Dr. B. Lal Institute of Biotechnology, Malviya Industrial
Area, Jaipur, Rajasthan, India.
Corresponding author email: parul.chowdhury@gmail.com
Article Publishing History
Received: 15/06/2021
Accepted After Revision: 25/09/2021
Biostimulants are substances when applied to plants seeds, or soil stimulates the natural process to improve water and nutrient use effectively and increase tolerance to biotic and abiotic stress by enhancing primary and secondary metabolism. Application of plant biostimulants in a state of environmental stress can reduce the effects of stress and improve soil water holding capacity, root growth, and yield. Particularly, they reduce the application of mineral fertilizers by increasing the number of micro-and macronutrients taken up by plants, positively affecting root morphology and plant growth. In this study, aqueous extract of different parts like shoot and bud of C. Album is used for observing role in salt stress tolerance of wheat seedling grown in vitro. The wheat seedling was germinated in moistened filter paper in petriplates, with 3 petriplates for each treatment and 10 seeds in each petriplate (the study was done in triplicates).
The work plan includes the observing seed germination in control set in which no plant extract was used and two concentration of salt was taken i.e., 50mM and 100mM, along with no salt stress (0mM). In another set, the same salt stress was used but seeds were also treated with shoot and bud extract of C. album. After their growth for 7 days, seedlings were measured for root length, shoot length, wet weight, and dry weight. Again the same sets of experiments were repeated for measuring biochemical parameters like protein, sugar, and proline. It was observed that aqueous extract of Chenopodium can induce salt stress by increasing protein, proline, and sugar content along with better growth characteristics of seedlings. In conclusion, an aqueous extract of Chenopodium can be used as a biostimulant for fighting stress tolerance in the wheat seedling. Further, this study can be extended to other plant species other than wheat, and a gene expression study can be done for further validation.
Biostimulants, Chenopodium Album, Physical And Biochemical Parameters, Salt Stress, Wheat
Mehta A, Chowdhury P. Effect of Exogenous Application of Chenopodium album Aqueous Extract on Salt-Stressed Wheat Seedling –Mitigating Salt Stress. Biosc.Biotech.Res.Comm. 2021;14(3).
Mehta A, Chowdhury P. Effect of Exogenous Application of Chenopodium album Aqueous Extract on Salt-Stressed Wheat Seedling –Mitigating Salt Stress. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3mISnju“>https://bit.ly/3mISnju</a>
Copyright © Mehta Chowdhury This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
A foremost threat to world agriculture involves the assembly of 70% more food crops for an additional 2.3 billion people by 2050 globally. With population expansion, the demand for food is greater than ever. Though, large and small crop yields fluctuate yearly, because of several abiotic stresses, causing the amplification in world food prices and food scarcity. Land plants are living in an intrinsically harsh atmosphere ever since their appearance. A huge variety of physical or chemical factors are antagonistic to them, including low or high temperature, undersupplied or excessive water, high salinity, heavy metals, and ultraviolet (UV) radiation, among others.
These stresses jointly referred to as abiotic stresses, are posing a brutal threat to agriculture and the ecosystem, accounting for great crop yield loss (Wang et al., 2003; Wania et al., 2016). Abiotic stresses lead to a sequence of morphological and physiological, biochemical, and molecular changes that significantly influence plant productivity (Wang et al., 2004). Salinity is the chief stress restraining the rise in the requirement for food crops. More than 20% of cultured land worldwide (~ about 45 hectares) is exaggerated by salt stress and the quantity is escalating day by day. Plants based on adaptive evolution can be segregated roughly into two most significant types: the halophytes (that can hold up salinity) and the glycophytes (that cannot hold up salinity and ultimately die).
Commonly major crop species fit into this second category. Therefore salinity generally is one of the fierce environmental stresses that slow down crop productivity universally (Flowers 2004; Tester and Munns 2008). A plant biostimulant is any substance or microorganism useful for plants to improve nutrition effectiveness, abiotic stress tolerance, and/or crop quality traits, despite its nutrients content (Patrick 2015; Gupta et al. 2021).
In current years, plant biostimulants are being widely used in farming and cultivation. Positive effects of its application have yielded unexpected results as confirmed by many studies (Poincelot 1993; Jelacic et al. 2007; Smolen and Sady 2010; Smolen et al. 2010; Matzsiak et al. 2011; Beata et al. 2013). Plant biostimulants are organic materials that come into view to impact some metabolic procedures such as respiration.
photosynthesis, nucleic acid synthesis, and ion uptake and when applied in small quantities, improve the plant growth and development or specifically, a blend of two or more PGRs or a mixture of these with other substances (amino acids, nutrients, vitamins) is acknowledged as a plant growth promoter or plant biostimulant. Plant biostimulants are effective when applied in small doses, consequently leads to plant growth, and production enhancement (Li and Ni 1996; Castro and Vieira 2001; Saa-Silva et al. 2013; Gupta et al. 2021).
Moreover, the application of plant biostimulants in a state of environmental stress can reduce the effects of stress and improve soil water holding capacity, root growth, and yield. The performance of endophytic fungi applied to crops as a supplement to plant genetics or soil management to alleviate salt stress in crops. They reduce the application of mineral fertilizers by increasing the number of micro-and macronutrients taken up by plants, positively affecting root morphology and plant growth (Fisher and Wilson 1975; Kunicki et al. 2010; Ziosi et al. 2013; Nardi et al. 2009; Ertani et al. 2013).
The salt-sensitive and tolerant A. tricolor variety behaved differently under salt stress regarding growth, anatomical, physiological, ROS accumulation, enzymatic and non-enzymatic antioxidative defence mechanisms, and attributes associated with tolerance to oxidative stress (Sarker and Oba 2020). As there are many reports of biostimulants playing role in promoting growth and development of plants, in this study a weed plant C. album is chosen as it grows in bulk amount in field and they coexist in the field with the wheat (Gupta et al. 2021). Therefore, this study has explored its effect in in vitro grown salt-stressed wheat seedlings.
MATERIAL AND METHODS
Grains of Triticum aestivum (Wheat) was collected from RARI, Durgapura, Jaipur, Rajasthan. Chenopodium album weed was selected as the accessibility of this weed was easy and grown with Wheat seeds. Plants of Chenopodium album was collected from the garden of Dr B. Lal Institute, Jaipur, and Rajasthan. Different parts of C. album was separated and washed three times with distilled water and then transferred in a beaker containing HgCl2 and was left for 1 min, again washed 3-4 times with distilled water. Plant material was being spread on blotting paper and was left 10-15 days for drying (Abid et al. 2017). After drying different parts of the plant were crushed separately in mortar and pestle and were stored in a well labelled clean and dry falcon. To prepare the extract 1gm powder of plant material was soaked overnight in 100ml of autoclaved distilled water. During this soaking, the plant metabolites are released in distilled water after that the filtrate is filtered with the help of filter paper and stored at 4°C.
From the latter extract, different concentrations of the shoot (50 mg and 200mg) and bud (50 mg and 100mg) was prepared using distilled water as these concentrations of plant parts showed the best results in both morphological parameters and biochemical parameters when compared to the control of all the concentrations used of C. album in the previous study done by us.
A homogenous set of grains of the wheat plant was selected for uniformity of size, shape, and viability. Before germinating, sterilization of seeds was done under laminar airflow, where seeds were washed 3-4 times with autoclaved distilled water then with HgCl2 for 1 min, and then again washed with distilled water 3-4 times. The grains were transferred to sterile petriplates containing two sheets of filter paper, in between a thin layer of cotton.Each petriplate contained 10 grains and each treatment was replicated 3 times.
The petriplates were moistened with different concentrations of NaCl solutions (50mM and 100 mM) and different concentrations of plant extract of the shoot (50 mg and 200mg) and bud (50 mg and 100 mg). These petriplates were wrapped in aluminium foil and incubated in dark for two days at an average room temperature of 25-27° C. After two days these petriplates were placed in plant tissue culture racks under 16 hours of photoperiod. The results of root length, shoot length, wet weight, and dry weight of both roots, shoots and different biochemical parameters of salt-stressed wheat seedlings was calculated after 7 days of transfer to plant tissue culture racks.
Protein evaluation was done according to Bradford’s method (Bradford 1976). Firstly preparation of individual components was done: Bradford’s Reagent (100mL): 0.01g G-250 in 5 ml, ethanol+8.5 mL ortho – Phosphoric acid + 87.5 ml Distilled water. Standard solution of BSA: 50 mg (0.05 g) BSA in 2.5 ml Bradford Reagent and 5 ml Buffer was added in each tube. Blank: 0.1 ml Distilled water + 0.9 ml buffer + 5 ml Bradford reagent.
Test sample: 0.1 ml sample in 0.9 ml buffer and add 5 ml Bradford reagent. After that protein estimation was carried out using the following steps: 0.1 ml of sample solution was taken and 0.9 ml 0.1M Na-P buffer was added. Suitable aliquots of BSA solution were pipetted out. Blank was prepared for calibration. 5 ml Bradford reagent was added to each tube. Absorbance was recorded at 595 nm. Protein concentration was determined by the standard curve of Bovine Serum Albumin (BSA) by using the following equation: y = 1.073x – 0.068; where y is absorbance recorded of the sample and x is protein concentration in mg/ml.
Sugar estimation was done according to Dubois method (Dubois et al. 1956). 1 ml of plant extract was taken in a test tube and 3 ml of 96% H2SO4 was added to it after that 1 ml of 5% Phenol was added to it. The solution was mixed and was kept in a water bath for 20 min at 25-300C. Absorbance was taken at 490nm. Blank was prepared using 1 ml D.W+ 3ml 96% H2SO4+1 ml phenol.
The total concentration of sugar was determined by using the equation from the standard curve: y = 0.033x – 0.003; where y is absorbance recorded of the sample and x is sugar concentration in mg/ml. Proline estimation was done according to Bates (Bates et al. 1973). 0.5g of plant material was extracted by homogenizing in 10 ml of 3% aqueous Sulphosylic acid. The homogenous was filtered through filter paper. 2 ml of filtrate was taken in a test tube and 2ml of glacial acetic acid and 2 ml of ninhydrin was added into it.
The solution was heated in the boiling water bath for 1 hr. The reaction was ended by placing the tube in an ice bath. 4 ml of toluene was added to the reaction mixture and was stirred well for 20-30 sec. The toluene layer was separated and was warmed at room temperature. The red colour intensity was measured at 520nm. Amount of proline in the test sample was calculated from the standard curve by using the following equation: Proline = [(μg proline/mL × mL toluene)]/ [115.5 μg/umole]/ [(g sample/5].
RESULTS AND DISCUSSION
Morphological Analysis: : Different concentrations of extracts of shoot and bud of C. album showed varied results in the following physical parameters like root length, shoot length, fresh and dry weight of roots and shoots when treated with different concentrations of salt.
Effect of different salt concentrations on wheat seedlings (Control set): In control set, two concentration of salt was taken i.e., 50mM and 100mM and no plant extract was used. Stimulatory effects were seen in control in all the parameters that are root length, shoot length, fresh and dry weight of roots and shoots while at higher concentration of salt (100mM) it showed inhibitory effects (Fig.1).
Out of two concentrations of salt, 50mM was found to be as optimum concentration for the growth of the wheat seedlings in the control set (Figure 1). CHS1 ameliorated the adverse effect of high NaCl stress and rescued soybean plant growth by regulating the endogenous plant hormones and antioxidative system. CHS1 isolate could be exploited to increase salt resistant and yield in crop plants (Asaf et al. 2018; Zelm et al. 2020).
Figure 1: Effects of salt stress (control set) on root length (a) shoot length (b), fresh weight of roots and shoots (c), and dry weight of roots and shoots of wheat seedlings (d). Values are mean ± SD (n = 30 seedlings).
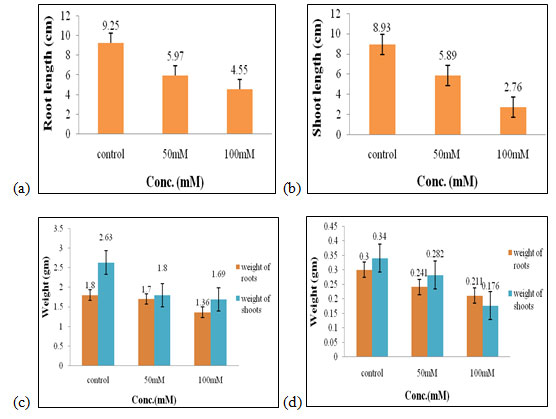
Effect of salt stress on physical parameters of wheat seedlings containing 50mg/ml of shoot extract: The maximal root length (5.59 cm, Fig. 2a), shoot length (7.67 cm, Fig. 2b), fresh weight of shoots and roots (1.9 gm and 1.4 gm, Fig. 2c), shoot dry weight (0.27 gm, Fig. 2d), were obtained in 50 mM of salt concentration, which increased by 0.66 cm,1.16 cm, 0.08 gm, and 0.5 gm correspondingly when compared to control. The optimal dry weight of roots (0.2 gm, Fig. 2d) was found to be in control one where no salt stress was given.
Therefore, 50mM of salt concentration was found to be the suitable concentration when 50mg/ml of shoot extract was used for the growth of wheat seedlings (Figure 2). Salt induces multiphase changes in growth rate, as well as changes in root system architecture and a salt avoidance response of the main root. These responses are mediated by several hormones, including auxin and ABA (Zelm et al. 2020).
Figure 2: Effects of salt stress on physical parameters of wheat seedlings containing 50mg/ml of shoot extract on root length (a) shoot length (b), fresh weight of roots and shoots (c), and dry weight of roots and shoots of wheat seedlings (d). Values are mean ± SD (n = 30 seedlings).
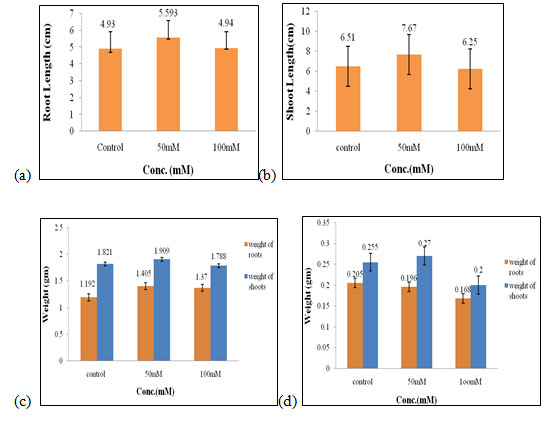
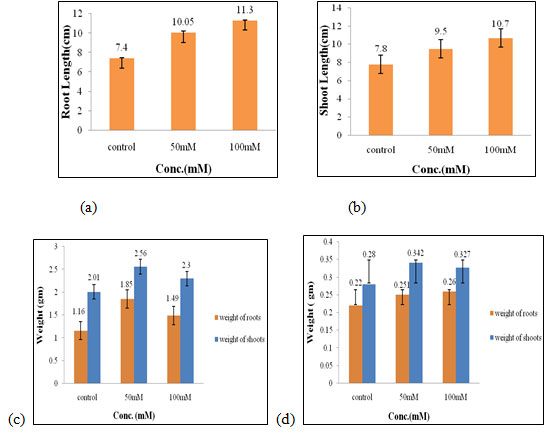
Effect of salt stress on physical parameters of wheat seedlings containing 200mg/ml of shoot extract: The maximal fresh weight of shoots and roots (2.56 gm and 1.85 gm, Fig. 3c), dry weight of shoots (0.34 gm, Fig. 3d) were obtained in 50mM of salt concentration, which increased by 0.55 gm, 0.69 gm, 0.06 gm correspondingly when compared to control. The optimal root length and shoot length (11.4 cm, 10.7 cm, Fig. 3a, 3b), dry weight of roots (0.26 gm, Fig. 3d) were found to be in 100mM of salt concentration.
Hence, both 50mM and 100mM of salt concentration were found to be the suitable concentration when 200mg/ml of shoot extract was used for the growth and development of wheat seedlings (Figure 3). C. crassa is a highly tolerant taxon to salinity that can also survive the presence of a low to moderate amount of Cd and Pb at the seedling stage (Samiei et al. 2020).
Figure 3: Effects of salt stress on physical parameters of wheat seedlings containing 200mg/ml of shoot extract on root length (a) shoot length (b), fresh weight of roots and shoots (c), and dry weight of roots and shoots of wheat seedlings (d). Values are mean ± SD (n = 30 seedlings).
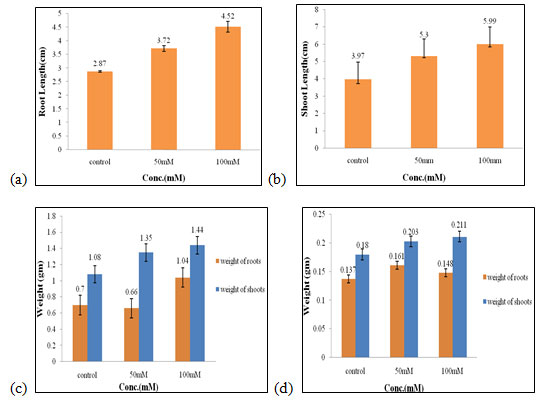
Effect of salt stress on physical parameters of wheat seedlings containing 50mg/ml of bud extract: The maximum root and shoot length (4.52 cm and 5.99 cm, Fig. 4a, 4b), fresh weight of shoots and roots (1.44 gm and 1.04 gm, Fig. 4c), dry weight of shoots (0.21 gm, Fig. 4d) were obtained in 100mM of salt concentration, which increased by 1.65 cm, 2.02 cm, 0.36 gm, 0.34 gm, 0.03 gm respectively when compared to control. The optimal dry weight of roots (0.16 gm, Fig. 4d) was found to be in 50mM of salt concentration.
Therefore, 100mM of salt concentration was found to be the suitable concentration when 50mg/ml of bud extract could effectively improve the growth performance of wheat seedlings (Figure 4). The salinity stress does not only hinder the growth and development of plants but also affects some physiological and metabolic activities as it affects osmolyte and ionic concentrations in plants. To prevent the crops from these losses various mitigation strategies are adopted to combat the harmful effects of saline stress (Bhardwaj and Kumar 2020).
Figure 4: Effects of salt stress on physical parameters of wheat seedlings containing 50mg/ml of bud extract on root length (a) shoot length (b), fresh weight of roots and shoots (c), and dry weight of roots and shoots of wheat seedlings (d). Values are mean ± SD (n = 30 seedlings).
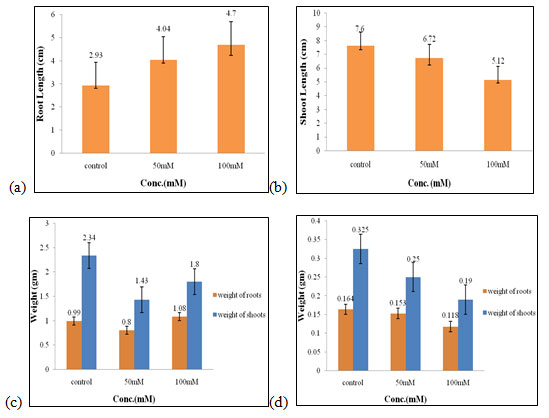
Effect of salt stress on physical parameters of wheat seedlings containing 100mg/ml of bud extract: The maximum root length (4.7 cm, Fig. 5a), fresh weight of roots (1.08 gm, Fig. 5c) were obtained in 100mM of salt concentration, which increased by 1.77 cm, 0.9 gm respectively when compared to control. The optimal shoot length (7.6 cm, Fig. 5b), fresh weight of shoots (2.34 gm, Fig. 5c), and dry weight of shoots and roots (0.32 gm and 0.16gm, Fig. 5d) were found to be in control.
Therefore, the case of 100mg/ml of bud extract control set shown the best results in the growth and development of wheat seedlings whereas 100mM of salt concentration was inhibiting its effect (Figure 5). The application of ascorbic acid and humic acid as a foliar spray and inoculation with PGPR treatment gave the highest significant yield, and chemical constituents as it may provide a useful way to reduce the adverse effects of salinity stress on wheat plants grown in saline soil (El-Sayed and Hagab 2020).
Figure 5: Effects of salt stress on physical parameters of wheat seedlings containing 100mg/ml of bud extract on root length (a) shoot length (b), fresh weight of roots and shoots (c), and dry weight of roots and shoots of wheat seedlings (d). Values are mean ± SD (n = 30 seedlings).
Biochemical Analysis: The biochemical analysis of the extract of C. album (shoot and bud) total soluble sugar estimation, protein estimation, and proline estimation was carried out using as mentioned in materials and method. Biochemical analysis of plants was necessary to check the quality of seeds when they are exposed to a different extract of weed of varying concentrations. In this study, along with the control two concentrations were used as selected for performing the above physical analysis.
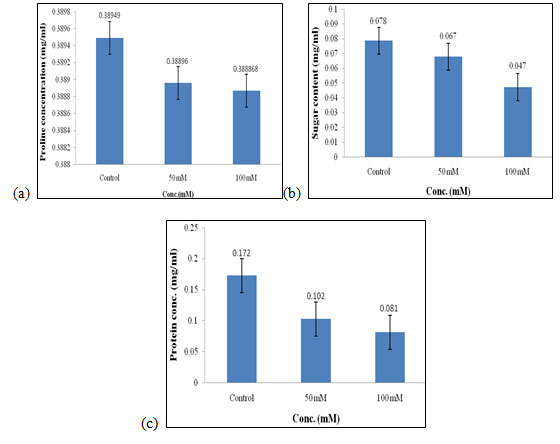
Effect of salt stress on biochemical parameters of wheat seedlings (Control set): In the control set, different biochemical parameters were measured when wheat seedlings were treated with varying concentrations of salt that are 50mM and 100mM, and no plant extract was used in them. Proline content in wheat seedlings was found to be highest in control as compared to the 50mM and 100mM of salt concentration. Content of proline in control was 0.389 mg/ml, (Fig. 6a), respectively.
Increased Proline concentration generally protects the plant from a different kind of stress. Sugar content was also found to be highest in control when compared to the other concentration of salt used which is 0.078 mg/ml (Fig. 6b). Sugar was mainly the essential component of plant nutrition found during photosynthesis and its amount gets decreased as it was highly sensitive to environmental stress (Ami et al. 2020).
Protein concentration also gets increased in control i.e., 0.172 mg/mL (Fig. 6c) as compared to other concentrations of salt used. Protein concentration in plants gets decreased due to the inhibition of incorporation of amino acids caused due to stress. Protein content also increases due to the activity of genes involved in various enzymatic activities get increased. Of all, proline, sugar, and protein content was found to be highest in control as compared to the other concentration of salt used i.e., 50mM and 100mM, and also at higher concentrations of salt i.e. at 100mM the quality of seed was getting inhibited. Salt stress can be partially alleviated by proline in the drought-resistant cultivar MBB, even though it is relatively salt-sensitive (Ami et al. 2020).
Figure 6: Effect of salt stress on biochemical parameters of wheat seedlings (Control set) Proline (a), soluble sugar (b), and soluble protein (c).
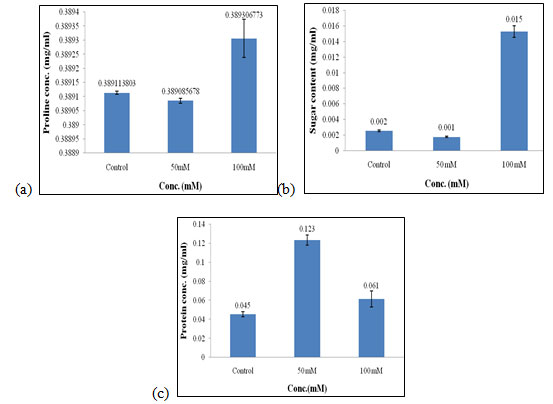
Effect of salt stress on biochemical parameters of wheat seedlings containing 50mg/ml of shoot extract: Wheat seedlings when treated with 50mg/ml of shoot extract gave the following results, Proline content in wheat seedlings was found to be almost similar in all control, 50mM and, 100mMof salt concentration. Content of proline did not show any significant effect when treated with 50mg/ml of shoot extract (Fig. 7a). Sugar content was also found to be highest in 100mM when compared to the other concentration of salt used which was 0.015 mg/ml (Fig. 7b).
Protein concentration gets increased in 50mM of salt concentration i.e., 0.123 mg/mL (Fig. 7c) as compared to other concentrations of salt used. Of all, proline, sugar content was found to be highest in 100mMand protein content was found to be highest in 50mM as compared to the other concentration of salt used i.e., control and 50mM. The production of phytohormones, particularly auxins, have been demonstrated by PGPR, even the pathogenic bacteria and fungi which also modulate the endogenous level of auxins in plants, subsequently enhancing plant resistance to various stresses (Ullah et al. 2021).
Figure 7: Effect of salt stress on biochemical parameters of wheat seedlings containing 50mg/ml of shoot extract, Proline (a), soluble sugar (b), and soluble protein (c).
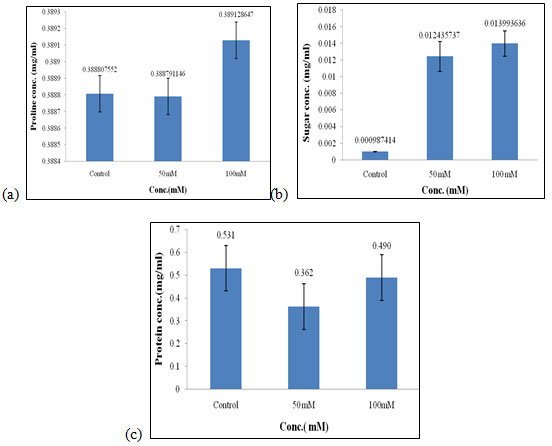
Effect of salt stress on biochemical parameters of wheat seedlings containing 200mg/ml of shoot extract: Wheat seedlings when treated with 200mg/ml of shoot extract gave the following results, Proline content in wheat seedlings was found to be highest in 100mM i.e., 0.389mg/ml (Fig. 8a) and almost equal in control, and 50mM of salt concentration. Here also, the content of proline did not show any considerable effect when treated with 200mg/ml of shoot extract. Sugar content was also found to be highest in 100mM when compared to the other concentration of salt used which was 0.013 mg/ml (Fig. 8b).
Protein concentration was found to be increased in control i.e., 0.531 mg/mL (Fig. 8c) as compared to other concentrations of salt used. Of all, proline and sugar content was found to be highest in 100mM as compared to the other concentration of salt used i.e., control and 50mM, and protein content did not show any significant effect as it was highest in control. Salt stress increased total soluble sugars (TSS) in all parts of the kiwifruit genotypes. The High accumulation of TSS in roots explains the overproduction of TSS by leaves transported and stored in roots via the phloem. (Abid et al. 2020).
Figure 8: Effect of salt stress on biochemical parameters of wheat seedlings containing 200mg/ml of shoot extract, Proline (a), soluble sugar (b), and soluble protein (c).
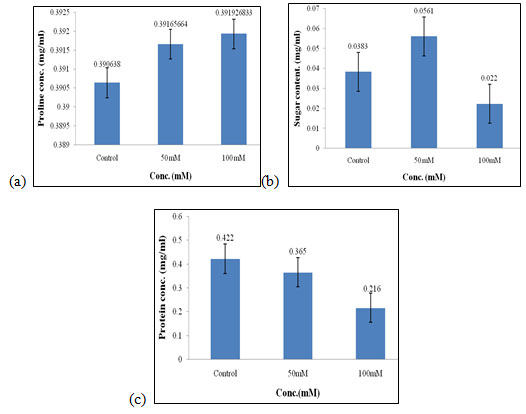
Effect of salt stress on biochemical parameters of wheat seedlings containing 50mg/ml of bud extract: Wheat seedlings when treated with 50mg/ml of bud extract gave the following results, Proline content in wheat seedlings was found to be highest in 50mM and 100mM i.e., 0.391mg/ml (Fig. 9a) of salt concentration. Sugar content was found to be highest in 50mM when compared to the other concentration of salt used which was 0.056 mg/ml (Fig. 9b). Protein concentrations were found to be increased in control i.e., 0.422 mg/mL (Fig. 9c) as compared to other concentrations of salt used but it gets decreased at higher salt concentrations.
Figure 9: Effect of salt stress on biochemical parameters of wheat seedlings containing 50mg/ml of bud extract, Proline (a), soluble sugar (b), and soluble protein (c).
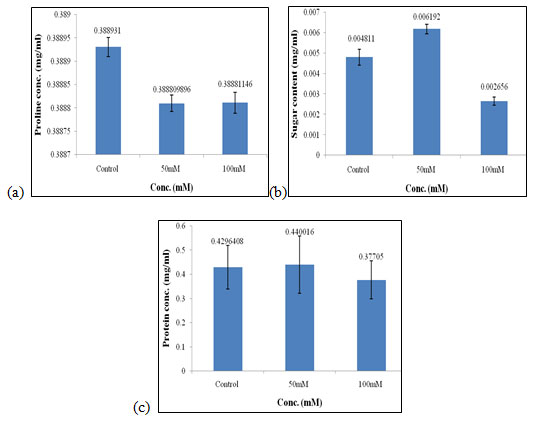
Effect of salt stress on biochemical parameters of wheat seedlings containing 100mg/ml of bud extract: Wheat seedlings when treated with 100mg/ml of bud extract gave the following results, Proline content in wheat seedlings didn’t show any considerable effect as it was similar in all the concentrations of salt used and also in control i.e., 0.388mg/ml (Fig. 10a) of salt concentration. Sugar content was found to be highest in 50mM when compared to the other concentration of salt used which was 0.006 mg/ml (Fig. 10b). Protein concentration was found to be increased in 50mM of salt concentration i.e., 0.440 mg/ml (Fig. 10c) as compared to other concentrations of salt used but it gets decreased at higher salt concentrations.
Of all, proline, sugar, and protein content were found to be highest in 50mM as compared to the other concentration of salt used i.e., control and 100mM. Cysteine treatments had a beneficial role in alleviating the adverse effect of salinity stress on the soybean plant (Sadak et al. 2020). Vanillic acid significantly improves salinity tolerance and plant growth performance by involving the actions of plant antioxidant defence and glyoxalase systems (Parvin et al. 2020).
Figure 10: Effect of salt stress on biochemical parameters of wheat seedlings containing 100mg/ml of bud extract, Proline (a), soluble sugar (b), and soluble protein (c).
CONCLUSION
The findings of the present study shows that bud and stem extract mitigates salt stress in wheat seedlings. It was shown by physical, and various biochemical characteristics like sugar, protein, and proline. We saw at the maximum places the increase in these biochemical parameters in the presence of C. album extracts. Plants growing in the desert have the potential to tolerate adverse climatic change. Chenopodium album grows naturally as a weed in fields of wheat, barley, etc. Therefore, this study can find the potential of weed plants that can be used for abiotic stress tolerance, and these plant extracts combined with reduced doses of herbicides could be the promising strategy not only for abiotic stress tolerance but for sustainable agriculture in the future.
ACKNOWLEDGEMENTS
This research was financially supported by the Centre for Innovation Research and Development (CIRD). Moreover, authors would like to thank Dr B. Lal Institute of Biotechnology, Jaipur for the assistance provided for this research.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
REFERENCES
Abid M, Zhang YJ, Li Z, et al. (2020). Effect of salt stress on growth, physiological and biochemical characters of four kiwifruit genotypes. Scientia Horticulturae:271: pp.109473.
Abid M, Hakeem A, Shao Y, et al. (2017) Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environmental and Experimental Botany:145:pp.12-20.
Ami K, Planchais S, Cabassa C, et al. (2020) Different proline responses of two Algerian durum wheat cultivars to in vitro salt stress. Acta Physiol Plant:42:pp. 21.
Asaf S, Hamayun M, Khan AL, et al. (2018). Salt tolerance of Glycine max. L induced by the endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiology and Biochemistry:128:pp.13-23.
Bates LS, Waldren RP and Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant and soil:39(1): pp.205-207.
Beata S, Agata M, Ewa R, et al. (2013) The influence of particular bio stimulators on some biochemical parameters in broccoli (Brassica oleracea L. var. Botrytis italic Plenck). Environmental Protection and Natural Resources:3(57): pp.25-7.
Bhardwaj S and Kumar P (2020). Salinity stress, its physiological response and mitigating effects of microbial bio inoculants and organic compounds. Phytopathology:9:pp.1297-1303.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry:72(1-2): pp.248-254.
Castro PRC and Vieira EL (2001) Aplicações de reguladores vegetais na agricultura tropical. Guaíba, Editora Agropecuária:pp.132.
Dubois M, Gilles KA, Hamilton JK, et al. (1956) Colorimetric method for determination of sugars and related substances. Analytical chemistry:28(3): pp.350-356.
El-Sayed SY and Hagab RH (2020). Effect of organic acids and plant growth-promoting rhizobacteria (PGPR) on biochemical content and productivity of wheat under saline soil conditions. Middle-East J. Agric. Res:9(2):pp.227-242.
Ertani A, Nardi S and Altissimo (2013) A Review: long-term research activity on the biostimulant properties of natural origin compounds. Acta Hort.:1009:pp.181-88.
Fisher KS and Wilson GL (1975) Effect of fertilizer on growth and yield in Sorghum bicolour. Aust. J. Agric. Res:26: pp.31-41.
Flowers T.J. (2004) Improving crop salt tolerance, Journal of Experimental Botany:55(396): pp.307-319.
Gupta S, Schillaci M, Walker R, et al. (2021) Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil:461:pp.219-244.
Jelacic S, Beatovic D, Lakic N, et al. (2007) The effect of natural bio stimulators and slow-is integrating fertilizers on the quality of rosemary seedlings (Rosmarinus officinalis L.). J. Agricultural Sciences:52(2): pp.85-94.
Kunicki E, Grabowska A, Sekara A, et al. (2010) The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic:22:pp. 9-13.
Li WJ and Ni YZ (1996) Researches on the application of microbial inoculant in crop production. In: Researches and application of En technology, Agriculture University Press, Beijing, China:pp.42-84.
Matzsiak K, Adamcyweski K and Kaczmarek S (2011) Wpływ biostymulatora Asahi SL na plonowanie i wybrane cechy ilościowe I jakościowe niektórych roślin rolniczych uprawianych w warunkach Wielkopolski, Progress in Plant Protection/ Postępy w Ochronie Roślin:51(4):pp.1849-57.
Munns R and Tester M (2008) Mechanisms of salinity tolerance, Annual Review of Plant Biology:59: pp.651-681.
Nardi S, Carletti P, Pizzeghello D, et al. (2009) Biological activities of humic substances.Biophysico-chemical processes involving natural nonliving organic matter in environmental systems. Fundamentals and impact of mineral-organicbiota interactions on the formation, transformation, turnover, and storage of natural nonliving organic matter (NOM). John Wiley and Sons, Hoboken, NJ: (1) pp.301-35.
Parvin K, Nahar K, Hasanuzzaman M, et al. (2020). Exogenous vanillic acid enhances salt tolerance of tomato: insight into plant antioxidant defence and glyoxalase systems. Plant Physiology and Biochemistry:150:pp.109-120.
Patrick DJ (2015) Plant biostimulants: Definition, concept, main categories and regulation.Scientia Horticulture:196:pp.3-14.
Poincelot RP (1993) The use of a commercial organic biostimulant for bedding plant production. Journal of Sustainable Agriculture:3(2): pp.99-110.
Saa-Silva S, Brown PH and Ponchet M (2013) (eds). First World Congress on the Use of Biostimulants in Agriculture. Leuven: International Society of Horticultural Science.
Sadak MS, Abd El-Hameid AR, Zaki FS, et al. (2020). Physiological and biochemical responses of soybean (Glycine max L.) to cysteine application under sea salt stress. Bulletin of the National Research Centre:44(1): pp.1-10.
Samiei L, Pahnehkolayi MD, Karimian Z, et al. (2020). Morpho-Physiological Responses of Halophyte Climacoptera crassa to Salinity and Heavy Metal Stresses in In Vitro Condition. South African Journal of Botany:131:pp.468-474.
Sarker U and Oba S (2020) The Response of Salinity Stress-Induced A. tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 11:559876.
Smolen S and Sady W (2010) Effect of plant biostimulation with pentakeep v fertilizer and nitrogen fertilization on the content of macro and micronutrients in spinach. J. Elementol:15(2): pp.343-53.
Smolen S, Sady W and Wierzbinska J (2010) The effect of plant biostimulation with pentakeep v and nitrogen fertilization on yield, nitrogen metabolism and quality of spinach, Acta Scientiarum Polonorum, Hortorum Cultus:9(1): pp.25-36.
Ullah A, Bano A and Khan N (2021) Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress Front. Sustain. Food Syst.:5:618092.
Wang W, Vinocur B and Altman A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta:28: pp.1-14.
Wang W, Vinocur B, Shoseyov O, et al. (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response, Trends in Plant Science:9(5): pp.244-252.
Wania SH, Kumar V, Shriram V, et al. (2016). Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J.:4:pp.162-176.
Zelm EV, Zhang Y and Testerink C (2020) Salt Tolerance Mechanisms of Plants, Annual Review of Plant Biology:71(1): pp.403-433.
Ziosi V, Zandoli R and Di Nardo A (2013) Biological activity of different botanical extracts as evaluated using an array of in vitro and in vivo bioassays. Acta Hortic:1009:pp.61-66.


