1Department Of Plant Pathology And Agricultural Microbiology, Post Graduate Institute, Mahatma Phule Krishi Vidyapeeth Rahuri -413722, Ahmednagar, Maharashtra, India.
2Department Of Agricultural Biochemistry, Post Graduate Institute, Mahatma Phule Krishi Vidyapeeth Rahuri -413722, Ahmednagar, Maharashtra, India.
3Department Of Soil Science And Agricultural Chemistry, Post Graduate Institute, Mahatma Phule Krishi Vidyapeeth Rahuri -413722, Ahmednagar, Maharashtra, India.
Corresponding author Email: Krishna_pagare@rediffmail.com
Article Publishing History
Received: 25/03/2019
Accepted After Revision: 17/06/2019
A field experiment was conducted with a view to see the effect of inoculation of salt tolerant rhizobium on nodulation and leghaemoglobin content of soybean at Post Graduate Institute, Mahatma Phule Krishi Vidyapeeth, Rahuri (M.S) during the year 2015-2017. Legumes and the process of nodule initiation are both more sensitive to salinity than are rhizobia Both N2 fixation activity and nodule respiration were inhibited sharply on exposure of plants to saline condition. The decrease in N2 fixation has been ascribed to direct effect on nitrogenase activity or an indirect effect to decrease in leghaemoglobin content, respiratory rate, malate concentration, nodules and altered their ultrastructure. Treatment T7 (Liquid consortium + 100 % N) was singnificantly superior in number of effective nodules over rest of treatments however it was at par with treatment T6 (Liquid consortium + 75 % N). It was also found that treatment T7 (Liquid consortium + 100 % N) significantly superior in number of non- effective nodules over rest of the treatments however it was at par with treatment T6 (Liquid consortium + 75 % N). The treatment absolute control recorded the least number of effective and highest number of non-effective nodules during flowering and harvesting stages respectively. The treatment T7 (Liquid consortium + 100 % N) was significantly superior in Leghaemoglobin content of nodule over rest of all the treatments and it was at par with treatment T6 (Liquid consortium + 75 % N). The treatment absolute control recorded the least in Leghaemoglobin content of nodule at 45 and 60 days. So there need to isolate strains and inoculation of salt tolerant Rhizobium which will enhance the nodule formation in legume crops.
Soybean, Leghaemoglobin content, salt tolerant Rhizobium, Nodulation
Pagare K. A, Navale A. M, Naik R. M, Durgude A. G. Effect of Inoculation of Salt Tolerant Rhizobium on Nodulation and Leghaemoglobin Content of Soybean. Biosc.Biotech.Res.Comm. 2019;12(2).
Pagare K. A, Navale A. M, Naik R. M, Durgude A. G. Effect of Inoculation of Salt Tolerant Rhizobium on Nodulation and Leghaemoglobin Content of Soybean. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2MwhBBf
Copyright © Pagare et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
The decrease in N2 fixation activity has been ascribed to a direct effect on nitrogenase (Burns et al, 1985) or an indirect effect through decreases in leghaemoglobin content, respiration rate and malate concentration in nodules (Delgado et al, 1993, 1994). Legumes and the process of nodule initiation are both more sensitive to osmotic stress than are rhizobia (Russell, 1976; Tu, 1981; Velagaleti et al., 1990). Salt tolerant rhizobia might have the potential to improve yield of legumes under salinity stress (EI-Mokadem et al., 1991). Numerous studies have shown that soil salinity decreased rhizobial colonization and nodulation and dramatically reduced N2 fixation and nitrogenase activity of nodulated legumes (Elsheikh and Wood, 1995; Zahran, 1999). The osmotic environment within the rhizosphere may affect root colonization, infection thread development, nodule development, and the formation of effective N2-fixing nodules (Miller and Wood, 1996). Rhizobia induce the formation of nodules on the roots of legume plants, in which atmospheric nitrogen is fixed and supplied to the host plant, thereby enhancing growth under nitrogen- limiting conditions. The symbiotic interaction between rhizobia and their cognate leguminous plants is important for agricultural productivity. However, physiological stresses, such as salinity, negatively affect these symbiotic interactions by limiting nitrogen fixation (Zahran, 1999). An efficient Rhizobium-legume symbiosis under salt stress required also the selection of salt-tolerant rhizobia (Zahran, 1999). Nodulation and nodule dry weight was promoted markedly by inoculation with Rhizobium triolii in Berseem crop and depressed significantly with consistent increase in salinity (Hussain et al., 2002). There is now increasing evidence that the use of beneficial microbes could enhance the resistance of plants to adverse environmental stresses, e.g., drought, salts, nutrient deficiency, and heavy metal contaminations (Glick et al., 2007). An increase of tolerance to salinity of rhizobial bacteria might constitute another approach to improve plant productivity under symbiosis (Kenenil et al., 2010).
Inoculation with RhM11 improved plant and nodule growth compared with those inoculated with RhM14 and CIAT 899 under saline condition in some common bean (Faghire et al. 2011). An alternate strategy to improve crop plants for salt tolerance is to introduce salt-tolerant plant growth promoting rhizobacteria (PGPR) that enhance crop growth in saline soil. It is suggested that root-colonizing bacteria that produce phytohormones may stimulate plant growth and help in nutrient recycling in the rhizosphere microcosm and thus the microbes can alleviate the effects of salinity in the environment. The evaluation of saline – tolerant bacterial strains to stimulate saline tolerance and promote growth of crop plants leading to better productivity in saline soil (Vivekanandan et al, 2015).
Therefore, inoculation of the salt tolerant Rhizobium under conditions of slat stress may help in nodule formation promote biological nitrogen fixation as well as leghaemoglobin content leguminous crops.
Material and Methods
Total 40 root nodules samples along with rhizosphere soil samples with intact root nodules of soybean plants were collected from saline tract of five districts of Western Maharashtra viz., Kolhapur, Sangali, Satara, Solapur and Ahmednagar to isolate salt tolerant Rhizobium. Total of 33 salt tolerant Rhizobium isolates were obtained from the root nodules of soybean grown in saline soils of Western Maharashtra. Isolation of Rhizobium from root nodule was done by the method of Samosegaran and Hoben (1985).The reference salt tolerant Rhizobium strain was used for comparison. To confirm the salt tolerance of Rhizobium isolates, they were tested against different concentrations of NaCl salt. For this, YEMA medium supplemented with 0.075, 0.15, 0.3, 0.6, 1.2, 1.8, 2.1, 2.4, 3.0, 3.6, 4.2, 4.8, 5.4, 6.0, 7.2, 8.4, 9.6 and 10.8 per cent NaCl. Out of 33 salt tolerant isolates, 20 isolates were categorized under extremely salt tolerance, (more than 5.4 % salt tolerance limit) only three efficient salt tolerant Rhizobium (STR-4. STR-14, STR-18) were selected to develop the liquid consortium. Classification of salt tolerant Rhizobium was done on the basis of salt tolerance limit (Cardoso et al., 2014).
Using liquid consortium of salt tolerant Rhizobium in comparison with reference strain liquid formulation obtained from liquid biofertilizers production unit, Department of Plant Pathology and Agricultural Microbiology, M.P.K.V., Rahuri to study their performance on nodulation and leghaemoglobin content of soybean as detailed below. The land selection for experimental purpose was ploughed once and two harrowing were given. The farm yard manure (FYM) @ 10 ton ha-1 was uniformly spread all over the land before preparatory tillage operation. The soil was brought to fine tilth condition. The experiment was carried out in kharif -2016 in Randomized Block Design with three replications and eight treatments as given in (Table No. 1). The gross and net plot size were 1.80 x 3.0 m2 and 1.20 x 2.60 m2 respectively. The initial chemical properties of soil in terms of salinity, pH (1:2.5) and EC (dSm-1) was 8.24 and 2.74 respectively.
The seeds of soybean (JS-335) were treated with consortium of salt tolerant Rhizobium @ 25 ml/kg of seeds at the time of sowing seed were dried in shade for 30 minutes and were sown in lines with spacing 30 cm x10 cm and 1.5 cm deep) in each plot. Salt tolerant Rhizobium consortium was applied to the seeds and through drenching @ 3.0 lit/ha @ 108 cfu/ml after 45 days of sowing as per the application of N. 50: 75:00 N:P2O5:K2O (kg ha-1 ) were used to supply N, P2O5 and K2O also initial chemical and biological properties of soil were studied.
Table 1: Treatment details
| Treatment
No. |
Treatment details |
| T1 | Absolute control |
| T2 | Reference strain + 50 % N |
| T3 | Reference strain + 75 % N |
| T4 | Reference strain + 100 % N |
| T5 | Liquid consortium + 50 % N |
| T6 | Liquid consortium +75 % N |
| T7 | Liquid consortium+100 % N |
| T8 | Only 100 % N |
T- Treatment
N- Nitrogen dose
The observations on nodulation were recorded at the flowering and harvesting stage. Before uprooting plants, light irrigation was given to plot so that it became easy for uprooting. The root system was dipped in water for removal of soil adhered to roots and then washed with water. The nodulation count for effective and non-effective nodules was taken for randomly selected five plants and then average figure was taken. The observations on lehaemoglobin content were recorded at 45 days and 60 days after sowing.
Colorimetric estimation of Leghaemoglobin as pyridine haemochromogen
Leghaemoglobin content in the nodules of soybean was determined by using pyridine reagent as described by Hartree (1957). The standard working solution af haemin (1 mg haemin per ml solution) was prepared. The standard solution was pipette out corresponding to (0, 0.1, 0.2, 0.3….1.0 mg) concentrations. The colour was developed and the absorbance was measured at 500 nm as above. The calibration curve of optical density (O.D.) against mg of haemin was plotted.
 |
Figure 1: Calibration of a standard curve for leghaemoglobin (haemin) determination |
The statistical analysis of data was carried out by employing Randomized Block Design (RBD). The critical differences were calculated at P = 0.05 level of significance for the in-vivo experiment. Wherever F test were significant and interpretation of the results was carried out in accordance by Panse and Sukhatme (1967).
Results and Discussion
Number of effective and non-effective nodule
It is revealed from the data that, the mean number effective nodules of soybean decreased with increase in the age of crop up to harvesting stage and the mean number non- effective nodules of soybean increased with increase in the age of crop up to harvesting stage. Effect of application of salt tolerant Rhizobium consortium on effective and non-effective nodules was significant at all intervals.
At flowering
From the data presented in (Table 2 and Fig. 2) it was found that treatment T7 (Liquid consortium + 100 % N) was singnificantly superior in number of effective nodules (25.25) over rest of treatments however it was at par with treatment T6 (Liquid consortium + 75 % N) (24.70). It was also found that treatment T7 (Liquid consortium + 100 % N) significantly superior in number of non- effective nodules (7.52) over rest of the treatments however it was at par with treatment T6 (Liquid consortium + 75 % N) (8.75). The treatment absolute control recorded the least number of effective and highest number of non-effective nodules (15.88) and (16.60) respectively.
Table 2: Effect of liquid consortium of salt tolerant Rhizobium on number of effective and non effective nodules per plant of soybean at flowering and harvesting stages
| Treatment | Number of effective nodules/plant | Number of non- effective nodules/plant | |||
| At flowering | At
harvesting |
At flowering | At
Harvesting |
||
| T1 | Absolute control | 15.88 | 5.79 | 16.60 | 21.80 |
| T2 | Reference strain + 50 % N | 17.74 | 8.20 | 15.12 | 19.17 |
| T3 | Reference strain + 75 % N | 21.64 | 11.33 | 11.55 | 15.47 |
| T4 | Reference strain + 100 % N | 23.32 | 12.24 | 10.76 | 14.21 |
| T5 | Liquid consortium + 50 % N | 18.49 | 9.12 | 13.87 | 18.33 |
| T6 | Liquid consortium + 75 % N | 24.70 | 14.93 | 8.75 | 12.20 |
| T7 | Liquid consortium + 100% N | 25.25 | 15.24 | 7.52 | 11.55 |
| T8 | Only 100 % N | 19.25 | 10.53 | 12.61 | 16.70 |
| S.E. + | 0.18 | 0.10 | 0.41 | 0.21 | |
| CD at 5 % | 0.55 | 0.32 | 1.25 | 0.65 | |
At harvesting
The treatment (Table 2 and Fig. 2) T7 (Liquid consortium +100 % N) was significantly superior in number of effective nodules (15.24) over rest of treatments however it was at par treatment T6 (Liquid consortium + 75 % N) (14.93). Also it was found that treatment T7 (Liquid consortium + 100 % N) significantly superior in number of non- effective nodules (7.52) over rest of treatments however it was at par treatment T6 (Liquid consortium + 75 % N) (12.20). The treatment absolute control recorded the least number of effective and highest number non-effective nodule (5.79) and (21.80) respectively.
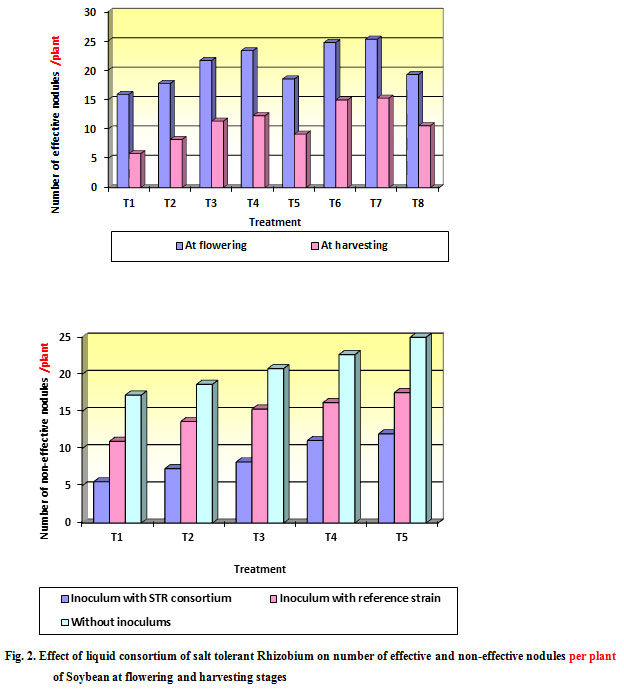 |
Figure 2: Effect of liquid consortium of salt tolerant Rhizobium on number of effective and non-effective nodules per plant of Soybean at flowering and harvesting stages
|
Effect of liquid consortium of salt tolerant Rhizobium on Lehaemoglobin content of nodule
The data (Table 3 and Fig. 3) showed that, the mean Leghaemoglobin content of soybean nodule increased with increase in the age of crop. Effect of application of salt tolerant Rhizobium consortium on Leghaemoglobin content was significant at all intervals.
Table 3: Effect of liquid consortium of salt tolerant Rhizobium on Leghaemoglobin content of soybean at 45 and 60 days
| Treatment | Leghaemoglobin content
(mg g-1 fresh weight of nodule) |
||
| At 45 days | At 60 days | ||
| T1 | Absolute control | 0.184 | 0.202 |
| T2 | Reference strain + 50 % N | 0.204 | 0.224 |
| T3 | Reference strain + 75 % N | 0.251 | 0.276 |
| T4 | Reference strain + 100 % N | 0.269 | 0.293 |
| T5 | Liquid consortium + 50 % N | 0.219 | 0.243 |
| T6 | Liquid consortium + 75 % N | 0.276 | 0.305 |
| T7 | Liquid consortium + 100% N | 0.290 | 0.321 |
| T8 | Only 100 % N | 0.235 | 0.257 |
| S.E. + | 0.001 | 0.001 | |
| CD at 5 % | 0.004 | 0.003 | |
The treatment T7 (Liquid consortium + 100 % N) which was significantly superior in Leghaemoglobin content of nodule (0.290 mg g-1 fresh of nodule weight) over rest of all the treatments and it was at par with treatment T6 (Liquid consortium + 75 % N) (0.276 mg g-1 fresh of nodule weight). The treatment absolute control recorded the least in Leghaemoglobin content of nodule (0.184 mg g-1 fresh of nodule weight).
 |
Figure 3: Effect of liquid consortium of salt tolerant Rhizobium on fresh weight of shoot and root of soybean at flowering and harvesting stages |
The treatment T7 (Liquid consortium + 100 % N) was significantly superior in Leghaemoglobin content of nodule (0.321 mg g-1 fresh of nodule weight) over rest of all the treatments and it was at par with treatment T6 (Liquid consortium + 75 % N) (0.305 mg g-1 fresh of nodule weight). The treatment absolute control recorded the least in Leghaemoglobin content of nodule (0.202 mg g-1 fresh of nodule weight).
Similarly, Hussain et al. (2002) reported that nodule formation inferred that mean number of nodules decreases significantly with an increase in salinity level of soil and increased significantly by seed inoculation with Rhizobium. Inoculation of seed increased the nodule formation significantly at control, 12 dS m-1 and 16 dS m-1 salinity levels, but at 8 dS m-1 increase in number of nodules per pot is non-significant statistically. Similarly, Adewusi et al., (2008) reported that rhizobial inoculation increased nodule biomass thus encouraged sustainable environmental friendly agriculture by responding perfectly in biological nitrogen fixation. Similarly Faghire et al. (2011) showed that in controls, inoculation with RhM11 improved plant and nodule growth compared with those inoculated with RhM14 and CIAT 899. NaCl treatment generally had a negative affect on plant and nodule growth. Under The nodular phosphoenolpyruvate carboxylase (PEPC) and malate dehydrogenase (MDH) exhibited higher activities and were less affected by salinity in plants inoculated with the reference strain CIAT899 than those inoculated with local strains and concluded that plants inoculated with CIAT899 and RhM11 showed more salinity stress tolerance than those inoculated with RhM14.
The results are in line with, Rejili et al. (2012) reported that on the selection and characterization of salt-tolerant Rhizobia strains, able to fix nitrogen symbiotically under salt conditions, might constitute a strategy for improving legume symbiosis in adverse conditions and might constitute a better economic and sustainable alternative to chemical fertilization. Similarly, Sharma et al. (2013) reported that on the salinity tolerance of naturally occurring rhizobia, isolated from the root nodules of three leguminous plants, viz., sesbania (Sesbania sesban), lablab (Lablab purpureus) and pigeonpea (Cajanus cajan), growing at research farm in Dubai (United Arab Emirates). The Rhizobial isolates were also found to be effective in nodulating 21-day old seedlings grown in potting soil and irrigated with saline water up to 12 dSm-1 after inoculation. The tolerance to high levels of salinity and the survival and persistence in severe and harsh desert conditions made these Rhizobia highly valuable inoculum to improve productivity of the leguminous plants cultivated under extreme environments.
Present finding correlates, Vishal et al. (2013) reported that the inoculation with Rhizobium culture had invariably and significantly promoted nodulation and leghaemoglobin content at both durations particularly at lower EC levels and minimized the deleterious effect of salinity at 10 to 14 dSm-1.Salinity is considered a limiting factor in nodulation and leghaemoglobin content in legume-Rhizobium associations, which can adversely affect the yield of legume crops. Rhizobia can tolerate high concentrations unlike legume plants. Therefore, in saline soils, the multiplication of these Rhizobium strains will not be affected in the rhizosphere of the plant host. So, there is currently need isolation and development salt tolerant Rhizobium strains would enhance the plant growth through nodulation and leghaemoglobin content of plants under saline conditions which indirectly increase the nitrogen fixing ability of legume crop.
References
Adewusi, H.G., Bada, S.O., Ladipo, D.O. and Husain, T. (2008). Nodulation in Millettia thonningii (Schum and Thonn.) Baker; native Rhizobia and seed interaction from southwest Nigeria. Pak. J. Bot., 40(5): 2237-2242.
Burns A, Watt GD, Wang ZC. (1985). Salt inhibition of nitrogenase catalysis and salt effects on the separate protein components. Biochemistry 24, 3932-6.
Cardoso P., Freitas R. and Figueira E. (2015). Salt tolerance of rhizobial populations from contrasting environmental conditions: understanding the implications of climate change. Ecotoxicology
Delgado MJ, Garrido JM, Ligero F, Lluch C. (1993). Nitrogen fixation and carbon metabolism by nodules and bacteroids of pea plants under sodium chloride stress. Physiologia Plantarum 89, 824-9
Delgado MJ, Ligero F, Lluch C. (1994). Effect of salt stress on growth and nitrogen fixation by pea, Faba-bean, common bean and soybean plants. Soil Biology and Biochemistry 26, 371-6.
El-Mokadem, M.T., Helemish, F.A. and Abdel-Wahab, S.M. (1991). Salt response of clover and alfalfa inoculated with salt tolerant strains of Rhizobium. Shams Sci. Bull., 28 B : 441-468.
El-Sheikh, E.A.E. and Wood, M. (1995). Nodulation and nitrogen fixation by soybean inoculated with salt-tolerant rhizobia or salt- sensitive, Brady rhizobia in saline soil. Soil Biol. Biochem., 27 : 657–661.
Faghire M, Bargaz A, Farissi M, Palma F, Mandri B, Lluch C, N.A. Tejera García, J.A. Herrera-Cervera, K. Oufdou and C. Ghoulam (2011). Effect of salinity on nodulation, nitrogen fixation and growth of common bean (Phaseolus vulgaris) inoculated with rhizobial strains isolated from the Haouz region of Morocco. Symbiosis 55 (2): 69-75.
Glick, B.R., Cheng, Z., Czarny, J. and Duan, J. (2007). Promotion of plant growth by ACC deaminase producing soil bacteria. Eur. J. Plant 119 : 329–339.
Hartree, E.F. (1957). Haematin compounds. In Modern Methods of Plant Analysis, Edts. K. Peach and M.V. Tracey, Springer-Verlag Publication.
N, Mujeeb. F, Tahir. M., Khan, G.D., Hassan, N.M. and Abdul Bari. (2002). Effectiveness of Rhizobium under salinity stress. Asian Journal of Plant Sciences. 1(1) : 12-14.
Kenenil, A., F. Assefa and P.C. Prabu, (2010). Characterization of acid and salt tolerant rhizobial strains isolated from faba bean fields of Wollo, Northern Ethiopia. J. Agric. Sci. Technol., 12: 365-376.
Miller, K. J., and J. M. Wood. (1996). Osmoadaptation by rhizosphere bacteria. Annu. Rev. Microbiol. 50:101–136.
Panse, V.G. and Sukhatme, P.V. (1967). Statistical methods for agricultural workers. IInd ICAR, New Delhi. pp. 135-136.
Rejili, M., Mahdhi, M. and Fterich, A. (2012). Symbiotic nitrogen fixation of wild legumes in Tunisia: soil fertility dynamics, field nodulation and nodules effectiveness. Agric. Ecosyst. Environ. 157 : 60–69.
Russell, J.S. (1976). Comparative salt tolerance of some tropical and temperate legumes and tropical grasses. Austr. J. Exp. Agric. Anim. Husbandry. 16 : 103-109.
Samosegaran, P. and Hoben, H.J. (1985). Handbook for rhizobia : Methods in Legume Rhizobium Berlin Hidelberg New York, Springer.
Sharma, S.R., Rao, N.K., Gokhale, T.S. and Ismail, S. (2013). Isolation and Characterization of Salt-Tolerant Rhizobia Native to the Desert Soils of United Arab Emirates. Emirates Journal of Food and Agriculture, 25 : 102-108.
Tu, J.C. (1981). Effect of Salinity on Rhizobium- root- hair interaction, nodulation and growth of Soybean. Can. J. Plant Science. 61 : 231-239.
Velagaleti, R.R., Marsh, S. and Kramer, D. (1990). Genotypic differences in growth and nitrogen fixation among soybean (Glycine max (L.) Merr.) cultivars grown under salt stress. Trop. Agric. 67 : 169-177.
Vishal Kumar Deshwal, Shipra Agarwal and Zubair Ahmad. (2013). Comparative Study of Berseem Cultivars (Trifolium alexandrium ) In Support of Nodulation and Leghaemoglobin content under Saline Conditions, International Journal of Applied Biology and Pharmaceutical Technology. 4 : 227-234.
Vivekanandan, M., Karthik, R. and Leela, A. (2015). Improvement of crop productivity in saline soils through application of saline-tolerant rhizosphere bacteria-Current Perspective. International Journal of Advanced Research. 3(7) : 1273-1283.
Zahran, H. H. (1999). Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev.63:968–989.


