1Kulti College, Kulti- 713343. West Bengal, India.
2Michael Madhusudan Memorial College, Durgapur-713216, West Bengal, India.
3Ramakrishna Mission Vivekananda Educational and Research Institute, Narendrapur, Kolkata 700 103 West Bengal, India.
Corresponding author email: urenuschatterjee@gmail.com
Article Publishing History
Received: 14/04/2020
Accepted After Revision: 29/05/2020
Breast cancer is one of the most frequently diagnosed cancers and the leading cause of cancer deaths in females worldwide. Several matrix metalloproteinases (MMPs) were reported to show stronger expression in breast cancer tissue compared to normal breast tissue. Some breast cancer cell lines also constitutively express a wide variety of MMPs. Thus targeting those MMPs can be a possible route to combat breast cancer. In different cancer cells curcumin was reported to exert both anti-proliferative and pro-apoptotic effect. In the present study, human breast cancer cells, MDA-MB-231 was exposed to curcumin and change in expression, localization and activity of different procancerous factors were studied using zymography, RT-PCR, Western blot, immunocytochemistry. It was found that, curcumin affects several cell signalling molecules like FAK, ILK, PI3K, Akt, NF-κB, and reduction of MMP-9 level, VEGF level, cell migration and increase in E-cadherin level. Since most of the molecules mentioned are part of the FAK mediated signalling, curcumin might exhibit here appreciable effects on tumor cell- ECM interaction with potential antagonist role on cell metastatic properties involving increase in E-cadherin and decrease in VEGF level.
Breast cancer , cell signalling, ECM, integrin, MMP
Pal S, Ganguly K. K, Chatterjee A. Effect of Curcumin on Cell- ECM Interaction in Human Breast Cancer Cells MDA-MB-231. Biosc.Biotech.Res.Comm. 2020;13(2).
Pal S, Ganguly K. K, Chatterjee A. Effect of Curcumin on Cell- ECM Interaction in Human Breast Cancer Cells MDA-MB-231. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/369EW1A
Copyright © Pal et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Curcumin is the yellow pigment of turmeric, the dried rhizome of the plant, Curcuma longa L. This pigment, with the chemical structure of polyphenol with a diarylheptanoid, has been reported to have chemopreventive as well as chemotherapeutic activities. The anti-cancer activities of this compound are exerted through affecting several pro-cancerous factors. These include downregulation of transcription factors like NF-κB, AP-1; pro-metastatic factors like uPA, MMPs; growth factor receptors like EGFR, HER2; cell signaling components like protein tyrosine kinases, protein serine/threonine kinases including c-Jun N-terminal kinase. Apart from these, curcumin has been found to protect DNA damage by carcinogens, promote “mild but yet significant activation of apoptosis”, and inhibit angiogenesis, invasion and metastasis, ( Chen and Tan, 1998 Aggarwal et al., 2003 Lopez-Lazaro, 2008 Tomeh et al., 2019).
Breast cancer is one of the most frequently diagnosed cancer and the leading cause of cancer death in females worldwide. Incidence rates are high in Australia/New Zealand, Northern and Western Europe, and North America; intermediate in South America, the Caribbean, and Northern Africa; and low in Asia and sub-Saharan Africa (Jemal et al., 2011; Bray et al 2018). Several matrix metalloproteinases (MMPs), which belongs to family of endopeptidases having the ability to degrade extracellular matrix (ECM) proteins and play a crucial role in tumor invasion and metastasis, were reported to show stronger expression in breast cancer tissue compared to normal breast tissue. Some breast cancer cell lines also constitutively express a wide variety of MMPs(Kohrmann et al., 2009; Katari et al, 2019).
Thus targeting those MMPs can be a possible route to combat breast cancer.
Curcumin has been found to downregulate expression and activity of MMPs in different experimental models. Treatment of highly metastatic murine melanoma cells B16F10 with curcumin significantly inhibited matrix metalloproteinase-2 (MMP-2) activity, involving reduction of expression of membrane type-1 matrix metalloproteinase (MT1-MMP), an important component of MMP-2 activation, and focal adhesion kinase (FAK), an important component of the intracellular signalling pathway (Banerji et al., 2004).
Curcumin can also reverse the effects of tumor promoting agent. Curcumin was found to significantly inhibit the MMP-9 expression and activity that was induced by phorbol ester PMA. Such inhibition of MMP-9 was attributed to curcumin mediated repression of the PMA-induced phosphorylation of ERK, JNK, p-38 MAP kinase and suppression of DNA binding activities of NF-κB and AP-1(Woo et al., 2005).Inhibitory effect of curcumin on expression of MMPs has been observed in mouse model also(Qi et al., 2009). Curcumin can enhance the expression of antimetastatic proteins, nonmetastatic gene 23 (Nm23), tissue inhibitor metalloproteinase (TIMP-2), and E-cadherin(Ray et al., 2003). In breast cancer cells curcumin was reported to exert both anti-proliferative(Liu et al., 2009) and pro-apoptotic effects(Choudhuri et al., 2002).
MATERIAL AND METHODS
Human breast cancer cell line MDA-MB-231 was procured from National Centre for Cell Sciences,(NCCS), Pune, India. Leibovitz’s L-15 medium, Fetal Bovine Serum (FBS), Trypsin, Gentamycin were purchased from GIBCOTM-Invitrogen. Curcumin was purchased from Sigma-Aldrich. Protease Inhibitor Cocktail Tablets were purchased from Roche, Germany. GelatinSepharose- 4B beads were purchased from GE Healthcare Biosciences AB, Uppsala, Sweden. All primary and secondary antibodies were purchased from Santa Cruz Biotechnology, Inc, USA and chemiluminescent substrate SuperSignal West Femto was purchased from Thermo Fisher Scientific Inc. Primers (MMP-9, TIMP-1 and GAPDH) were synthesized by Operon, Germany. RNAqueous 4 PCR (Total RNA isolation kit) and Retroscript (RT-PCR Kit) were purchased from Ambion, Austin, TX, USA. DAB substrate and stable peroxidase substrate buffer were purchased from Pierce Biotechnology, USA. Avidin-biotinylated peroxidase complex reagent (Vectastain ABC kit) was purchased from vector laboratories, Burlingame, CA. MTT reagent, DMSO were purchased from Amresco. Coomassie brilliant blue was purchased from Merck. Immobilon-P Membrane (PVDF) was purchased from Millipore, USA.MDA-MB-231, a highly metastatic human breast cancer cell line was grown and maintained in L15 medium, containing 10% FBS in a dry (CO2 free incubator) at 370C, (ATCC, 2020).
MDA-MB-231 cells were allowed to attach on 35 mm petridishes (300,000 cells/ml medium) and various concentrations of curcumin treatment were applied for different time periods. As cells remained attached upto 48 hrs in presence of 5, 10 µM curcumin treatment in L15 SFCM (serum free culture medium), this treatment condition was used for our experiments. As the curcumin stock solution was dissolved in DMSO, equal volume of this solvent was treated in control cells.
MDA-MB-231 cells were plated in 96-well plate and treated with curcumin of various conc. (0, 5, 10, 15, 20 µM) for 48 hrs. Wells with SFCM, but without cells were used as ‘blank’. After the treatment, 50 µl MTT reagent (100 µg) was added to each well and incubated for 4 hrs. Culture supernatant was carefully discarded and formazan was dissolved in DMSO. Absorbance of the solution was measured at 570 nm against the blank wells and percentage of viability was calculated assuming that of 0 µM curcumin treated cells as 100%.
After the required treatments on MDA-MB-231 cells, the gelatinases were separated from the culture supernatants using gelatinsepharose 4B beads (shaking for 2 hrs at 40 C). The beads were washed twice with Tris-buffered saline with 0.02% tween-20 (TBS-T) and eluted with 1X Laemli buffer (0.075 gm Tris, 0.2 gm SDS, in 10 ml water, pH 6.8) for 30 mins at 370 C. The eluted supernatant (separated from bead by centrifugation) was subjected to zymography on 7.5% SDS-PAGE co-polymerized with 0.1% gelatin. Gel was washed in 2.5% Triton-X-100 for 30 mins to remove SDS and was then incubated in reaction buffer (50 mMTris-HCl pH 7.5, 4.5 mM CaCl2, 0.2 M NaCl) for overnight. After incubation, the gel was stained with 0.25% Coomassie Blue in 40% methanol and 10% glacial acetic acid.The bands were visualized by washing the gel in water and photograph was taken (Sen et al., 2009).
RNA was extracted from 1×106 MDA-MB-231 cells treated with DMSO (Control) and 10 µM curcumin (Experimental) for 48 hrs. Total RNA was extracted (RNAqeous, Ambion, USA). 2 steps RT-PCR (Retroscript, Ambion, USA) was done with equal amounts of total RNA using specific primers for human MMP-9 and TIMP-1 genes. The steps followed have been previously described (Pal et al., 2013). The primer sequences are:-
hMMP-9: 5’-CGCTACCACCTCGAACTTTG-3’ (forward),
5’GCCATTCACGTCGTCCTTAT-3’-(reverse);
hTIMP-1: 5’-CACCCACAGACGGCCTTCTGC-3’- (forward)
5’-AGTGTAGGTCTTGGTGAAGCC-3’-(reverse);
GAPDH: 5’-CGGAGTCAACGGATTTGGTCGTAT- 3’ (forward)
5’-AGCCTTCTCCATGGTGGTGAAGAC- 3’ (reverse).
Conditions used for PCR consisted of 40 cycles for TIMP-1 and MMP-9 at 94°C for 30 sec, 58°C for 30 sec and 72°C for 90 sec in thermal cycler (Ganguly et al., 2012).
Whole Cell Extraction :- MDA-MB-231 cells were treated with DMSO (C) and 10 µM curcumin (E) for 48 hrs. The cells were trypsinized, washed with PBS (containing 10% NaF, 10% Na3VO4) and resuspended in RIPA extraction buffer. Extraction was done by snap-freeze method. Protein contents of the whole cell extracts were estimated by Lowry’s method.
Western blot assay:- Equal amount (100 μg) of protein from whole cell extracts from control and experimental sets was loaded in each lane on the protein gel. After SDS-PAGE the proteins were electrophoretically transferred onto PVDF membrane (Millipore). Nonspecific binding sites on the membrane were blocked in 4% BSA. After treatment with required primary antibodies [anti-FAK, anti-PI3K (p85), anti-Akt, anti-MMP-9, anti-E-cadherin, anti-VEGF, anti-ILK; 1µg/2ml dilution for each] and their respective HRP- labeled secondary antibodies (1µg/200ml dilution), blots were developed by ECL method as previously described (Ganguly et al., 2012).
Immunocytochemistry:- The MDA-MB-231 cells were allowed to attach on coverslips (few thousand cells/coverslip) followed by curcumin treatment for 48 hrs. After PBS wash, cells on coverslips were fixed with 3.5% formaldehyde and treated with 0.5% Triton X-100. Non-specific sites were blocked using 1% BSA solution. Immunocytochemistry was done with anti-NF-κB p65 primary antibodies (1 µg/ml dilution), and respective biotin labeled secondary antibodies (1 µg/ml dilution) in a method described in earlier reports (Pal et al., 2013).
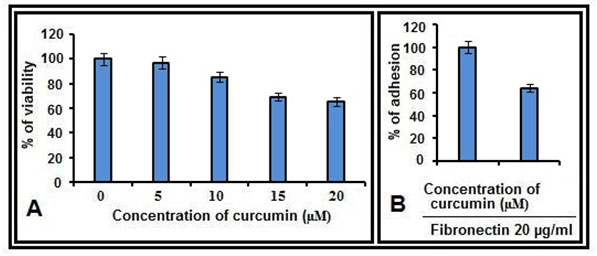
Figure 1: Cell adhesion and cell viability assays:A. MDA-MB-231 cells, treated with curcumin (0, 5, 10, 15, 20 µM for 48 hrs) were subjected to MTT assay and percentage of survival was calculated. B. MDA-MB-231 cells were treated with curcumin (0, 10 µM) for 48 hrs and percentage of adhesion on FN coated (20 µg/ml) wells were calculated.
RESULTS AND DISCUSSION
MTT and cell adhesion assay results:- The effect of curcumin on cell viability was studied by MTT assay. Among the concentrations studied, the maximum concentration which resulted >80% cell survival was 10 µM (Fig 1A) and this concentration was used in the subsequent experiments. By the cell adhesion assay it was found that, MDA-MB-231 cells bind efficiently to fibronectin (FN), however this binding is reduced in cells treated with curcumin (Fig. 1B).
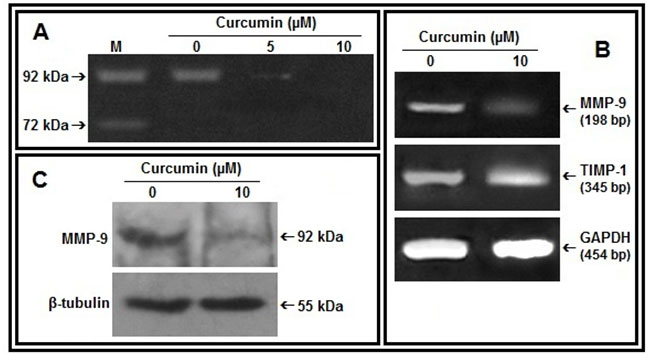
Figure 2: Effect of curcumin on MMP-9, TIMP-1:A. MDA-MB-231 cells were treated without (0) or with curcumin (5, 10 µM) in SFCM for 48 hrs. Lane M is the marker lane. The gelatinases in all the cases were separated from SFCM by mixing Gelatin Sepharose 4B beads and subjected to gelatin zymography. B. 2 steps RT-PCR was done with equal amounts of total RNA isolated from control and curcumin treated (10 µM, 48 hrs) cells and using MMP-9, TIMP-1 specific primers. G3PDH primers were used to check for equal loading. C. MDA-MB-231 cells were treated with curcumin (0, 10 µM for 48 hrs). The cells were collected, extracted and equal protein (100 µg) was subjected to western blot analysis with anti-MMP-9 antibody and respective HRP-coupled secondary antibody. The blots were developed by ECL method and β-tubulin was used as loading control.
Curcumin reduces MMP-9 expression and secretion in MDA-MB-231 cells:-MDA-MB-231 cells treated with curcumin for 48 hrs showed decrease in MMP-9 gelatinolytic activity in the culture supernatant with appreciable downregulation at 10 µM concentration (Fig 2A). RT-PCR analysis of curcumin treated MDA-MB-231 cells (10 µM for 48 hrs) indicates decrease in expression of MMP-9 mRNA and increase in TIMP-1 mRNA (Fig 2B). Upregulation of MMP-9 protein expression under same condition was further confirmed by western blot assay (Fig 2C).
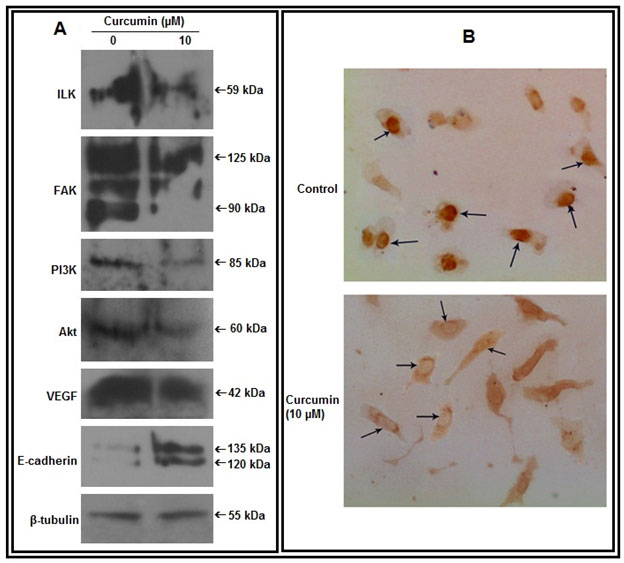
Figure 3: Effect of curcumin on cell signaling components:A. Cell extracts from untreated and curcumin treated (10 µM for 48 hrs) MDA-MB-231 cells were subjected to western blot assay with anti-ILK, anti-FAK, anti-PI3K, anti-Akt, anti-VEGF, anti-E-cadherin antibodies (1 µg/2 ml dilution). β-tubulin was used as loading control. B. Cells were grown in absence (Control) and presence of curcumin (10 µM, 48 hrs) on coverslips. Immunocytochemistry was done using anti-NF-κB primary and respective biotin-labeled secondary antibody
Effect of curcumin on cell signaling components:-Western blot analysis reveals that, curcumin treatment appreciably reduces expression of ILK, FAK, PI3K, Akt and VEGF and enhances expression of E-cadherin in MDA-MB-231 cells (Fig 3A). Immunocytochemical assay demonstrates that, curcumin treatment causes nuclear to cytosolic localization of NF-κB (Fig 3B).
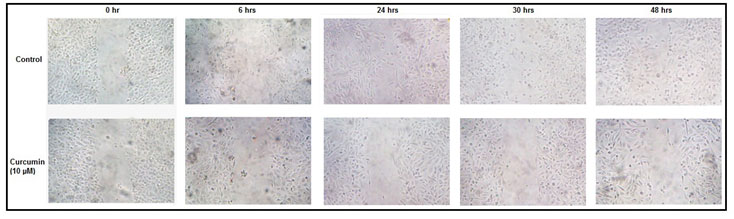
Effect of curcumin on cell migration:-Photographs taken at different time intervals indicate that, the ability of MDA-MB-231 cells to heal experimentally scratched wounds is reduced appreciably in presence of curcumin (Fig 4).
Figure 4: Effect of curcumin on cell migration: MDA-MB-231 cells were grown and treated without (control) or with 10 µM curcumin for 48 hrs before giving the scratch wound. Photographs were taken just after the scratch (0 hr), and then at 6, 24, 30, 48 hrs.
Signaling pathways leading to upregulation of MMPs have been studied within various cell lines in our laboratory. Among the breast cancer cell lines, MDA-MB-231 cells secrete MMP-9 and its upregulation at a certain ECM environment, i.e. in presence of fibronectin, require activation of ILK, FAK, PI3K and nuclear translocation of NF-κB(Maity et al., 2011). ]. In our present study curcumin was found to reduce the ability of MDA-MB-231 cells to bind the same ECM ligand. Curcumin treatment also downregulated MMP-9 secretion in MDA-MB-231 cells. We further studied the effects of curcumin on the associated signaling molecules, specially those involved in regulation of MMP-9.
Several integrin receptors, specially the β1 integrins were found to play important role to initiate MMP-9 upregulating signal (Pal et al., 2012). ILK is a Ser/Thr kinase and can interact with the cytoplasmic domains of β1integrins to promote suppression of apoptosis, cell survival, cell migration and invasion (Yoganathan et al., 2002, Zheng et al, 2019).
In our study, curcumin treated cells showed appreciable downregulation of ILK expression. FAK is another cell signalingmolecule, which is regulated by integrin mediated signaling and is a potent regulator of MMP-9 (Ganguly et al., 2012, Sein et al., 2000). Here FAK is found to be downregulated in MDA-MB-231 cells in presence of curcumin. FAK activity is coupled to assembly of focal adhesions and plays major role in cell attachment and migration (Gilmore and Romer, 1996, Mitra et al., 2005). In the present study, the natural ability of MDA-MB-231 cells to migrate and heal experimental scratch wounds was greatly reduced in curcumin treated cells.
PI3K, one of the downstream targets of FAK, was also found to be downregulated in curcumin treated cells. PI3K can exert its effects by activating Akt, followed by several transcription factors, including nuclear factor kB (NF-kB) (Bader et al., 2005, Torrealba et al, 2019).
In presence of curcumin, appreciable downregulation of PI3K, Akt and translocation of NF-kB out of the nucleus is observed in MDA-MB-231 cells. NF-kB was reported to modulate expression of MMP-9 gene in various cell lines (Tai et al., 2008; Guarneri et al, 2017). Reduced mRNA expression of MMP-9 (RT-PCR data) found in our study may be due to lack of availability of NF-kB as transcription factor within nucleus. In addition, enhanced mRNA expression of TIMP-1, a negative regulator of MMP-9, in presence of curcumin may affect cumulatively in downregulation of MMP-9 activity.
Among the other related signaling proteins studied, E-cadherin was found to be upregulated in curcumin treated cells. It is a cell adhesion molecule and known to form powerful invasion suppressor complex(Noe et al., 2001). Thus curcumin may lead to a situation that prevents invasion and metastasis. Successful metastasis require sufficient angiogenesis and VEGF is a potent inducer of angiogenesis. MMP-9 can release biologically active VEGF from the extracellular matrix of cancer cells(Hawinkels et al., 2008). Here downregulation of VEGF in curcumin treated cells may be due to the reduced MMP-9 activity.
Therefore it may be concluded that in MDA-MB-231 cells curcumin affects several cell signaling molecules like FAK, ILK, PI3K, Akt, NF-κB, the effect of which may be the reduction of MMP-9 level and cell migration. In addition, treatment with this natural drug enhances E-cadherin and reduces VEGF level. Curcumin exhibits here appreciable effect on tumor cell ECM interaction with potential antagonist role on cell metastatic properties.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the Vice Chancellor, Ramakrishna Mission Vivekananda Educational and Research Institute and Principal, Kulti College for the research support.
Conflict of Interest: The authors declare no conflicts of interest.
REFERENCES
Aggarwal, B. B., Kumar, A. & Bharati, A. C. (2003). Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res, 23, 363-98.
ATCC culture method for MDA-MB-231 cells. Last accessed (12th May, 2020) from https://www.atcc.org/products/all/HTB-26.aspx#culturemethod.
Bader, A. G., Kang, S., Zhao, L. & Vogt, P. K. (2005). Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer, 5, 921-9.
Banerji, A., Chakrabarti, J., Mitra, A. & Chatterjee, A. (2004). Effect of curcumin on gelatinase A (MMP-2) activity in B16F10 melanoma cells. Cancer Lett, 211, 235-42.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 68, 394-424.
Chen, Y. R. & Tan, T. H. (1998). Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene, 17, 173-8.
Choudhuri, T., Pal, S., Agwarwal, M. L., Das, T. & Sa, G. (2002). Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett, 512, 334-40.
Ganguly, K. K., Sen, T., Pal, S, Biswas J. & Chatterjee, A. (2012). Studies on Focal Adhesion Kinase in human breast cancer cell MDA-MB-231. Adv Biol Chem 2, 29-42.
Gilmore, A. P. & Romer, L. H. (1996). Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell, 7, 1209-24.
Guarneri, C., Bevelacqua, V., Polesel, J., Falzone, L., Cannavo, P. S et al. NFkappaB inhibition is associated with OPN/MMP9 downregulation in cutaneous melanoma (2017). Oncol Rep, 37, 737-746.
Hawinkels, L. J., Zuidwijk, K., Verspaget, H. W., de Jonge-Muller, E. S., van Duijn, W., Ferreira, V. et al. (2008). VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer, 44, 1904-13.
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E. & Forman, D. (2011). Global cancer statistics. CA Cancer J Clin, 61, 69-90.
Katari, S.K., Pasala, C., Nalamolu, R.M., Vankadoth, U.N., Alexander, S.P., Pakala, S.R., et al. (2019). Pathophysiology of matrix metalloproteinases in breast cancer progression. J Clin Sci Res, 8, 145-50
Kohrmann, A., Kammerer, U., Kapp, M., Dietl, J. & Anacker, J. (2009). Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer, 9, 188.
Liu, Q., Loo, W. T., Sze, S. C. & Tong, Y. (2009). Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine, 16, 916-22.
Lopez-Lazaro, M. (2008). Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res, 52 Suppl 1, S103-27.
Maity, G., Choudhury, P. R., Sen, T., Ganguly, K. K., Sil, H. & Chatterjee, A. (2011). Culture of human breast cancer cell line (MDA-MB-231) on fibronectin-coated surface induces pro-matrix metalloproteinase-9 expression and activity. Tumour Biol, 32, 129-38.
Mitra, S. K., Hanson, D. A. & Schlaepfer, D. D. (2005). Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol, 6, 56-68.
Noe, V., Fingleton, B., Jacobs, K., Crawford, H. C., Vermeulen, S., Steelant, W.et al. (2001). Release of an invasionpromoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci, 114, 111-118.
Pal, S., Ganguly, K. K., Chatterjee, A. (2013). Extracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3. Cell Commun Adhes, 20, 105-14.
Pal, S., Ganguly, K. K., Moulik, S. & Chatterjee, A. (2012). Modulation of MMPs by cell surface integrin receptor alpha5beta1. Anticancer Agents Med Chem, 12, 726-32.
Qi, Q., Dai, M., Fan, H., Zhang, B. & Yuan, X. (2009). Inhibitory effect of curcumin on MMP-2 and MMP-9 expression induced by polyethylene wear particles and its mechanism. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 23, 677-82.
Ray, S., Chattopadhyay, N., Mitra, A., Siddiqi, M., Chatterjee, A. (2003). Curcumin exhibits antimetastatic properties by modulating integrin receptors, collagenase activity, and expression of Nm23 and E-cadherin. J Environ Pathol Toxicol Oncol, 22, 49-58.
Sein, T. T., Thant, A. A., Hiraiwa, Y., Amin, A. R., Sohara, Y., Liu, Y. et al. (2000). A role for FAK in the Concanavalin A-dependent secretion of matrix metalloproteinase-2 and -9. Oncogene, 19, 5539-42.
Sen, T., Moulik, S., Dutta, A., Choudhury, P. R., Banerji, A., Das, S. et al. (2009). Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci, 84, 194-204.
Tai, K. Y., Shieh, Y. S., Lee, C. S., Shiah, S. G., Wu, C. W. (2008). Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene, 27, 4044-55.
TOMEH, M. A., HADIANAMREI, R. & ZHAO, X. 2019. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int J Mol Sci, 20.
Torrealba, N., Vera, R., Fraile, B., Martinez-Onsurbe, P., Paniagua, R., Royuela, M. (2019). TGF-beta/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male epub: 1-11.
Woo, M. S., Jung, S. H., Kim, S. Y., Hyun, J. W., Ko, K. H. (2005). Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun, 335, 1017-25.
Yoganathan, N., Yee, A., Zhang, Z., Leung, D., Yan, J., Fazli, L. et al. (2002). Integrin-linked kinase, a promising cancer therapeutic target: biochemical and biological properties. Pharmacol Ther, 93, 233-42.
Zheng, C. C., Hu, H. F., Hong, P., Zhang, Q. H., Xu, W. W. et al (2019). Significance of integrin-linked kinase (ILK) in tumorigenesis and its potential implication as a biomarker and therapeutic target for human cancer. Am J Cancer Res, 9, 186-197.