Cell and Molecular Biology Laboratory, Department of Zoology, Rammohan College,
Raja Rammohan Sarani, Kolkata, India
Molecular Biology and Tissue Culture Laboratory, Post Graduate Department
of Vidyasagar College, Vidyasagar College, Kolkata, India
Department of Infertility, Institute of Reproductive Medicine, Kolkata, India
Corresponding author email: kaustavduttachowdhury@gmail.com
Article Publishing History
Received: 25/09/2023
Accepted After Revision: 14/12/2023
Study on occupational injuries indicates the industrial exposure to air-pollutants, asthmagens, carcinogens, and noise for extended hours as leading risk factors directing to death. This exposure generally occurs by inhalation, ingestion, or via dermal contact. Out of which inhalation is the most rapid route of uptake through breathing in the air that is contaminated with particulate matter/dust, vapours of volatile or semi-volatile contaminants and aerosols due to outdoor and indoor industrial activities. Irritational lung injury, asphyxia, respiratory depression, tachycardia, pulmonary edema may develop as long-lasting systemic effects even after completion of the working life of a worker.Most occupational lung diseases are caused by repeated, long-term exposure.
Therefore, our study was conducted to analyze the effect of 40 days of chronic calcium carbide exposure in a close chamber through inhalation in lung of Swiss-albino mice. ALT, AST, SOD and catalase activities were estimated spectrophotometrically. Spectrofluorimetric estimation was performed for reactive oxygen species determination. Flow cytometric analysis was performed to examine cell death and cell cycle. Pro-apoptotic and anti-apoptotic protein levels were estimated by immunoblot. Data demonstrated altered body homeostasis as marked by AST/ALT assay. 3gm CaC2 exposure indicated activation of antioxidant enzymes, increased cell death causing sustained animal survivability. 5gm and significantly 7gm CaC2 exposure displayed antioxidant enzymatic activities along-with decreased cell death and animal survivability. While in 9gm CaC2 exposure total antioxidant enzymes were collapsed with increased cell death leading to probably maintenance of animal survivability to some-extent in the said group.
Cac2, Cell Death, Lungs, Mice, Ros
Banerjee S, Ghosh P, Patra D, Chakraborty P, Chowdhury K. D. Effect of Calcium Carbide Exposure Through Inhalation in Lungs of Mus musculus. Biosc.Biotech.Res.Comm. 2023;16(4).
Banerjee S, Ghosh P, Patra D, Chakraborty P, Chowdhury K.D. Effect of Calcium Carbide Exposure Through Inhalation in Lungs of Mus musculus. Biosc.Biotech.Res.Comm. 2023;16(4). Available from: <a href=”http://surl.li/pefyd“>http://surl.li/pefyd</a>
INTRODUCTION
Recent time witnessed an increase in respiratory distress due to environmental pollution, lifestyle as well as occupational exposures. In this context, the lung is the most affected organ due to its delicate endothelial network being constantly involved in gaseous exchange with the environment. Report suggests that 1 in 20 people suffers from chronic respiratory diseases (CRDs) globally, attributing CRDs as the third leading cause of death in the world (Momtazmanesh et al., 2019).
Amongst all other causes of CRDs, professional hazard (i.e., breathing in chemicals, dust or noxious gases in industrial zones), is the most overlooked and neglected one. Occupational lung diseases may take a long time to develop and may have lasting effects on lungs even after the worker stops working. According to the World Health Organization (WHO), 125 million people worldwide are exposed to asbestos at work. According to global estimates, at least 90,000 people die each year from asbestosis, asbestos-related lung cancer and mesothelioma (Chen et al., 2022). Despite all efforts to prevent silicosis, it still afflicts tens of millions of workers and kills thousands of people every year, all over the world (Hoy et al., 2022).
Calcium carbide (CaC2) also known as calcium acetylide being a source of acetylene and other noxious gases is considered as hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). It is mainly used to manufacture acetylene and other industrial compounds. Pure CaC2 produces acetylene when it reacts with water. Commercial grade CaC2 is used in welding, desulphurization of steel, production of cyanamide and this grade of CaC2 contains impurities like arsenic, sulfur and phosphorus which emits harmful gaseous compounds when dissolved in water (Bini et al., 2021, Okeke et al., 2022).
CaC2 generated acetylene which is an analogue of ethylene is vastly used as fruit ripening agent in many developing countries as it is cheap and easily available (Maduwanthi and Marapana, 2019, Okeke et al., 2022). Reports suggest that ingestion of CaC2 through edibles may affect the neurological system via inducing prolonged hypoxia (Okeke et al., 2022). Moreover, calcium carbide being an alkali compound causes irritation in mouth, nasal pathways, gastric discomfort, as well as damages mucosal tissues in abdomen (Okeke et al., 2022).
Unwitty handling of CaC2 may also cause ocular burn injury to blindness (Bandyopadhyay et al., 2013). The range of hazards also includes dizziness, fatigue, difficulties in breathing, seizures. It has been reported that exposure for three weeks with crude acetylene has possible deleterious effects on heart (Grek et al., 1976), liver and kidney (Frang et al., 1967) as well as blood constituents. Survey from a welding industry also reported that the exposure of acetylene might have been increasing the risk of respiratory cancer among the workers (Riboli et al., 1983).Even though CaC2 shows potent toxicity through contaminated ingestion, much significant data is unavailable about its effect from direct inhalation which is common in industrial zones.
Considering the industrial atmosphere which is usually full of various fumes and other gaseous substances, occupational exposure to some extent is unavoidable for workers especially through respiration, in spite of proposed preventive measures. Regarding that, our study has been conducted to mimic such ambience and to investigate how chronic exposure of CaC2 and CaC2 generated obnoxious gases brings out deleterious effects in lungs.
MATERIAL AND METHODS
The technical grade calcium carbide (CaC2) was obtained from Sigma-Aldrich (21039).
Rest of the reagents were obtained from Sigma-Aldrich.
Animal maintenance & Schedule for exposure: Animal experiments were carried out in male Swiss albino mice (Mus musculus of 4‐6 weeks age; 20-25g) maintained at 27 ± 2 °C with relative humidity of 44–56% and in 12 h light/darkness cycle as well as free access of food and water in a cross-ventilated room. All animal experiments were performed following the ethical guidelines of the Institutional Animal Ethics Committee (IAEC), Rammohan College, Committee for Control and Supervision of Experiments on Animals (CCSEA), MoFAHD DAHD, Government of India. Experiments were designed to minimize animal suffering and to use the minimum number necessary for valid statistical evaluation.
All animals were separated into two groups, one CaC2 exposed group and a control group. The exposed group was further divide into four groups which were exposed to 3gm, 5gm, 7gm and 9gm of calcium carbide per day with requisite volume of water (w:v :: 1:10) for 15 minutes in a leak proof glass container of 24 litre volume. This exposure procedure was scheduled for 40days.
Survivability & Body weight-Organ weight Analysis: Animal survival was monitored daily and reported as the survivability (%) until 40 days. Each group consisted of five Swiss albino male mice (Mus musculus). Body weight and organ weight of every animal were recorded before sacrifice and after sacrifice respectively.
ALT and AST Activity: Blood was collected from animals and serum was prepared to estimate the activities of Aspartate transaminase (AST), alanine transaminase (ALT). Assays were performed following respective manufacturing kit protocols at room temperature. ALT and AST (TECO Diagnostics, CA, USA) activities were measured by estimating NADH oxidation at 320 nm wavelength for 30s intervals up to 2min.
SOD & Catalase: Superoxide dismutase (SOD) activity was measured from chloroform methanol extract following the standard protocol (Sengupta et al., 2014). Values were quantified spectrophotometrically (UV-1240 Pharma Spec, Shimadzu, Kyoto, Japan) by calculating the changes in pyrogallol auto-oxidation at 420 nm in presence of catalase enzyme. One unit of SOD activity is equal to the 50% suppression of superoxide mediated oxidation of pyrogallol. Results were represented in unit/mg protein.Catalase activity was evaluated spectrophotometrically (UV-1240 Pharma Spec, Shimadzu, Kyoto, Japan) by measuring degradation of H2O2 in presence of tissue lysate as a source of enzyme. Values were quantified by measuring absorbances at 240 nm in 10s intervals. Data were represented in unit/mg protein (Sengupta et al., 2017).
Estimation of ROS: Cells of the lung were isolated from experimental groups by Collagenase-IV digestion. Intracellular ROS was measured by incubating 5% cell suspension with 5mM 2,7-dichlorofluorescein diacetate (DCFDA) (Sigma-Aldrich, St. Louis, Missouri,USA), a fluorogenic dye, at 37°C for 15 min. After diffusion it was deacetylated by cellular esterase and turned into highly fluorescent 2′,7′-dichlorofluorescin (DCF) upon oxidation. Emitted fluorescence (Ex:485 nm/ Em: 535 nm) was estimated in RF-6000 Fluorescence Spectro-fluorometer (Shimadzu, Kyoto, Japan). Values were presented by Relative Fluorescence Unit (RFU) (Sengupta et al., 2014).
Flow Cytometric Analysis: Flow cytometric analysis was performed to analyze cell death and cell cycle by using FACS Calibur (BD Biosciences, Mountain View, CA, USA). Cells were suspended in Binding buffer (pH 7.4 containing 10mM HEPES–Na, 136mM NaCl, 2.7mM KCl, 2mM MgCl2, 1mM NaH2PO4, 5mM glucose, 5mg/ml BSA and 2.5 mM CaCl2). Annexin V-FITC and PI were then added to a concentration of 1μg/ml to each of the samples. Cell suspensions were then incubated for 15 min at room temperature in the dark. After incubation, samples were taken out for flow-cytometric analysis. Percentage of apoptotic cells were calculated using CellQuest software attached with the flow cytometer. (Sengupta et al., 2014).
In the process of cell cycle analysis, the samples were centrifuged at 500xg for 5 minutes. Supernatant was aspirated without disturbing the pellet. The cells were then washed by 1x PBS. Pellets were resuspended in 200μL 1x propidium iodide and RNase staining solution. The incubation was continued for 20-30 minutes in the dark at 37°C. In the next step, samples were placed in ice (still in dark) and cell cycle phases were determined by calculating PI fluorescence using flow cytometer.
Western Blot Analysis: Whole cell lysates were used as protein source for quantitative analysis of cellular Bcl2, Bax, p53 expressions and caspase3 activity. SDS-PAGE resolved proteins were transferred to the nitrocellulose membrane. Then the membrane was blocked in 3% BSA in TBST (50 mmol/L Tris‐HCl, pH 7.5, 150mmol/L NaCl, 0.1% Tween20) and subsequently incubated with respective primary antibodies (Santa Cruz Biotechnology) (1:500 to 1:1000 dilutions in TBST). Next the membrane was incubated with alkaline phosphatase tagged secondary antibody (Santa Cruz Biotechnology) (1:2000 dilutions in TBST) and binding signals were visualized with NBT-BCIP. Respective band densitometric analysis was performed with ImageJ software (NIH, Bethesda, MD, USA).
Cell Count: Cell count was performed by calculating cell number using ImageJ software in the 1mm x 1mm area of the histological section of lung tissue. The process was repeated for six independent observations (n= 5) for each tissue to get statistically significant data on average (Grishagin, 2015).
Statistical Analysis: Conventional methods were used for calculation of means and SEM. Statistically differences among exposed groups were evaluated by Student’s t-test. Data analysis was carried out using the GraphPad Prism software, La Jolla, CA, USA.
RESULTS AND DISCUSSION
To determine the harmful effect of CaC2, our experimental model Swiss albino male mice (Mus musculus) were divided into four different groups and chronically exposed with 3gm, 5gm, 7gm and 9gm of CaC2/day. After 40 days of exposure the percentage of survivability visibly altered (Fig. 1a). Survivability bar of the 3gm CaC2 exposed group of animals decreased slightly and the change seemed non-significant with respect to the control group of animals (p<0.05). Animal survivability further sloped down gradually in 5gm and 7gm consecutively and significantly spiked in 9gm CaC2 exposed animals.
As the route of exposure was through inhalation, the whole-body weight and targeted organ i.e., lung weight were estimated from respective groups of exposed animals. Values indicated that both the parameters have been increased in 3gm and 5gm of CaC2 exposed group with respect to control, whereas, in 7gm the body weight drastically declined and organ weight increased (Fig. 1b). This sequence was reflected in the ratio-metric analysis of organ weight and body weight (Fig. 1c) where 3gm and 5gm CaC2/day exposed groups depicted increasing patterns and 7gm showed a sharp spike. The trend of alteration in body weight, organ weight exactly reversed in 9gm of exposed group and the organ weight-body weight ratio decreased.
Considering the visible changes of body and organ weight, cell count was performed by calculating cell number in the 1mm x 1mm area of the histological section of lung tissue. Data were obtained from six independent experiments (n=5) for each tissue to get statistically significant data on average. Result showed that cell number slightly increased in 3gm and further increased in 5gm CaC2/day exposed group. Value suggested significant increase in cell count in 7gm and 9gm of CaC2 exposed group with respect to control group of animals (Fig. 1d).
Figure 1: Change in survivability, body weight, organ weight and cell
count along the course of calcium carbide (CaC2) exposure.
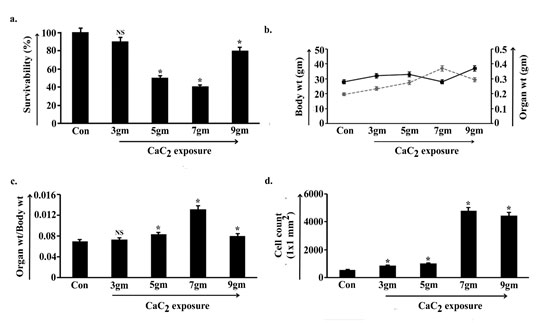
Survivability analysis was measured and represented as survivability percentage against control mice in 3gm, 5gm, 7gm and 9gm CaC2 exposed group (a). Body weights and organ (lung) weights of the animals of control and four CaC2 exposed groups were measured in gm (b) and ratio-metric analysis of organ (lung) weights and body weights were demonstrated (c). Number of cells at lung collected from control, 3gm, 5gm, 7gm and 9gm CaC2 exposed group, were represented in cell count (1X1mm2) (d). Data were expressed as mean+SD and were obtained from six independent experiments (n=5). NS, *p<0.05 vs Control. Con= Control.
The non-sequential variation in body weight-organ weight as well as cell number analysis clearly indicated pulmonary alterations which instigated us to estimate the activities of liver enzymes ALT (Alanine Aminotransferase) and AST (Aspartate Aminotransferase) as markers of body specific stability in mice. In both the observations activity levels of enzymes gradually elevated in the four groups of animals viz. 3gm, 5gm, 7gm, 9gm with respect to control, showing an ascending staircase trend along with the course of CaC2 exposure (Fig. 2a, 2b). Such a result being an obvious indicator of disrupted body homeostasis and level of ROS (Reactive Oxygen Species) was quantified which serves as a regulatory index of the same.
ROS content was found higher in 3gm of CaC2 exposed group of animals than rest of the exposed groups. 5gm and 7gm showed visibly lower ROS compared to 3gm CaC2 exposure. In 9gm it was moderately high yet considerably lower than 3gm, exhibiting a dose responsive non- linear pattern of oxidative stress in four exposed groups (Fig. 2c). Activity of widely known antioxidant enzymes SOD and catalase were determined to validate the variation of the said oxidative stress.
SOD was activated in 3gm CaC2 exposure and catalase activity was maintained to some extent with respect to control. As SOD activity increased in 5gm compared to 3gm, evidently potent ROS was generated but the maintained catalase activity in 5gm might have a role in lowering the oxidative stress. SOD activity reached its highest peak in 7gm and catalase activity was sustained. This phenomenon implied that a higher amount of CaC2 exposure gave rise to an extra amount of ROS which was converted into H2O2 by high SOD activity, followed by persistent catalase activity that turned the additional H2O2 into H2O and O2. Activity of both SOD and catalase significantly decreased in 9gm insinuating the probable crumpling of the protective antioxidant system (Fig. 2d)
Figure 2: Estimation of oxidant-antioxidant homeostasis in the body
along the course of calcium carbide (CaC2) exposure.
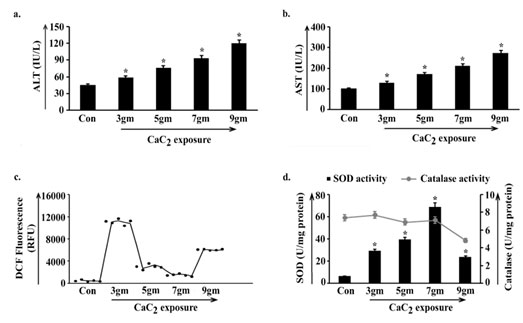
Liver stress specific bio-markers ALT (a), AST (b) were estimated in blood serum isolated from control and 3gm, 5gm, 7gm and 9gm CaC2 exposed group of mice. Values were represented in IU/L. Level of reactive oxygen species was estimated by measuring DCF fluorescence (c) and was represented in RFU. Lysates were processed from lungs isolated from control, 3gm, 5gm, 7gm and 9gm CaC2 exposed groups. Status of SOD and catalase activities (d) were measured and were represented in U/mg protein. Data were expressed as mean+SD and were obtained from six independent experiments (n=5). *p<0.05 vs Control. Con= Control.
Different levels of ROS are the determining factors of cell fate via inducing either cell survivability or apoptosis. That instigated us to check the balance between anti-apoptotic and pro-apoptotic proteins. In Fig. 3b Densitometric analysis of anti-apoptotic Bcl2 protein showed significant decrease in 3gm of exposed group with respect to control. Values increased in 5gm and further in 7gm and a significant fall was portrayed in 9gm. Parallely, band intensity analysis of pro-apoptotic Bax protein demonstrated sufficient increase in 3gm and consequent decrease in 5gm and significantly in 7gm while value again escalated in 9gm CaC2 exposure. Chief regulator of apoptosis p53 nearly mirroring the data of Bax, elevated in 3gm and gradually decreased in 5gm and 7gm exposure. A visible rise in intensity was found at 9gm after scheduled exposure (Fig. 3b). Band intensity of executioner caspase, the caspase3 was augmented in 3gm and 9gm, on the other hand value was reduced in 5gm and further in 7gm with respect to 3gm of exposure (Fig. 3c).
Changes in pro-apoptotic and anti-apoptotic balance instigated us to estimate the percentage of apoptotic cells under chronic CaC2 exposure. Data depicted that FITC positive cells increased in 3gm (both in early and late) while a decreasing trend was observed in 5gm. Value of early apoptosis was further reduced while the number of late apoptotic cells were increased in 7gm exposure. The 9gm CaC2 exposed group again exhibited sufficiently increased early apoptotic cells with a non-significant change in late apoptotic group with respect to control (Fig. 3a).
Cell cycle analysis indicated increased subG1 phase, reduced G1 and S as well as a proficient increase in G2/M in 3gm signifying increased cell death, cell proliferation and G2/M arrest. 5gm represented reduced subG1 to some extent, increased G1 and sustained S as well as reducing trend in G2/M arrest. 7gm depicted further reduced subG1, significant increase in G1, reduction in S and G2/M arrest indicating a trend towards cell survivability. 9gm indicated significant increase in subG1 with a decreasing trend in G1 and S as well as a trend towards increase in G2/M arrest representing cell death (Fig. 3d).
Figure 3: Apoptotic parametric analysis along the course of
calcium carbide (CaC2) exposure
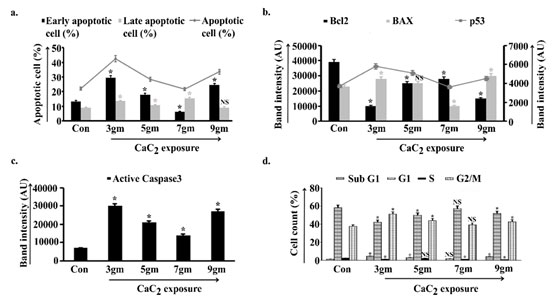
obtained from six independent experiments (n=5). NS, *p<0.05 vs Control. Con= Control.
Apoptotic cell percentage were estimated from the pulmonary cells isolated from control, 3gm, 5gm, 7gm, 9gm CaC2 exposed group (a). Bcl2, Bax and p53 expression (b), as well as caspase3 activity were measured in whole cell lysate of lung isolated from control and all of the four CaC2 exposed groups of mice (c). Densitometric analysis of target specific bands were represented in AU. The cell percentage in different stages of cell cycle were estimated in control and CaC2 exposed groups of mice (d). Data were expressed as mean+SD and were
Countless reports have shown that technical grade CaC2 contains traces of arsenic, phosphorus and other impurities. Assessment of CaC2 treated mangoes via inductively coupled plasma mass spectrometry has also found traces of heavy metals like lead, chromium, cadmium (Hassan et al., 2019). The adverse effects of these impurities cause both acute and chronic conditions when ingested with CaC2 contaminated food. Mostly in developing countries, due to lack of awareness, CaC2 is used by many food vendors to boil eggs, soften the beans and to keep the food warm at the time of selling.
Previous in vivo studies suggest that oral intake of CaC2 induces an array of abnormalities like derangement of hematopoiesis and organ toxicity (Ouma et al., 2022), disruption in reproductive system in both male and female mice (Bafor et al., 2019), hematological alterations (Appah et al., 2019), alteration in plasma electrolyte concentration and kidney function (Ugbeni and Alagbaoso, 2023). Compared to the effect of ingestion, toxicity through inhalation is more of a silent killer to the internal system.
Through inhalation the toxicant directly reaches the lungs and hampers the normal gaseous exchange leading to accumulation of CO2 and oxygen deprivation. As a result, blood pH falls which reduces tissue specific metabolic rate, also alters peripheral homeostasis. A study on the effects of chronic exposure of crude acetylene on white rabbits showed a significant increase in SOD activity heart, ALT and AST activities in serum while catalase activity was suppressed in heart, liver and kidney (Okolie et al., 2005). In this context our study dealt with the effect of CaC2 exposure through respiration and therefore the primary affected organ was obviously lungs.
Activities of ALT and AST were altered under CaC2 exposure which indicated unsteady body homeostasis. This finding led to the estimation of oxidative stress in targeted organ, lungs, as the chosen route of exposure was inhalation. The outcome showed that the modification of ROS happened in four CaC2 exposed groups of animals in a dose dependent manner. Such variation of generated ROS probably was corroborated by the estimated status of SOD and Catalase activities.
CaC2 exposure activated the SOD in the 3gm exposed group and further increased in 5gm. A balanced catalase activity was maintained in both the said exposed groups which perhaps helped in lowering the ROS generated under exposure. Activity status of SOD was expressed its peak value in 7gm and lowest value in 9gm of CaC2/day exposed group. On the other hand, catalase increased in 7gm and visibly decreased in 9gm.
Elevation of SOD and sustained activities of catalase possibly mitigated the additional ROS generated from increasing doses of CaC2 exposure. This mechanism perhaps shielded the harmful effects of exposure up to a certain level and collapsed in the extreme condition. Excess cellular levels of ROS damages cell organelles which can lead to activation of programmed cell death. Moderate ROS contributes to the control of cell proliferation and differentiation (Perillo et al., 2020). In this study, alterations in the antioxidant system might lead to development of a stressed environment which is generally associated with the change in apoptotic balance shifting under experimental condition.
Here enhancement of cell death in lowest dose (3gm/day) of CaC2 exposure and reduction of cell death in two median doses (5gm/day and 7gm/day) likely indicated the pro- to anti-apoptotic shifting of cellular fate. Further, the highest dose of exposure (9gm/day) indicated the augmentation of cell death due to another shifting of anti- to pro-apoptotic balance. Alteration in cell death probably reflected in organ weight since organ weight/body weight ratio was enhanced in 5gm and most effectively in 7gm CaC2/day exposed group. More over increase in cell death perhaps helped in restoration of organ weight, as organ weight/body weight ratio in the 9gm CaC2/day exposed group nearly returned to the control group of animals.
Compared to apoptotic status, the cell counts are almost maintained in 3gm and 5gm CaC2 exposed groups with respect to control. Even though the early onset of apoptosis in both the cases were visibly significant, the accumulation of inflammatory cells under exposure probably played a role in this maintenance. In the 7gm of exposed group, low percentage of apoptotic cells indicated the way of direction towards cell survival and cell count were found high as it might be expected. Apoptotic cell percentage increased in 9gm but cell count also notably augmented. This was probably due to the accumulation of a higher number of inflammatory cells under a higher extent of exposure.
CONCLUSION
A number of physio-biochemical parameters were altered under CaC2 exposure, but survivability of exposed animals was the most noticeable factor. In spite of high ROS and augmented cell death, survival rate in 3gm was highest amongst all exposed groups, perhaps due to 3gm being a considerably low and endurable dose of exposure. In 5 and 7 gm exposed groups, lower apoptosis probably indicated retention of damaged cells which might have resulted in low survival capacity of said groups. Higher rate of apoptosis in 9 gm possibly pointed towards elimination of exposure affected cells, thus increasing the survivability of mentioned group.
SAuthor’s Contribution: KDC and PC were responsible for conceptualization and designing of the study. SB and PG were responsible for model development, planning of experiments and data collection. KDC, SB, PG and DP were responsible for result analysis and interpretation. All authors equally contributed in literature research, manuscript preparation, editing and review.
ACKNOWLEDGEMENTS
Authors are thankful to Dr. Tuli Biswas, Retired Scientist, CSIR-Indian Institute Chemical Biology, Kolkata, West Bengal; Dr. Gargi Sen, University of Kalyani, Kalyani, Nadia, West Bengal; Dr. Samarendra Nath Banerjee, Department of Zoology, Rammohan College, Kolkata, West Bengal; Mr. Mriganka Biswas from Chota Jagulia High School (H.S), Chhota Jagulia, North 24 Parganas, West Bengal; Dr. Sujan Chatterjee, University of Nevada, Las Vegus, USA for critical comment, scientific discussion and helpful suggestions.
Statement of Ethics: This study was approved by the Institutional Animal Ethics Committee (IAEC), Rammohan College.
Declaration of Conflicting Interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/ or publication of this article.
Funding: The project was funded by SCIENCE & TECHNOLOGY AND BIOTECHNOLOGY, Government of West Bengal (WB-DST), MEMO NO. 198(Sanc.)/ ST/P/S&T/9G-45/2017 DATED 21/03/2018.
REFERENCES
Appah, J., Aina, V.O., MUDI, I. and Auta, R., 2019. Effects Of Industrial Grade Calcium Carbide On Haematological Parameters Of Wister Albino Rats. Journal of Pharmaceutical & Allied Sciences, 16(1).
Bafor, E.E., Greg-Egor, E., Omoruyi, O., Ochoyama, E. and Omogiade, G.U., 2019. Disruptions in the female reproductive system on consumption of calcium carbide ripened fruit in mouse models. Heliyon, 5(9).
Bandyopadhyay, S., Saha, M., Biswas, S., Ranjan, A., Naskar, A.K. and Bandyopadhyay, L., 2013. Calcium carbide related ocular burn injuries during mango ripening season of West Bengal, eastern India. Nepalese Journal of Ophthalmology: a Biannual Peer-reviewed Academic Journal of the Nepal Ophthalmic Society: NEPJOPH, 5(2), pp.242-245.
Bini, M., Rajesh, B. and Babu, T.D., 2021. Chronic exposure of industrial grade calcium carbide and ethylene glycol exert genotoxic effect in Wistar albino rats. Journal of Basic and Clinical Physiology and Pharmacology, (0), p.20200360.
Chen, J., Wang, C., Zhang, J., Zhang, T., Liang, H., Mao, S., Li, H. and Wang, Z., 2022. A comparative study of the disease burden attributable to asbestos in Brazil, China, Kazakhstan, and Russia between 1990 and 2019. BMC Public Health, 22(1), pp.1-9.
Frang, D., Csata, S., Szemenyei, K. and Hamvasi, G., 1967. Renal damage following acetylene glycol poisoning. Orvosi Hetilap, 108(12), pp.553-556.
Grek, O.R., Dolgov, A.V. and Iziumov, E.G., 1976. Stabilization of biological membranes with various acetylene amines. Farmakologiia i Toksikologiia, 39(4), pp.483-487.
Grishagin, I.V., 2015. Automatic cell counting with ImageJ. Analytical biochemistry, 473, pp.63-65.
Hassan, S., Mazhar, W., Farooq, S., Ali, A. and Musharraf, S.G., 2019. Assessment of heavy metals in calcium carbide treated mangoes by inductively coupled plasma-mass spectrometry (ICP-MS). Food Additives & Contaminants: Part A, 36(12), pp.1769-1776.
Hoy, R.F., Jeebhay, M.F., Cavalin, C., Chen, W., Cohen, R.A., Fireman, E., Go, L.H., León‐Jiménez, A., Menéndez‐Navarro, A., Ribeiro, M. and Rosental, P.A., 2022. Current global perspectives on silicosis—Convergence of old and newly emergent hazards. Respirology, 27(6), pp.387-398.
Maduwanthi, S.D.T. and Marapana, R.A.U.J., 2019. Induced ripening agents and their effect on fruit quality of banana. International journal of food science, 2019.
Momtazmanesh, S., Moghaddam, S.S., Ghamari, S.H., Rad, E.M., Rezaei, N., Shobeiri, P., Aali, A., Abbasi-Kangevari, M., Abbasi-Kangevari, Z., Abdelmasseh, M. and Abdoun, M., 2023. Global burden of chronic respiratory diseases and risk factors, 1990–2019: an update from the Global Burden of Disease Study 2019. E Clinical Medicine, 59.
Okeke, E.S., Okagu, I.U., Okoye, C.O. and Ezeorba, T.P.C., 2022. The use of calcium carbide in food and fruit ripening: Potential mechanisms of toxicity to humans and future prospects. Toxicology, 468, p.153112.
Okolie, N.P., Ozolua, R.I. and Osagie, D.E., 2005. Some biochemical and haematological effects associated with chronic inhalation of crude acetylene in rabbits. J. Medical Sci, 5, pp.21-25.
Ouma, P.A., Mwaeni, V.K., Amwayi, P.W., Isaac, A.O. and Nyariki, J.N., 2022. Calcium carbide–induced derangement of hematopoiesis and organ toxicity ameliorated by cyanocobalamin in a mouse model. Laboratory Animal Research, 38(1), p.26.
Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., Castoria, G. and Migliaccio, A., 2020. ROS in cancer therapy: The bright side of the moon. Experimental & molecular medicine, 52(2), pp.192-203.
Riboli, E., Bai, E., Berrino, F. and Merisi, A., 1983. Mortality from lung cancer in an acetylene and phthalic anhydride plant: A case-referent study. Scandinavian journal of work, environment & health, pp.455-462.
Sengupta, D., Chowdhury, K.D., Chatterjee, S., Sarkar, A., Paul, S., Sur, P.K. and Sadhukhan, G.C., 2017. Modulation of adenylate cyclase signalling in association with MKK3/6 stabilization under combination of SAC and berberine to reduce HepG2 cell survivability. Apoptosis, 22, pp.1362-1379.
Sengupta, D., Chowdhury, K.D., Sarkar, A., Paul, S. and Sadhukhan, G.C., 2014. Berberine and S allyl cysteine mediated amelioration of DEN+ CCl4 induced hepatocarcinoma. Biochimica et Biophysica Acta (BBA)-General Subjects, 1840(1), pp.219-244.
Ugbeni, O.C. and Alagbaoso, C.A., 2023. Calcium carbide-ripened plantain induced alterations in plasma electrolytes concentration and kidney function in rats. Brazilian Journal of Nephrology.


