1Department of Biological Sciences, Faculty of Sciences, Sana’a University, Yemen.
2Department of Arid Land Agriculture, Collage of Meteorology, Environment and Arid Land Agriculture – King Abdulaziz University, Saudi Arabia,
3Department of Biological Sciences, Faculty of Sciences, King Abdulaziz University,Saudi Arabia,
4Department of Biological Sciences, Faculty of Sciences, University of Bisha, Bisha 61922, P. O. Box 551- Saudi Arabia and
5Department of Biological Sciences, Faculty of Sciences, Thamar University- Yemen.
Corresponding author email: abbasazab2000@gmail.com
Article Publishing History
Received: 09/01/2020
Accepted After Revision: 27/02/2020
Aedes aegypti is the vector mosquito of dengue fever, which is an endemic disease in Saudi Arabia, Jeddah. The use of conventional insecticides in water sources introduces risks to people and/or the environment. In this study, mosquitocidal activity and adult development inhibition were investigated using four medicinal plants (Rhazya stricta, Lantana camara, R. chalepensis, and Punica granatum) against Ae. aegypti under laboratory conditions.A laboratory bioassay was conducted to assess larvicidal activity of leaves and peels crude acetonic extract of L. camara, Ruta chalepensis, Rh. stricta and P. granatum. Under controlled laboratory conditions, late 3rd or early 4th instar larvae were exposed to various concentrations – ranging from 150ppm to 1000 ppm – of the extracts to obtain the median lethal concentration (LC50) values for each plant extract tested.Mortalities were observed to increase with the increase in concentrations. Acetone extract of Rh. Stricta revealed high activity against Ae. aegypti compared to the rest of the extracts. The larval mortality rates of mosquito larvae ranged from 25 to 97% for Rh. stricta, 23 to 94% for L. camara, 17 to 96% for R. chalepensis, and 10 to 72% for P. granatum. In terms of effects on adult emergence, the inhibition percentage of the mosquito ranged from 26 to 100% for Rh. stricta, 13 to 100% for L. camara, 10 to 100% for R. chalepensis, and 22 to 92% for P. granatum. The evaluated medicinal plants seemed to be a better alternative to synthetic insecticides for controlling Ae. aegypti.
Aedes aegypti, Bioassay, Dengue Fever, Lantana camara, Medicinal Plants.
Al-Azab A. M, Zaituon A. A, Al-Ghamdi K. M, Al-Galil F. M. A. Effect of four Indigenous Medicinal Plants on Dengue Fever Vector, Aedes aegypti from Jeddah, Saudi Arabia. Biosc.Biotech.Res.Comm. 2020;13(1).
Al-Azab A. M, Zaituon A. A, Al-Ghamdi K. M, Al-Galil F. M. A. Effect of four Indigenous Medicinal Plants on Dengue Fever Vector, Aedes aegypti from Jeddah, Saudi Arabia. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2RT9MWP
Copyright © Al-Azab et al.,, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Dengue fever is an arthropod-borne viral disease found in many tropical and subtropical countries throughout the world (Gubler, 2018; Hosseini et al., 2018). Aedes aegypti (L) is presently considered the main vector of dengue, yellow fever, Zika, and chikungunya viruses in many parts of the world (Kraemer et al., 2015; Mayer et al., 2017). For many decades, synthetic pesticides have been employed to successfully control the infection cycle. However, the development of insect resistance to synthetic pesticides has also been documented globally, ( Smith et al., 2016, Goindin et al., 2017; David et al., 2018). High operational costs and environmental pollution have created the need for developing alternative approaches for controlling vector-borne disease (Ohia and Ana, 2015). In early historical time, humans used plant extracts and concentrated compounds to control certain pests and disease-causing insects (Pavela, 2016). Overall, the search for such compounds has been directed extensively at the plant kingdom, (Veer and Gopalakrishnan, 2016,; Ninan et al., 2017 Lakshmi et al., 2018).
Medicinal plants are common sources of bioactive compounds, especially in tropical and subtropical countries (Van Wyk and Wink, 2017). The use of phytochemicals is one such strategy that may be suitable for mosquito control. The first, commonly used plant extract against adult mosquitoes was the flower extract of Chrysanthemum cinerariaefolium in South Africa and India, (Maheswaran and Ignacimuthu 2012 and Govindarajan, 2016 ) Many authors across the world started large screening activities for using extracts of medicinal and herbaceous plants to control mosquitoes. Extracts and oils from leaves, flowers, and roots of plants were found to display mosquito larvicidal activity (Chansang et al., 2018; Pavela et al., 2018).
Several researches from all over the world have documented the toxic effect of plant extracts such as L. camara and Rh. stricta and those of essential oils in controlling Ae. aegypti L mosquito (Ghosh et al., 2012; Hari and Mathew, 2018; Kalita et al., 2012; Kumar and Maneemegalai, 2008; Mappau and Ganing, 2018; Muangmoon et al., 2018; Ved et al., 2018). Lantana camara Linn. (Verbenaceae) is a hardy, evergreen, straggling shrub with a characteristic odor; it has antibacterial, antifungal, and insecticidal properties, and its oil provides protection from Aedes mosquitoes (Mossa et al., 1987). R. chalepensis L. (Rutaceae), commonly known as “ruda” is a perennial herb, which is widely distributed in the Mediterranean region and the Kingdom of Saudi Arabia area (Verma, 2018). The toxic effect of the R. chalepensis extract against insects, particularly mosquitoes, have been reported previously, ( Morsy et al., 1998, Al-Myah et al., 2011; Madkour et al., 2012). It is an ancient medicinal plant still used in the traditional medicine of many countries as recently reported by Bedini et al., (2018).
There is a scarcity of literature regarding the insecticidal potential of P. granatum. Its reported larvicidal activity was observed against the instar larvae of Chrysomya albiceps decades ago in Egypt (Sameeh et al., 2010). Moreover, its larvicidal activity was also reported recently against the larvae of Anopheles pharoensis (Eidi et al., 2005). To the best of the author’s knowledge, this is the first study to address the larvicidal properties of P. granatum and Rh. stricta against Ae. aegypti. In this study, mosquitocidal activity and adult development inhibition were investigated using four medicinal plants (Rh. stricta, L. camara, R. chalepensis, and P. granatum) against the dengue fever vector Ae. aegypti under laboratory conditions.
MATERIALS AND METHODS
Mosquito strain source and rearing :Ae. aegypti (Diptera; Culicidae) was obtained from the mosquito laboratory at Al-Amana, Jeddah, Saudi Arabia. The Ae. aegypti (L) larvae were reared under laboratory conditions for over 12 generations using diet media fish food. Larvae from the culture were used for the different treatments described in this study. The stock colony was maintained at room temperature (27±1°C) and relative humidity of 70±5% with a 14:10 (LD) photoperiod throughout the study. The adults were fed on a 10% sugar solution.
Plant collection and extraction: The medicinal plants L. camara, R. chalepensis, Rh. stricta, and P. granatum were collected from the campus of King Abdulaziz University, Jeddah and were identified by an experienced botanist at the department of Botany. Fresh leaves from the plants were washed and shade-dried at room temperature for one week. They were then prepared to extract the effective ingredients according to the published standard method (WHO, 2005). Forty to sixty gm of leave tissues were finely ground and loaded into a 250 ml glass stoppered Soxhlet apparatus. Absolute Acetone (200 ml) was added to the glass and the extraction was performed for 6 hours. The rotary evaporator was used to concentrate the extracts to turn them into a semi-dry material. The extracted components were kept at –10°C until use for testing against selected insect stages.
Bioassay of plant extract against Ae. aegypti (L):The larvicidal bioassay followed the WHO standard protocols (Finney, 1972). The stock solution of each plant extract was prepared by adding 1 ml of re suspended extracts to 99 ml of distilled water containing 0.3% dimethyl sulphoxide (DMSO). From this stock solution, concentrations of 150, 250, 500, 800, and 1000 ppm were prepared in distilled water. Larval treatments were carried out by exposing the late 3rd or early 4th instar larvae continuously to various concentrations of the plant extracts. Tests were performed in groups of 250 ml glass beakers containing 100 ml tap water. Four replicates of 25 larvae for each concentration and control trail were assembled. The larvae were given the usual larval food during these experiments. After 24 hours of exposure time, the percent of mortality was recorded. The cumulative mortalities of larvae and pupae were recorded daily for the tested plants. Live pupae were transferred to untreated water in new beakers for further observation. X Actelic 50% EC (Primiphosmethyle) was tested as a positive control, while acetone was the negative control.
Statistical Analysis: This study used a completely randomized design (CRD) in a factorial experiment. The collected data were statistically analyzed using analysis of variance (ANOVA) tools, and the means were compared by LSD at p ≤ 0.05 using the SAS software program [SAS Institute (2006) version 9.3]. IC50 and IC95 were calculated according to the Probit analysis program ( Finney 1972, Abbott, 1925). The 95% confidence intervals, values, the χ2 goodness of fit test, and regression equations were estimated by a computerized log-probit analysis. The mortalities were corrected for control mortality using Abbott’s formula (Tisgratog et al., 2016).
RESULTS AND DISCUSSION
The screening of herbal bioactive insecticides is a rapidly emerging research field that aims to find potential mosquito control agents with advantages such as low costs, ease of administration, and risk-free properties that can overcome the resistance to synthetic insecticides, and safety to the environment (Remia and Logaswamy, 2010). In the present study, crude leaves / peel extracts of Rh. stricta, L. camara, R. chalepensis and P. granatum showed larvicidal activity against Ae. aegypti when the larvae were exposed to concentrations of the plant extract ranging from 150 to 1000 ppm for 24 hours. The detailed results of the larvicidal activity of, Ae. aegypti larvae are presented in Tables 1 and 2 and depicted graphically in Figs. 1–4.
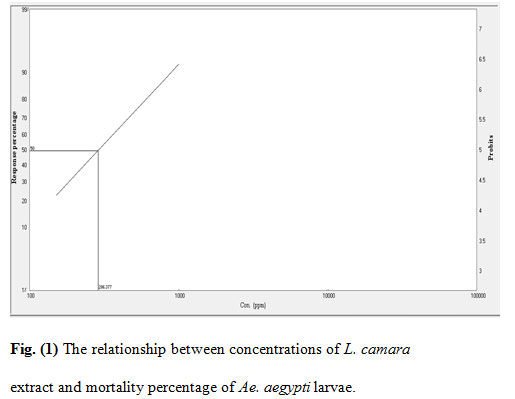
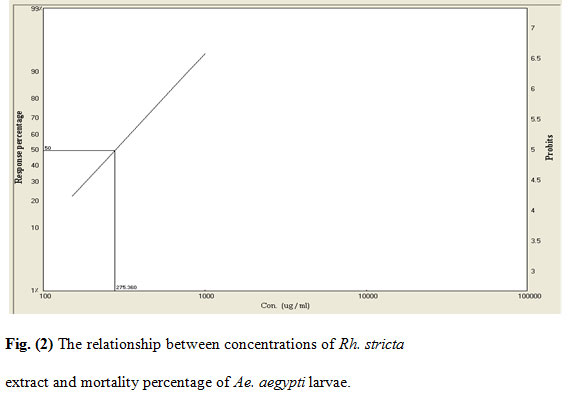
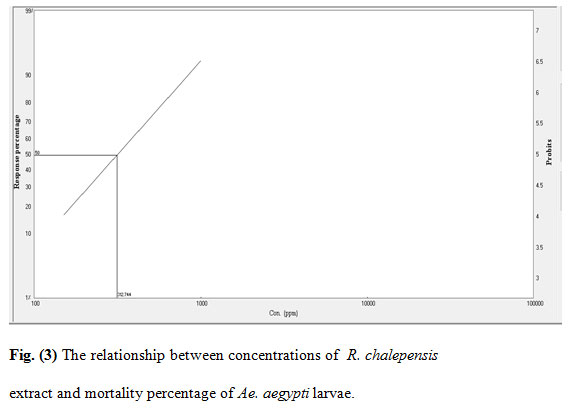
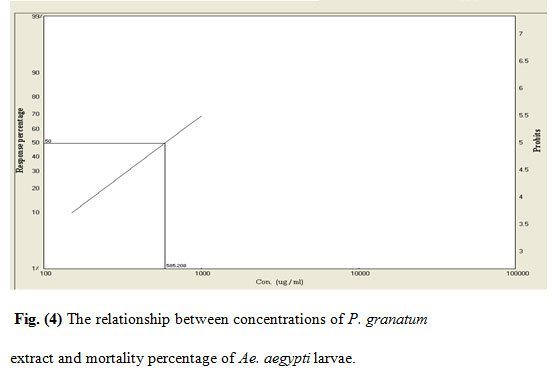
The mortality rates of the larvae exposed to various concentrations of the extracts were 25–97% (Rh. stricta), 23–94% (L. camara), 17–96% (R. chalepensis), and 10–72% (P. granatum). The acetone extract of Rh. stricta showed a significantly high larvicidal activity against Ae. aegypti compared with the other three extracts. The LC50/ LC95 for the four plant values were 275/729, 286/1203, 312/1095 and 585/2050 ppm for Rh. stricta, L. camara, R. chalepensis, and P. granatum respectively.
The findings of the present study are comparable to several earlier studies, ( Remia and Logaswamy, 2010, Ghosh et al., 2012; Mappau and Ganing, 2018). Indeed, Ghosh and associates reported that the LC50 value of the Rh. stricta plant extract against mosquitoes was 251 ppm, while Remia and Logaswamy reported that the highest mortality of acetone extracts of L. camara leaves against Ae aegypti larvae with LC50 value was 230.7 ppm (Ghosh et al., 2012; Remia and Logaswamy, 2010). However, a higher value for LC50 (468.5 ppm) was reported by Indian scholars (Jayaraman et al., 2015). On the other hand, a higher mortality rate (85% at 150 ppm conc.) of Ae. aegypti 4th instar larva was reported in a recent study (Hemalatha et al., 2015).
Currently, the larvicidal mechanisms of phytochemicals are poorly understood. Recently, it has been reported that some alkaloids inhibit in vitro activity of glutathione S-transferase (GST) and acetylcholinesterase (AChE) of Lymantria dispar and the octopaminergic system / receptor in Ae. aegypti (Wang et al., 2019; Zou et al., 2017). However, subsequent studies revealed that protein carbonylation mediates an oxidative damage to the proteins involved in the energy metabolism of Ae. aegypti larvae (Rodríguez-Cavallo et al., 2018). The presence of certain phytochemicals (alkaloids, steroids, and phenolics) in plant extracts might be the reason for their larvicidal activity (Lakshmi et al., 2018).
Unfortunately, scientific literature is unavailable regarding the efficacy of R. chalepensis and P. granatum against Ae. aegypti. However, their effects against other species of mosquitoes have been tested. For instance, P. granatum has been evaluated for its larvicidal activities against Culex gelidus and Culex quinquefasciatus mosquitoes. Methanol extracts showed absolute mortality of the larvae at 500 μg/ml after 24 hours (Kamaraj and Rahuman, 2010). Strikingly, a recent Libyan study found the acetone extract of R. chalepensis’ aerial parts to demonstrate powerful larvicidal activity (LC50 at 1.08 ppm) against Culex pipiens (El-Bokl, 2016).
The cumulative mortalities during larval development to pupae and adults were recorded for the evaluation of the tested plants. The inhibition percentage of adult emergence were 26–100%, 27–100%, 33–100%, and 22–92% for Rh. stricta, L. camara, R. chalepensis, and P. granatum respectively (Table 1). Furthermore, mortality rates were observed at both the pupal stage and incomplete adult emergence. This could be due to morphological aberrations leading to failure of emergence from the exuviae of the pupal stage (Yu et al., 2015). The R. chalepensis extract exhibited the highest inhibition of adult emergence (75%) at LC50 (275 ppm), whereas the Rh. stricta extract showed the lowest activity (48%) at LC50 value (585 ppm) (Table 2).
As shown in Table 2, when compared with earlier studies, these results revealed that the experimental plant extracts were effective in controlling Ae. aegypti and showed promising results (Ghosh et al., 2012; Morsy et al., 1998). The activity of plant extracts is often attributed to the complex mixture of active compounds (Kumar et al., 2012). The high efficacy of Rh. stricta and L. camara extracts may be due to the presence of chemical components including furanonaphthaquinones regioisomers and camaric acid as reported previously (Kamaraj et al., 2011). However, the biological effect of R. chalepensis against mosquitoes might be attributed to its high content of biologically active alkaloids (Sousa and Costa, 2012).
Table 1: Toxicity effect of four plant extracts against different stages of Ae. aegypti.
| Plant extract | Conc.
(ppm) |
Larval
Mortality (%)A |
Pupation
% |
Adult emergence | |
| Total | Inhibition (%)B | ||||
| Rh. stricta | 150 | 25 | 75 | 67 | 26 |
| 250 | 42 | 58 | 50 | 44 | |
| 500 | 77 | 23 | 17 | 80 | |
| 800 | 90 | 10 | 10 | 89 | |
| 1000 | 97 | 3 | 0 | 100 | |
| Control | 8 | 92 | 90 | 10 | |
| L. camara | 150 | 23 | 77 | 69 | 27 |
| 250 | 44 | 66 | 38 | 60 | |
| 500 | 74 | 26 | 23 | 76 | |
| 800 | 86 | 14 | 7 | 93 | |
| 1000 | 94 | 6 | 0 | 100 | |
| Control | 3 | 97 | 95 | 5 | |
| R. chalepensis | 150 | 17 | 83 | 77 | 33 |
| 250 | 39 | 61 | 40 | 60 | |
| 500 | 72 | 28 | 21 | 79 | |
| 800 | 87 | 13 | 8 | 92 | |
| 1000 | 96 | 4 | 0 | 100 | |
| Control | 2 | 98 | 96 | 4 | |
| P. granatum | 150 | 10 | 90 | 72 | 22 |
| 250 | 23 | 77 | 51 | 47 | |
| 500 | 41 | 59 | 36 | 61 | |
| 800 | 60 | 40 | 20 | 78 | |
| 1000 | 72 | 28 | 7 | 92 | |
| Control | 5 | 95 | 92 | 8 | |
| Positive control | 2 | 100 | |||
A Four replicates of 25 larvae each B Corrected with Abbott’s formula [35]
Table 2: Statistical parameters of plant extract against Ae. aegypti.
| Statistical parameters | Rh. stricta | L. camara | R. chalepensis | P. granatum |
| LC50 (ppm) | 275 | 286 | 312 | 585 |
| Inhibition of adult development (%) | 48 | 68 | 75 | 71 |
| 95% (*F. L) | 233–298 | 251–320 | 280–345 | 550–696 |
| LC95 (ppm) | 729 | 1203 | 1095 | 2050 |
| 95% (*F. L) | 648–842 | 990–1560 | 931–1149 | 1628–2873 |
| Slope | 2.8 | 2.63 | 3.02 | 2.43 |
| Calculated (Chi)2 | 1.26 | 0.87 | 1.3 | 1.04 |
| Tabulated (Chi)2 | 7.8 | 7.8 | 7.8 | 7.8 |
| R2 | 0.96 | 0.90 | 0.92 | 0.98 |
Figure 1: The relationship between concentrations of L. camara extract and mortality percentage of Ae. aegypti larvae.
Figure 2: The relationship between concentrations of Rh. stricta extract and mortality percentage of Ae. aegypti larvae.
Figure 3: The relationship between concentrations of R. chalepensis extract and mortality percentage of Ae. aegypti larvae.
Figure 4: The relationship between concentrations of P. granatum extract and mortality percentage of Ae. aegypti larvae.
CONCLUSION
The acetone extract of Rh. stricta, L. camara, R. chalepensis, and P. granatum could comprise other approaches to be utilized for controlling the dengue vector mosquito, Ae. aegypti. These results could encourage the search for new active natural compounds offering a suitable alternative to insecticides synthesized from other medicinal plants; these alternatives would be safer and easier to handle than synthetic insecticides.
Authors’ ContributionsA Al-Azab performed the experiments, analyzed the data, and wrote and revised the manuscript. Both A Zaituon and K Al-Ghamdi conceptualized the research idea designed the study, coordinated the work and wrote and revised the manuscript. F. Abd Al Galil assisted in collecting the literature search, read and revised the final manuscript. All authors read and approved the final manuscript for publication
ACKNOWLEDGMENTS
We gratefully acknowledge the King Abdulaziz City for Science and Technology (KACST) for their financial support under grand No. A-S-10-0013.
Competing interests: The authors declare that they have no competing interests.
REFERENCES
Abbott, W.S., (1925). A Method of Computing the Effectiveness of an Insecticide. Journal of Economic Entomology, 18, 265–267.
AL-Myah, A., Al-Mansour, N., Al-Dhahir, A., (2011). Effect of some plant extracts on the mortality of the larval mosquitoes Culex pipiens molestus Forskal. Basrah Journal of Science, 1, 47–61.
Bedini, S., Flamini, G., Ascrizzi, R., Venturi, F., Ferroni, G., Bader, A., Girardi, J., Conti, B., (2018). Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Scientific Reports, 8. 17857
Chansang, A., Champakaew, D., Junkum, A., Amornlerdpison, D., (2018). Potential of natural essential oils and cinnamaldehyde as insecticides against the dengue vector Aedes aegypti (Diptera: Culicidae). Southeast Asian Journal of Tropical Medicine and Public Health, 1, 6–22.
David, M.R., Garcia, G.A., Valle, D., Maciel-de-Freitas, R., (2018). Insecticide Resistance and Fitness: The Case of Four Aedes aegypti Populations from Different Brazilian Regions. Biomed Research International, 2018, https://doi.org/10.1155/2018/6257860
Eidi, M., Eidi, A. and Zamanizadeh, H., 2005. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. Journal of Ethnopharmacology, 100, 310-313.
El-Bokl, M.M., (2016). Toxicity and bioefficacy of selected plant extracts against the mosquito vector Culex pipiens L. (Diptera: Culicidae). Journal of Entomology and Zoology Studies, 4, 483–488.
Finney DJ (1972) Probit Analysis, 2nd ed. Cambridge University Press, New York.
Ghosh, A., Chowdhury, N., Chandra, G., 2012. Plant extracts as potential mosquito larvicides. Indian Journal of Medical Research, 135, 581.
Goindin, D., Delannay, C., Gelasse, A., Ramdini, C., Gaude, T., Faucon, F., David, J.-P., Gustave, J., Vega-Rua, A., Fouque, F., (2017). Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infectious Diseases and Poverty, 6, 38.
Govindarajan, M., (2016). Mosquito larvicidal potential of medicinal plants. In: Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization. Pp 25–61, Springer India.
Gubler, D.J., (2018). Pandemic yellow fever: a potential threat to global health via travelers. Journal of Travel Medicine, 25.
Hari, I., Mathew, N., (2018). Larvicidal activity of selected plant extracts and their combination against the mosquito vectors Culex quinquefasciatus and Aedes aegypti. Environmental Sciences and Pollution Research, 25, 9176–9185.
Hemalatha, P., Elumalai, D., Janaki, A., Babu, M., Velu, K., Velayutham, K., Kunyil Kaleena, P., (2015). Larvicidal activity of Lantana camara aculeata against three important mosquito species. Journal of Entomology and Zoology Studies, 3, 174-181
Hosseini, S., Oliva-Ramírez, J., Vázquez-Villegas, P., Rodriguez-Garcia, A (2018). Dengue Fever: A Worldwide Threat An Overview of the Infection Process, Environmental Factors for a Global Outbreak, Diagnostic Platforms and Vaccine Developments. Current Topics in Medicinal Chemistry, 18, 1531–1549.
Jayaraman, M., Senthilkumar, A., Venkatesalu, V., (2015). Evaluation of some aromatic plant extracts for mosquito larvicidal potential against Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi. Parasitology Research, 114, 1511–1518.
Kalita, S., Kumar, G., Karthik, L., Rao, K.V.B., (2012). A Review on Medicinal Properties of Lantana camara Linn. Research Journal of Pharmacy and Technology, 6, 711.
Kamaraj, C., Bagavan, A., Elango, G., Zahir, A.A., Rajakumar, G., Marimuthu, S., Santhoshkumar, T., Rahuman, A.A., (2011). Larvicidal activity of medicinal plant extracts against Anopheles subpictus & Culex tritaeniorhynchus. Indian Journal of Medical Research, 134, 101–6.
Kamaraj, C., Rahuman, A.A., (2010). Larvicidal and adulticidal potential of medicinal plant extracts from south India against vectors. Asian Pacific Journal of Tropical Medicine, 3, 948–953.
Kraemer, M.U.G., Sinka, M.E., Duda, K.A., Mylne, (2015). The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife 4, e08347.
Kumar, M., Maneemegalai, S., (2008). Evaluation of Larvicidal Effect of Lantana camara Linn Against Mosquito Species Aedes aegypti and Culex quinquefasciatus. Advances in Biological Research, 2, 39–43.
Kumar, S., Wahab, N., Mishra, M., Warikoo, R., (2012). Evaluation of 15 Local Plant Species as Larvicidal Agents Against an Indian Strain of Dengue Fever Mosquito, Aedes aegypti L. (Diptera: Culicidae). Frontiers in Physiology, 3. 104.
Lakshmi, K. V., Sudhikumar, A. V., Aneesh, E.M., (2018). Larvicidal activity of phytoextracts against dengue fever vector, Aedes aegypti – A review. Plant science Today, 5, 167–174.
Madkour, M.H., Zaitoun, A.A., Singer, F., (2012). No TitleEfficacy of three plant species extracts in the control of Trogoderma granarium Everts (Coleoptera: Dermestidae). Journal of Food Agriculture and Environment, 2, 1200–1203.
Maheswaran, R., Ignacimuthu, S., (2012). A novel herbal formulation against dengue vector mosquitoes Aedes aegypti and Aedes albopictus. Parasitology Research, 110, 1801–1813.
Mappau, Z., Ganing, A., (2018). The Effect of Tembelekan leaves extract (Lantana camara L) as electrical rechargeable of mosquitoes repellent to eliminate Aedes Aegypti. Journal of Physiotherapy and Occupational Therapy-An International Journal, 12, 261.
Mayer, S. V., Tesh, R.B., Vasilakis, N., (2017). The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and Zika fevers. Acta Tropica, 166, 155-163.
Morsy, T.A., Mazyad, S.A., el-Sharkawy, I.M., (1998). The larvicidal activity of solvent extracts of three medicinal plants against third instar larvae of Chrysomyia albiceps. Journal of the Egyptian Society of Parasitology, 28, 699–709.
Mossa, J., Al-Yahya, M., Al-Meshal, I., (1987). Medicinal plants of Saudi Arabia. King Saud University Press, Riyadh. 212-225.
Muangmoon, R., Junkum, A., Chaithong, U., Jitpakdi, A., Riyong, D., Wannasan, A., Somboon, P., Pitasawat, B., (2018). Natural larvicides of botanical origin against dengue vector Aedes aegypti (Diptera: Culicidae). Southeast Asian Journal of Tropical Medicine and Public Health 2, 227–239.
Ninan, S., Krishnakumar, K., Dineshkumar, B., (2017). Larvicidal activity of herbals: a review. Journal of drug Discovery and Therapeutics 5, 10–14.
Ohia, C., Ana, G.R.E., (2015). Pesticide toxicology and ameliorative interventions View Project Safety and Toxicological evaluation of African Medicinal Plants. Journal Biology, Agriculture, and Healthcare, 5, 22–26.
Pavela, R., (2016). History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects – A review. Plant Protection Science, 52, 229–241.
Pavela, R., Maggi, F., Lupidi, G., Mbuntcha, H., Woguem, V., Womeni, H.M., Barboni, L., Tapondjou, L.A., Benelli, G., (2018). Clausena anisata and Dysphania ambrosioides essential oils: from ethno-medicine to modern uses as effective insecticides. Environmental Science and Pollution Research, 25, 10493–10503.
Remia, K.M., Logaswamy, S., (2010). Larvicidal efficacy of leaf extract of two botanicals against the mosquito vector Aedes aegypti (Diptera: Culicidae). Indian Journal of Natural Products Resources, 1, 208–212.
Rodríguez-Cavallo, E., Guarnizo-Méndez, J., Yépez-Terrill, A., Cárdenas-Rivero, A., Díaz-Castillo, F., Méndez-Cuadro, D., (2018). Protein carbonylation is a mediator in larvicidal mechanisms of Tabernaemontana cymosa ethanolic extract. Journal of King Saud University- Science, 31, 464-471.
Sameeh, A.M., Reda, F.B., Latha, S.H., Reman, I.M., (2010). Toxic and Synergistic Properties of Several Botanical Extracts against Larval and Adult Stages of the Mosquito, Anopheles pharoensis. Biopesticides international, 2, 129–145.
Smith, L.B., Kasai, S., Scott, J.G., (2016). Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pesticide biochemistry and physiology, 133, 1-12.
Sousa, E.O., Costa, J.G.M., (2012). Genus Lantana: Chemical aspects and biological activities. Brazilian Journal of Pharmacognosy, 22, 1155–1180.
Tisgratog, R., Sanguanpong, U., Grieco, J.P., Ngoen-Kluan, R., Chareonviriyaphap, T., (2016). Plants traditionally used as mosquito repellents and the implication for their use in vector control. Acta Tropica, 157, 136-144.
Van Wyk, B., Wink, M., (2017). Medicinal Plants of the World, Revised Ed. ed. CABI.
Ved, A., Arsi, T., Prakash, O., Gupta, A., (2018). A Review on phytochemistry and pharmacological activity of Lantana camara Linn. International Journal of Pharmaceutical Sciences and Research, 1, 37–43.
Veer, V., Gopalakrishnan, R., (2016). Herbal insecticides, repellents and biomedicines: Effectiveness and commercialization. Springer India.
Verma, S., 2018. Medicinal potential of Lantana camara: Verbenaceae. Journal of Drug Delivery and Therapy, 8. 62-64
Wang, Z., Perumalsamy, H., Wang, X., Ahn, Y.J., (2019). Toxicity and possible mechanisms of action of honokiol from Magnolia denudata seeds against four mosquito species. Scientific Reports, 9. 411.
WHO (2005). Guidelines for laboratory and field testing of mosquito larvicides. Geneva.
Yu, K.X., Wong, C.L., Ahmad, R., Jantan, I., (2015). Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae). Asian pacific Journal of Tropical Medicine, 8, 1006–1012.
Zou, C.S., Lv, C.H., Wang, Y.J., Cao, C.W., Zhang, G.C., (2017). Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar. Pesticide biochemistry and physiology, 142, 123–132.