physiological maturity, height was measured from the ground level to the top of the spike (excluding the awns) using a meter rod. Number of productive/ fertile tillers- Number of fertile tillers per selected plants were counted.
Spike length (cm)- Three spikes from each of the ten plants per plots selected and length of spikes were recorded from the base to the apex of the spike through a meter rod. Number of grains per spike- Three spikes of each selected ten plants from each replication were threshed and the grains were separated from the spikes and were counted manually. 1000 grain weight (gm) – Thousand grains were counted after harvest and weighed for each replication. After harvest, weight of thousand grains from each plot were taken using weighing balance. The mean value of three replication was used in figure. Grain yield/ (kg/ha)- After harvesting, grains were threshed and seperated, grain weight of all crop plant of each plot were taken in kg using electronic balance and subsequently converted into kg/ha.
Straw yield (kg/ha)-After harvesting weight of sun-dried above ground parts (excluding grains) of all crop plants from each plots were taken using electronic balance in kg and subsequently converted into kg/ha. Total biomass/ Biological yield (kg/ha) – All crops from each plot under that area were harvested, bundled, sun dried and then weight the bundles in kg using electronic balance for estimation of total biomass , afterward converted into kg/ha. Harvest index%- Ratio between grain yield and total biomass of all crops of each plot was determined by applying the following formula: HI (%) = (Grain yield each replication/ Total biomass (grain + straw) each replication ×100.
Histochemical deposition and distribution of lignin deposition in barley internodes: To estimate lignin deposition, a hand cut transverse section of fresh barley internode was mounted on a slide and stained with a solution of 0.5 percent saturated phloroglucinol (w/v) with addition of HCl and observed under an Olympus binocular microscope. The appearance of red-violet colour on the section defined the conformation of accurate lignin staining (Jensen 1962).
Statistical analysis: The IBM SPSS Statistics Ver.20 software was used for all statistical analysis and calculations. The statistical data were expressed as the mean of three independent replications, standard error of mean (SEM) of three replicates of each experiment, and thrice repetition data of each replicate, and were interpreted using one-way ANOVA followed by Duncan’s multiple range test at the P=0.001 significance level. The experiments in this study were carried out in triplicate, with each experiment being repeated three times using a completely randomised design.
Results and Discussion
Yield and yield attributed agronomic parameters: Grain yield performance is heavily influenced by agronomic traits. Seeds inoculated with PGPR strains P. punonensis, P. plecoglossicida, P.aeruginosa, A. faecalis alone and in combination (consortium) significantly increases plant height, number of fertile tillers, leaf surface area, spike length, number of grains per spike, 1000 grain weight, grain yield, straw yield, total biomass, and harvest index. Performance of the plants on all the studied parameters was superior in PGPR inoculated treatments in comparison to the non-PGPR inoculated plants. The maximum increase in all the agronomic and plant growth promoting parameters was recorded by consortium treated plants followed by P. punonensis, P. plecoglossicida, P. aeruginosa, A. faecalis, where as the least value of data was obtained from control plots. For all the field parameters, mean value of all the three replications of each treatments were consider for calculation and yield analysis (Fig. 1).
Figure 1: Barley crop grown in experimental field plot of botanical garden of Banaras Hindu University.

Leaf surface area (cm2): Seeds inoculated with PGPR significantly increases the leaf surface area in barley cultivars over untreated plants. The mean result of all the treatments revealed that the combined effect of all the PGPRs (consortium) produced the highest leaf surface area (72.13 cm2, followed by R1 (70.26 cm2), R4 (68.60 cm2), R2 (67.73 cm2) and DBHU5 (66.30 cm2), while the least value (59.73 cm2) was recorded by control treatment. The mean data with respect to leaf surface area have been summarized in Table No. 1 and Fig. 2a. PGPR treatments in barley plants results in increase in leaf surface area which may enhances the gaseous exchange hence rate of photosynthesis increases which promotes various plant metabolic activities. Purwanto et al. have reported that PGPR inoculation in rice plant can increase leaf surface area upto 91.10 percent compared to control plants (Purwanto et al. 2019).
Plant height (cm): All the PGPR treatments had significantly increased the plant height over untreated plants. The mean of plant height was observed to be in the ranges of 76.56-87.53 cm. The highest plant height (87.53 cm) was recorded by consortium followed by R1 (85.36 cm), R4 (84.60 cm), R2 (82.56 cm), and DBHU5 (80.80 cm), while the shortest plant height was showed by control plants (76.56 cm). Result of mean plant height indicated in (Table No. 1, Fig. 2a). Plant height is an important factor and positively correlated with grain yield.Increase in plant height by the inoculation of PGPR indicates that PGPR inoculation in barley plants can increase vegetative growth. Increase in plant height of barley, wheat, corn, by PGPR treatment was already reported (Shaharoona et al.2007; Gholami et al. 2009; Shirinzadeh et al. 2013; Hussain et al. 2020).
Number of productive/fertile tillers: Seeds treated by consortium produced maximum number of productive tillers (10.23), followed by R1 (8.76), R4 (7.40), R2 (6.30) an DBHU5 (6.00), the reduced number of productive tillers was produced by control plants.The average number of total fertile tillers as indicated in (Table No. 1, Fig. 2a).The number of fertile tillers is an important agronomic factor that influences grain yield. Similar reports were foundby Shaharoona et al. in wheat crop as the number of fertile or productive tillers in plant increases the number of spikes along with grains, which play a vital role in grain yield (Shaharoona et al. 2007). Increase in number of tillers is one of the chief agronomic character as this may compensate the difference in number of plants, partially or totally after crop establishment and may allow crop recovery from early frost (Acevedo et al. 1998; Hussain et al. 2020).
Figure 2: (a-b) Graph representing effect of different PGPR strains and their consortium on (a.) leaf surface area, plant height, number of fertile/productive tillers, (b.) spike length, grain per spike, 1000 grain weight of barley plants in comparision to control plants, illustrating increase over control in field condition. Data are means of three replicates along with standard error of mean bars. Different letters above the standard error bars denotes significant differences over control (p<0.001).
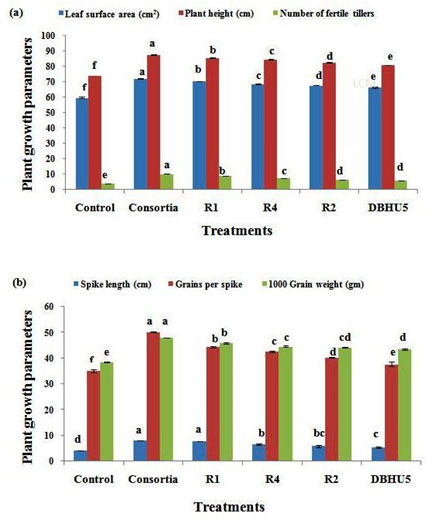
Spike length (cm): The impact of seed inoculation with PGPR treatment on spike length was significant. The maximum spike length (8.10) was obtained by consortium treated plant followed by R1 (7.80 cm), R4 (6.53 cm), R2 (5.86 cm), DBHU5 (5.30 cm) and minimum spike length (4.10 cm) was recorded by control plots indicated in (Table No. 1, Fig. 2b). PGPR inoculated plants showed significant increase in the grain number per spike in comparision to control plants so most highest number of grains per spike shown by consortium (50.10) treated plants, while R1 (44.40) showed the second most highest grains/spike followed by R4 (42.50), R2 (40.23) and DBHU5 (37.63).
The lowest number of grain/spike was recorded by control plots (34.96) as indicated in (Table No. 1, Fig. 2b). Increase in spike length, number of grains per spikeis directly proportional to the grain yield, increase in spike length by PGPR treatments, results in more production of grains in spike which consequently results in increase of grain yield of barley plant (Shahzad et al. 2007). In our study increase in spike length and number of grains per spike through the inoculation of PGPR is according to the findings of Shirinzadeh et al., on agronomic traits of barley (Shirinzadeh et al. 2013). Inoculated barley plants had more grain number per spike and hence more grain yield (Hussain et al. 2020).
1000 grain weight (gm)- Based on the data of all treatments of each of the three replicates, the highest 1000 grain weight was recorded by plants inoculated by consortium (47.86 gm), further R1 showed the second highest (45.80 gm), later on R4, R2 and DBHU5 showed (44.50 gm), (44.10 gm) and (43.46 gm) 1000 grain weight respectively. The lowest value was recorded by the grains produced by control plants (38.53) indicated in Table No. 1 and Fig. 2b. 1000-grain weight is an essential yield determining factor of barley. Inoculation of barley seeds with PGPR significantly increases the 1000-grain weight which results in improved seed quality, has been reported by cakmakci et al (cakmakci et al 2007). Increase in 1000-grain weight of barley plant, inoculated by Azotobacter,Azospirillium, Azotobacter+ Azospirillium is reported by Shirinzadeh et al. (Shirinzadeh et al. 2013). Wheat plant treated with consortia of Paenibacillus polymyxa, Bacillus subtilis and Bacillus aryabhattai as well as separate inoculation of these PGPRs significantly increased the 1000 grain weight of wheat (Hussain et al. 2020).
Grain yield/ Economic yield (kg/ha)- Grain yield varied between 3200 kg/ha in without treated till 7976 kg/ha in seed treated with PGPR. Maximum grain production was recorded by consortium treated plants (7976 kg/ha) followed by R1 (6976 kg/ha), R4 (6263 kg/ha), R2 (5400 kg/ha), and DBHU5 (4400 kg/ha), while control platts produced only 3200 kg/ha indicated in (Table No.1, Fig. 3a). Grain yield is the main goal of agricultural practices by farmers. Grain yield is one of the significant factor towards yield and yield attributing components. Increased grain yield is directly dependent on increase in number of productive tillers and grain per spike which is also supported by the study of Naeem et al. (Naeem et al. 2018). Enhancement in barley plant growth and grain yield through PGPR treatment is reported in a previous study (Cakmakci et al. 2007). Increase in yield of many cereals crops through application of diazotrophs has been in a previous study (Dobbelaere et al. 2003). Bacteria inoculated plants such as corn, sugarcane, rice increases the yield upto 10 to 30 percent as reported in a previous study (Kloepper et al. 1992). Hussain et al. (2020) reported that application of novel Bacillus and Paenibacillus species bio-inoculants separately and in combination has a positive influence on yield of wheat crop. Application of Pseudmonas spp.
and Burkholderia caryophylli in wheat plant leads to increase in yield of wheat is reported in a previous study (Shaharoona et al. 2007). Significant increase in barley grain yield by the application of Azotobacter and Azospirilliu is reported by Shirinzadeh et al. (Shirinzadeh et al. 2013). Seed inoculation with Azospirillum brasilense significantly affects the yield of barley and wheat as reported in a previous study (Ozturk et al. 2003). Similar result was found by combined application of indigenous PGPR; B. megaterium, A. chlorophenolicus and Enterobacter on wheat grain yield as reported in a previous study (Kumar et al. 2014). Several studies were found in support of significant increase in grain yield by PGPR inoculated plants (Tiwari et al. 1989). Imran et al. (2015) have reported increase in grain yield in Ochrobactrum ciceri and Mesorhizobium ciceri inoculated chickpea (PUSA-372) plant (Imran et al. 2015). The maximum increase in grain yield of wheat was observed due to the consortium application of PGPR; Paenibacillus polymyxa, Bacillus subtilis and Bacillus aryabhattai as investigated in a previous study (Hussain et al. 2020). Bacillus spp. significantly increased the grain yields of crops such as fingermillet, maize, amaranth, buckwheat and French bean (Pal 1988; Hussain et al. 2020).
Straw yield (kg/ha), Biological yield/Total biomass (kg/ha)– Among all treatments maximum significant straw yield (14990 kg/ha) was recorded in consortium treated plants, followed by R1 (14133 kg/ha), R4 (13433 kg/ha), R2 (12033 kg/ha) and DBHU5 (11333 kg/ha), while lowest straw yield (10200 kg/ha) was obtained in control plants indicated in Table 1, Figure 3a. The maximum biological yield were recorded in consortium treated plants (22966 kg/ha) followed by R1 (21110 kg/ha), R4 (19696 kg/ha), R2 (17433 kg/ha) and DBHU5 (15733 kg/ha) while the lowest biological yield (13400 kg/ha) was obtained from control plots (Table No. 1, Fig. 3a). Biological yield is also an important parameter because farmers were interested in straw in addition to grain (Tigabu and Asfaw 2016).
In our study maximum biological yield or total yield was produced from consortia treated barley plants and similar result was found in a previous study by the treatment of triple combination of PGPR B. megaterium A. chlorophenolicus and Enterobacter on wheat plant which significantly enhanced the straw yield in field conditions in comparision to uninoculated plant (Kumar et al. 2014). Increase in straw yield, total biomass and harvest index by application of phosphate solublizing bacteria on wheat in comparision to control plants (Turan et al. 2010). Combined effect of Azospirillum lipoferum, Arthrobacte mysorens and Agrobacterium radiobacter increases the grain and straw yield in 3 barley cultivars (Belimov et al. 1995). Seed inoculation with Bacillus polymyxa significantly enhanced total yield in rice and chickpea crops (Tiwari et al. 1989). Harvest index (%)- The maximum harvest index value was obtained from consortium treated plants (34.73%), followed by R1 (33.04 %), R4 (31.79 %), R2 (30.95 %) and DBHU5 (27.96%), while the lowest harvest index was recorded by control plants (23.85%), as explained in Table No. 1, Fig. 3b.
Figure 3(a-b). Graph representing effect of different PGPR strains and their consortium on (a.) grain yield, straw yield and total biomass/ biological yield, (b.) Harvest index % of barley plants in comparision to control plants, illustrating increase over control in field condition. Data are means of three replicates along with standard error of mean bars. Different letters above the standard error bars denotes significant differences over control (p<0.001).
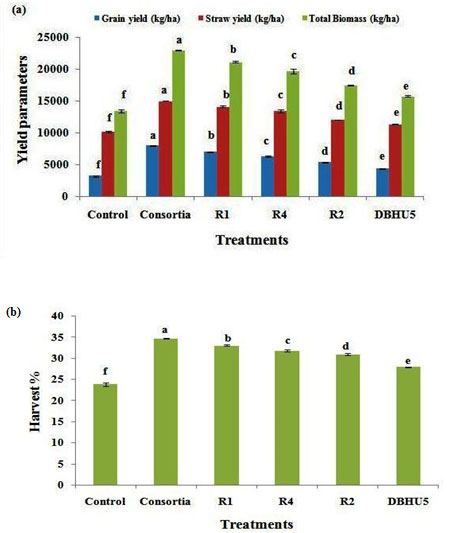
Table 1. Effect of inoculation with PGPR strains and their consortium on yield and yield attributing parameters of barley crop in comparision to control plants.
| Treatments | PH | LSA | FT | SL | GPS | TGW | GY | SY | TB | HI |
| Consortia | 87.53±0.20a | 72.13±0.08a | 10.23±0.14a | 8.10±0.05a | 50.1±0.05a | 47.86±0.03a | 7976±14.52a | 1499±5.77a | 22966±20.27a | 34.73±0.03a |
| R1 | 85.36±0.27b | 70.26±0.12b | 8.76± 0.13b | 7.8± 0.05a | 44.40±0.30b | 45.80±0.43b | 6976±14.52b | 1413±133.33b | 21110±145.25b | 33.04±0.17b |
| R4 | 84.60±0.20c | 68.60±0.15c | 7.40± 0.05c | 6.53± 0.2b | 42.50±0.28c | 44.50±0.28c | 6263±131.69c | 1343±260c | 19696±375.95c | 31.79±0.26c |
| R2 | 82.56±0.17d | 67.73±0.12d | 6.30± 0.24d | 5.86±0.44bc | 40.23±0.14cd | 44.10±0.05cd | 5400±57.73d | 1203±33.33d | 17433±66.66d | 30.95±0.23d |
| DBHU5 | 80.80±0.05e | 66.30±0.30e | 6.0± 0.06d | 5.30±0.25c | 37.63±0.91e | 43.46±0.26d | 4400±57.73e | 1133±88.19e | 15733±145.29e | 27.96±0.11e |
| Control | 76.56±0.28f | 59.79±0.37f | 4.10±0.16e | 4.10±0.05d | 34.96±0.54f | 38.53±0.2e | 3200±115.4f | 10200±115.47f | 13400±230.94f | 23.85±0.44f |
Here ‘ha’, hectare; ‘gm’, grams, PH- plant heigh, LSA- leaf surface area, FT- number of fertile tillers, SL- spike length, GPS-grain per spike, TGW-thousand grain weight, GY-grain yield, SY- straw yield, TB- total biomass, HI- harvest index. Different letters on mean±standard error denotes significant differences over control (p<0.001).
Barley seeds inoculation with PGPR Azotobacter and Azospirillium enhanced the plant growth promoting parameters such as plant height, spike length, number of spike per area, grains per spike, 1000 grain weight significantly and could enhance grain yield to an acceptable level. PGPR inoculation in crop plants can increase the crop productivity and yield as high as upto approx 57% depending on the crop plant (AsgharMet al. 2004; Shirinzadeh et al. 2013). The PGPR strains; Bacillus megaterium, Bacillus subtilis, and Azospirillum brasilense were reported to enhance the grain yield, straw yield, total yield, and plant nutrient element content of barley and wheat crop (Baris et al. 2014; Hussain et al. 2020).
Linear Correlations Between All Agronomic Parameters of all Treatments: Table No. 2 represents the types of correlations between the agronomic growth and yield parameters of barley. All the 10 agronomic parameters were positively and significantly correlated with each other of all the six treatments, this implies that increase in the value of one parameter leads to increase in the other parameter to which it is significantly correlated and their growth were dependent on each other. As grain yield is our main objective of this study and for the farmers it is the main factor so we recorded that plant height showed highly significant and maximum correlation with grain yield r =0.988**,1Leaf surface area showed r = 0.953** with grain yield, Number of fertile tiller have r =0.985** with grain yield, while spike length r =0.987**, grain per spike r =0.981**, thousand grain weight r =0.947** also showed significant correlation coefficient with grain yield. Here ‘r’ is correlation coefficient.
From the present correlation data it was concluded that in this study plant height and spike length is positively interlinked with grain yield, if plant height and spike length increases there will be increase in the grain yield also. It was also observed from the data that if plant height increases there will be increase in the harvest index %. Correlation between different agronomic traits provides necessay information and guidance to the farmers for the selection of yield enhancing traits. In the present study correlation between all the 10 traits; plant height, leaf surface area, number of fertile tillers, spike length, grains per spike, 1000 grain weight, grain yield, straw yield total biomass, harvest index% were analyzed for all the six treatments. All the traits have positive and highly significant interrelationship with each other (Hussain et al. 2020).
Table 2. Correlation (Pearson coefficients) among agronomic parameters of all the six treatments.
| Parameters | PH | LSA | FT | SL | GPS | TGW | GY | SY | TB | HI |
| PH | 1 | |||||||||
| LSA | .983** | 1 | ||||||||
| FT | .969** | .943** | 1 | |||||||
| SL | .970** | .947** | .985** | 1 | ||||||
| GPS | .953** | .908* | .983** | .957** | 1 | |||||
| TGW | .975** | .995** | .951** | .940** | .920** | 1 | ||||
| GY | .988** | .953** | .985** | .987** | .981** | .947** | 1 | |||
| SY | .978** | .933** | .984** | .987** | .974** | .926** | .995** | 1 | ||
| TB | .984** | .944** | .986** | .988** | .978** | .938** | .999** | .999** | 1 | |
| HI | .992** | .984** | .948** | .962** | .935** | .972** | .979** | .979** | .960** | 1 |
* Correlation is significant at the p ≤ 0.05 level
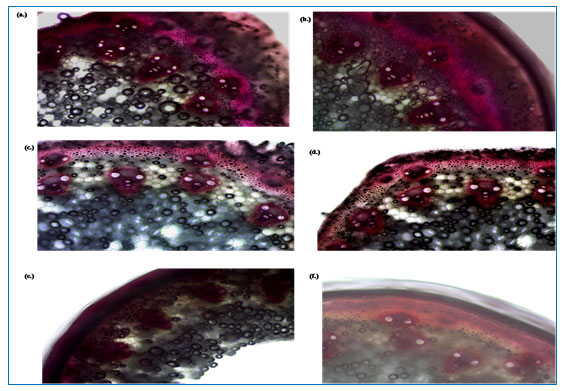
Deposition and Distribution of Lignin In Internodes of Barley: Deposition of lignin in pink to violet color were observed in the region of sclerenchyma ring of cortex, epidermis, parenchyma and vascular tissue of all the 4 rhizobacterial isolates (R1, R4, R2 and DBHU5) and their consortium treated barley plants, while there is light or moderate pink color were developed which showed thin or less lignin deposition on sclerenchyma ring of cortex, epidermis, parenchyma and vascular tissue of control plants. PGPR treated plants showed thickness in the cell wall due to lignin deposition in comparision to the non treated plants as observed in Fig 4 (a-f).
As barley is a monocot grass plant which was known for less lignin deposition but in the present result we found that stem vascular bundle section of all the four PGPR treated plant showed intense lignin deposition in comparision to control. Although it was studied and found that lignin synthesis in barley plants occurs with very low intensity and lower quantity, but in the present study it was found that all the four PGPR treated barley plants showed significant and uniform lignin deposition in their vascular bundle region and cell wall of internodes section of stem in comparision to control plants. PGPR treated plants develop maximum cell wall lignification which induces and activates higher concentrations of defense related enzymes, it was found that rhizospheric bacteria Bacillus megaterium enhances the lignin deposition in the cell wall of maize plants and protects against Aspergillus niger (Jha 2019).
Similar result was observed by the treatment of PGPR inoculants Pseudomonas and Rhizobium on lignin deposition in the vascular bundle of chickpea plant (Singh et al. 2013). Study of the pattern of lignin deposition in the cell wall of internodes of barley (Begovic et al. 2015). Maximum and dense lignin deposition was found in the secondary walls of xylem vessels of strawberry plant treated by Azospirillum brasilense (Viejobueno et al. 2021). Lignin deposition in plant stem perform vital role in conductance and movement of water which develop resistance ability in plants under abiotic stress and also provide rigidity to the cell wall (Ajao et al. 2018). Stem and root section of B. megaterium and P. fluorescens treated mungbean plants showed significant increase in lignin deposition which also protect the mungbean plant from the infection of M. phaseolina (Javed et al. 2021).
Figure 4. (a-f) Influence of PGPR strains (a.) Consortium (b.) R1, (c.) R4, (d) R2, (e.) DBHU5 on lignification in barley stem by histochemical staining in comparision to stem of (f.) non-inoculated control plant, illustrating increase lignin deposition over control.
CONCLUSION
The findings of the present finding showed significant increase in the yield and yield associated agronomic parameters of barley plants treated by separate inoculation of PGPRs P. punonensi, P. plecoglossicida, P. aeruginosa and A. faecalis along with combined inoculations of these PGPR strains in comparision to the control plants. PGPR treated barley stem vascular bundle have more intense lignin deposition layer as compared to the non treated plants. All the four PGPR treated isolates showed maximum lignin deposition in the cell wall of barley internodes and consequentialy enhances cell wall thickness in comparision to control. This is the first study on the effect of PGPR; P. punonensis, and P. plecoglossicida strains treated barley plants on their yield and yield attributing parameters under field condition. Also this the first study done on characterization of lignin deposition on barley plants treated by P.punonensis and P. plecoglossicida PGPR strains.
ACKNOWLEDGEMENTS
The study was supported by the department of botany, banaras hindu university, varanasi, uttar pradesh, india and human resource development group, council of scientificand industrial research, new delhi, india under the research fellowship as CSIR-JRF and CSIR-SRF.
Declarations
Funding: KD wish to thanks Council of scientific and Industrial Research (award no. 09/013(0689)/2017-EMR-I), New Delhi, India for financial support as CSIR JRF and CSIR SRF.
Conflicts of Interests: Authors declares no conflicts of interests to disclose.
Availability of data and Material: All data/ results/ information is available with the authors, were mentioned in the manuscript and can be shared on a reasonable request made to the corresponding author when required.
Code Availability: Not applicable
Authors’ Contributions: KD: Conceived the research, wrote the manuscript, analyzed the data, acquire the funding. KD: Performed the research. KD: Analyze the data. RSU: Wrote the manuscript, Supervised the research.
Ethics approval: Not applicable.
Consent to participate: All authors participated in the experiment.
Consent for Publication: All authors read and aware of publishing the manuscript in Bioscience Biotechnology Research Communications.
REFERENCES
Acevedo, E, Silva, H, Silva. P, et al. (1996). Tendencias actuales de la investigación en la resistencia al estrés hídrico de las plantas cultivadas 10 Reunion de la Sociedad Botanica de Chile. 34. Reunion Anual de la Sociedad de Biología de Chile, Viña del Mar, 8-10 Oct 1996 (1996).
Ajao, O, Jeaidi, J, Benali, M, et al. (2018). Quantification and variability analysis of lignin optical properties for colour-dependent industrial applications. Molecules. 23(2) 377.
https://doi.org/10.3390/molecules23020377
Antar, M, Lyu, D, Nazari, M, et al. (2021b). Biomass for a sustainable bioeconomy: an overview of world biomass production and utilization. Renew. Sustain. Energy Rev.
139:110691. doi: 10.1016/j.rser.2020.110691
Asghar HN, Zahir, ZA, Arshad, M, et al. (2004). Screening rhizobacteria for improving the growth, yield, and oil content of canola (Brassica napus L.).Aust. J. Agric. Res. 55(2), 187-194. https://doi.org/10.1071/AR03112
Baris, O, Sahin, F, Turan, M, et al (2014). Use of plant-growth-promoting rhizobacteria (PGPR) seed inoculation as alternative fertilizer inputs in wheat and barley production.
Commun Soil Sci Plant Anal. 45(18) 2457-2467. https://doi.org/10.1080/00103624.2014.912296
Begovic, L, Ravlic, J, Lepedus, H, et al. (2015). The pattern of lignin deposition in the cell walls of internodes during barley (Hordeum vulgare L.) development. Acta Biol Crac Ser Bot. 57(2). https://doi.org/10.1515/abcsb-2015-0017.
Behall, KM, Scholfield, DJ, Hallfrisch, J, et al. (2004). Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. The Am. J. Clin. Nutr. 80(5), 1185-1193 (2004). https://doi.org/10.1093/ajcn/80.5.1185
Belimov, AA, Kojemiakov, AP, Chuvarliyeva, CN, et al. (1995). Interaction between barley and mixed cultures of nitrogen fixing and phosphate-solubilizing bacteria. Plant and soil. 173(1), 29-37 (1995).
Bonawitz, ND and Chapple, C (2013).Can genetic engineering of lignin deposition be accomplished without an unacceptable yield penalty? Curr. Opin. Biotechnol. 24(2) 336-343. https://doi.org/10.1016/j.copbio.2012.11.004
Cakmakci, R, Dönmez, MF, Erdoğan, Ü, et al. (2007). The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turkish Journal of Agriculture and Forestry 31(3) 189-199.
Choudhry, AU. (2005). Higher value organics. Pakistan and Gulf Economist 24(15) 35-38.
Dobbelaere, S, Vanderleyden J, Okon, Y,et al. (2003).Plant growth-promoting effects of diazotrophs in the rhizosphere.Crit Rev Plant Sci. 22(2) 107-149. https://doi.org/10.1080/713610853
Elkoca, E, Turan, M, Donmez, MF, et al. (2010). Effects of single, dual and triple inoculations with Bacillus subtilis, Bacillus megaterium and Rhizobium leguminosarum bv. Phaseoli on nodulation, nutrient uptake, yield and yield parameters of common bean (Phaseolu vulgaris L. cv.‘elkoca-05’). J. Plant Nutr. 33(14), 2104-2119 https://doi.org/10.1080/01904167.2010.519084
Ghanbari, A, Babaeian, M, Esmaeilian, Y, et al. (2012).The effect of cattle manure and chemical fertilizer on yield and yield component of barley (Hordeum vulgareL).Afr. J. Agric. Res.. 7(3) 504-508https://doi.org/10.5897/AJAR11.1133
Gholami, A, Shahsavani, S, Nezarat, S, et al. (2009).The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. World Academy of Science Engineering and Technology 49 19-24.
Hai, L, Guo, H, Xiao, S, et al. (2005). Quantitative trait loci (QTL) of stem strength and related traits in a doubled-haploid population of what (Triticum aestivum L.). Euphytica141(1) 1-9 (2005). https://doi.org/10.1007/s10681-005-4713-2
Harwood, WA. (2009). An introduction to barley: the crop and the model. In Barley (pp. 1-5). Humana Press, New York NY. https://doi.org/ 10.1007/978-1-4939-8944-7-1
Hussain, A, Ahmad, M, Nafees, M, et al. (2020). Plant-growth-promoting Bacillus and Paenibacillus species improve the nutritional status of Triticum aestivum L. PLoS. One 15(12) e0241130. (2020).https://doi.org/10.1371/journal.pone.0241130
Imran, A, Mirza, MS, Shah, TM, et al. (2015). Differential response of kabuli and desi chickpea genotypes toward inoculation with PGPR in different soils. Front. Microbiol.6859.https://doi.org/10.3389/fmicb.2015.00859
Javed, S, Javaid, A, Hanif, U, et al. (2021). Effect of necrotrophic fungus and PGPR on the comparative histochemistry of Vigna radiata by using multiple microscopic techniques. Microscopy Research and Technique, 84(11), 2737-2748.
Jayamohan, NS and Kumudini, BS (2011). Host pathogen interaction at the plant cell wall. International Research Journal of Pharmacy and Pharmacology1, 242-249.
Jensen, WA. (1962). Botanical Histochemistry principles and practices. London : W.H Freeman and Co.
Jha, Y. (2019). Higher induction of defense enzymes and cell wall reinforcement in maize by root associated bacteria for better protection against Aspergillus niger. J.Plant Prot. Res. 341-349. https://doi.org/10.24425/jppr.2019.129746
Jones, L, Ennos, AR, Turner, SR, et al. (2001).Cloning and characterization of irregular xylem4 (irx4): a severely lignin‐deficient mutant of Arabidopsis. The Plant J. 26(2) 205-16.https://doi.org/10.1046/j.1365-313x.2001.01021.x
Kaymak, HC. (2010). Potential of PGPR in agricultural innovations. Plant growth and health promoting bacteria. 45-79.https://doi.org/10.1007/978-3-642-13612-2_3
Khan, N, Bano, A, Ali, S and Babar, M. A (2020). Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 90, 189–203. doi: 10.1007/s10725-020-00571-x
Kloepper, JW and Beauchamp, CJ (1992).A review of issues related to measuring colonization of plant roots by bacteria.Canadian journal of Microbiology 38(12), 1219-1232. https://doi.org/10.1139/m92-202
Kumar, A, Maurya, BR and Raghuwanshi, R (2014). Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.).Biocatalysis and Agricultural Biotechnology.3(4), 121-128. https://doi.org/10.1016/j.bcab.2014.08.003
Lalić, A, Kovačević, J, Novoselović, D, et al. (2005).Effects of selection for short stem on yield and yield components in barley. Poljoprivreda11(2), 5-11.
Liu, Q, Luo, L, Zheng, L, et al. (2018). Lignins: biosynthesis and biological functions in plants. International journal of molecular sciences19(2), 335 .https://doi.org/10.3390/ijms19020335
Ma, QH (2009).The expression of caffeic acid 3-O-methyltransferase in two whea genotypes differing in lodging resistance.Journal of Experimental Botany 60(9), 2763-2771. https://doi.org/10.1093/jxb/erp132
Naeem, M, Aslam, Z, Khaliq, A, et al. (2018). Plant growth promoting rhizobacteria reduce aphid population and enhance the productivity of bread wheat.Brazilian journal of microbiology 49 9-14(2018). https://doi.org/10.1016/j.bjm.2017.10.005
Nayak, KK, Parkhey, P, Sahu, R, et al. (2020). Analysis of Lignin Using Qualitative and Quantitative Methods. Lignin Biosynthesis and Transformation for Industrial Applications Springer Nature Switzerland AG. 115-138.
O’connell, PF (1992).Sustainable agriculture a valid alternative.Outlook on Agriculture21(1) 5-12.https://doi.org/10.1177/003072709202100103
Ozturk, A, Caglar, O, Sahin, F, et al. (2003). Yield response of wheat and barley to inoculation of plant growth promoting rhizobacteria at various levels of nitrogen fertilization. Journal of Plant Nutrition and Soil Science 166(2) 262-266. https://doi.org/10.1002/jpln.200390038
Pal, SS (1998).Interactions of an acid tolerant strain of phosphate solubilizing bacteria with a few acid tolerant crops. Plant and soil198(2), 169-177.
Agustono, T, Widiatmoko, T, W1idjonarko, BR, et al. (2019). The Effect of Plant Growth Promotion Rhizobacteria Inoculation To Agronomic Traits of Aromatic Rice (Oryza sativa CV. Inpago Unsoed 1). In IOP Conference Series:Environmental Earth Sciences. (Vol. 255, No. 1, p. 012023). IOP Publishing. April. doi:10.1088/1755-1315/255/1/012023
Sharma, AK (2003). Biofertilizers for Sustainable Agriculture. Agrobios. India. Shetty. S., Singhal KS and Kulkaria PR Antimicrobial properties of cumin.Journal of Microbiology and Biotechnology10, 230-233.
Shaharoona, B, Jamro, GM, Zahir, ZA, et al. (2007). Effectiveness of various Pseudomonas spp. and Burkholderia caryophylli containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.).Journal of Microbiology and Biotechnology17(8), 1300-1307.
Shahzad, MA, Sahi, ST, Khan, MM, et al. (2007). Effect of sowing date and seed treatment on grain yield and quality of wheat.Pakistan Journal of Agricultural Sciences (Pakistan).
Shirinzadeh, A, Soleimanzadeh, H, Shirinzadeh, Z, et al. (2013). Effect of seed priming with plant growth promoting rhizobacteria (PGPR) on agronomic traits and yield of barley cultivars. World applied science journal 21(5) 727-731. https://doi.org/10.5829/idosi.wasj.2013.21.5.1749
Singh, A, Sarma, BK, Upadhyay, RS, et al. (2013). Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities.Microbiological Research 168(1), 33-40.https://doi.org/10.1016/j.micres.2012.07.001
Tigabu, R and Asfaw, F (2016). Effects of seed rate and row spacing on yield and yield components of bread wheat (Triticum aestivum L.) in Dalbo Awtaru Woreda, Wolaita zone, southern Ethiopia.Journal of Biology, Agriculture and Healthcare6(7), 58-67.
Tiwari, VN, Lehri, LK, Pathak, AN, et al. (1989).Effect of inoculating crops with phospho-microbes. Experimental agriculture25(1), 47-50.https://doi.org/10.1017/S0014479700016434
Turan, M, Gulluce, M, Cakmakci, R, et al. (2010). The effect of PGPR strain on wheat yield and quality parameters. In Proceedings of the 19th World Congress of Soil Science: Soil solutions for a changing world, Brisbane, Australia (pp. 209-212).
Viejobueno, J, Albornoz, PL, Camacho, M, et al. (2021). Protection of Strawberry plants against charcoal rot disease (Macrophomina phaseolina) Induced by Azospirillum brasilense. Agronomy 11(2) 195. https://doi.org/10.3390/agronomy11020195
Vogel, J (2008). Unique aspects of the grass cell wall. Current opinion in plant biology 11(3) 301-307. https://doi.org/10.1016/j.pbi.2008.03.002
Whitfield, C (1988). Bacterial extracellular polysaccharides.Canadian Journal of Microbiology 34(4) 415-420 (1988)https://doi.org/10.1139/m88-073


