Department of Biotechnology, Dr. G.R. Damodaran College of Science (Autonomous), Coimbatore-641014, Tamil Nadu, India
Corresponding author email: sugantham2000@gmail.com
Article Publishing History
Received: 13/04/2020
Accepted After Revision: 27/05/2020
Anti-listerial bacteriocin producing bacteria were isolated and identified using 16S rRNA sequencing and the bacteriocin were purified by Amberlite XAD-16 absorption. The biofilm forming ability of Listeria monocytogenes MTCC657 was assessed using tube method and CVB assay. Biofilm formation on substrates were also checked using different substrates. Polystyrene possessed strong biofilm formation of (0.185) Listeria monocytogenes MTCC657. In biofilm eradication studies, the bacteriocin GRDS6 showed similar activity of Nisin on all the three substrates (polystyrene, glass and aluminum foil) at 45°C, 30°C, 4°C and -20°C. Based on CBD® assay, the activity of GRDS6 bacteriocin and Nisin were similar in both high (45°C) and freezing (-20°C) temperatures. This study shows that the GRDS6 bacteriocin is able to inhibit the biofilm formed by Listeria monocytogenes MTCC657 under different temperatures and different incubation periods. The activity of GRDS6 bacteriocin is similar to activity of Nisin. So we recommend to use this bacteriocin in the food preservation industry at higher (45°C) to freezing temperature (-20°C). In the present study, toddy was used as a source for isolation of bacteriocin producing bacteria. The isolate we assayed for its bacteriocin production. Listeria monocytogenes MTCC657 is able to produce biofilm which helps the microorganism to survive and grow for an extended period of time. Adhesive ability and biofilm formation ability of Listeria monocytogenes MTCC657 on solid substrates (polystyrene, glass and aluminum foil) at different temperatures was determined and found to be higher on polystyrene at 72 h followed aluminum foil and glass. The biofilm eradication potential of GRDS6 bacteriocin and Nisin were evaluated by microtiter plate method and CBD® assay. Based on our results the activity of GRDS6 bacteriocin was similar to Nisin. The incubation temperature doesn’t influence the activity of the bacteriocin, so we recommend this bacteriocin in food storage industry.
Bacteriocin, Biofilm, Listeria Monocytogenes, Nisin
Krishna A. R, Chockalingam J, Jayalekshmi S. K, Mary T, Antony P, Ramasamy S. Effect of Bacteriocin of Paenibacillus Polymyxa on Biofilm Forming Listeria Monocytogenes MTCC 657. Biosc.Biotech.Res.Comm. 2020;13(2).
Krishna A. R, Chockalingam J, Jayalekshmi S. K, Mary T, Antony P, Ramasamy S. Effect of Bacteriocin of Paenibacillus Polymyxa on Biofilm Forming Listeria Monocytogenes MTCC 657. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2yNAYQa
Copyright © Krishna et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Microorganisms can exist in the environment as planktonic cells or on the surface in biofilms enclosed with in a matrix predominantly made up of polysaccharide material (Gandhi et al, 2007). The existence of these bacteria can be termed as biofilms; a microbially derived sessile community characterized by the cells that are irreversively attached to a substratum or interface or to each other, embedded in a matrix of extracellular polymeric substance that they have produced and with an altered phenotype with respect to growth rate and gene transcription (Donlan et al, 2002). The formation of biofilms by some pathogenic bacteria such as Listeria monocytogenes have been reported. Such biofilms could be continuous sources of contamination of food and medical products in contact with them. That may also lead to spoilage of foods or transmission of food borne diseases (Harvey et al, 2006, Shivangi et al 2020).
Listeria monocytogenes has been recognized as a major food borne pathogen with the ability to survive in various environmental conditions such as extreme cold conditions (Donnelly et al, 1997), at lower pH such as 3.6 in foods (Parish et al, 1989), in salt concentration up to 10% (Mc Clare et al, 1989), in the presence of surfactant sanitizers (Frank et al, 1990) and at high temperatures (Mc Carty et al, 1990). It has been demonstrated that Listeria monocytogenes can grow and form biofilms on several food processing surfaces including rubber, plastics, glass and stainless steel (Wong et al, 1998, Di Bonaventura et al, 2008). It is also found in fermented products (cheese, yogurt, fermented milk, sausages etc.) made from raw materials contaminated with the organism (Berry et al, 1999). The infection caused by Listeria monocytogenes (listeriosis) can lead to gastroenteritis, septicemia, perinatal infections, miscarriages, meningitis and meningo-encephalitis in immune-compromised individuals (Barbuddhe et al, 2012, Eliot et al, 2016 Gurgu et al 2019).
Listeria monocytogenes can adhere rapidly and firmly to inert surfaces commonly found in the food processing industry (Frank et al, 1990) with protection against various physical and chemical stress is conferred on when they adhere. The cellular mechanisms underlying microbial biofilm formation and behavior are beginning to be understood and are targets for novel specific intervention strategies to control problems caused by biofilm formation in these different fields, and in particular for the food processing environments. Biofilm of Listeria monocytogenes is much more resistant to environment stress and can stay for long time on the surface. Bacteria in the form of biofilm will be protected against disinfectant as the efficiency of disinfectant declines in the presence of biofilm; sanitation faces serious difficulties in food industries (Chapman et al, 2003). Investigation of bacteriocins which inhibit pathogens such as Listeria monocytogenes has become particularly attractive for the food industry. Listeria monocytogenes in ready to eat (RTE) food, is of special concern because it can adhere to abiotic surfaces in food processing creating a cellular mass that joins nutritious residues and other microorganisms forming biofilms (Regiane et al, 2008 Gurgu et al., 2019 Shivangi et al 2020).
The various bacteriocins have shown great diversity in their effects on bacterial species (Radler et al, 1990). Bacteriocins are defined as extracellular bioactive peptides or peptide complexes that are bactericidal, because of the combined action of the bacteriocin and the host autolysin (Martinez cuesta et al, 2000) or bacteriostatic against other species, usually closely related to the producer strain. The ability to produce bacteriocin is associated with the presence of a stable genetic factor in the cell and is composed of DNA (De Witt et al, 1965). Bacteriocins produced by lactic acid bacteria have attracted increasing attention since they are active in a nanomolecular range and have no toxicity. Bacteriocins and similar metabolites produced by certain group of bacteria like lactic acid bacteria (LAB), isolated from different types of food, have been known to inhibit the growth of Listeria monocytogenes (Colak et al, 2007 Gurgu et al., 2019 Shivangi et al 2020).
These bacteriocins can be considered as an alternative to the use of chemical preservatives (Zhu et al, 2005). Bacteriocins produced by Lactococcus species, especially Nisin are widely used in foods since the LAB have generally been regarded as safe (GRAS) organisms. Nisin is active especially at the lower pH values of many fruits and some vegetables (Jung et al, 1992). In the food industry, Nisin is obtained from the culturing of certain LAB present in natural sources such as milk, milk products, meat etc. and it can’t be chemically synthesized. The anti-microbial activity of Nisin on planktonic cells of Listeria monocytogenes has been well identified (Buddee et al, 2000). Studies addressing the effect of Nisin producing strains on Listeria monocytogenes biofilms are less (Leriche et al, 1999). Paenibacillus polymyxa is a common soil bacterium which belongs to plant growth promoting (PGPR) rhizobacteria. (Ash et al, 1993). Paenibacillus polymyxa is widely distributed in the environment as well as food products (Gupta et al, 2014, Gurgu et al., 2019).
Several small peptide antibiotics, for e.g.: Polymyxin B produced by this organism (Kimura et al, 2009). Isolated from various sources the genus Paenibacillus comprises bacterial species relevant to humans and animals, plants and the environment. Paenibacillus derived anti-microbial substances also have application in medicines including polymyxins (bacteriocin like substance) and fusaricidins, which are non-ribosomal lipopeptides first isolated from strains of Paenibacillus polymyxa (Eliot et al, 2016). The main objective of this study is to find out the biofilm formation potential of Listeria monocytogenes on different substrates and its eradication using bacteriocin and Nisin by different methods like Micotitre plate method and Calgary biofim device method.
MATERIALS AND METHODS
Screening of bacteriocin producing bacteria: Toddy samples were collected from Palakkad district of Kerala, India. A tenfold dilution of the sample were serially diluted and plated in MRS agar (Man Rogosa De Sharpe Agar) using spread plate technique. The plates were incubated for 48 to 72h. Microorganism producing bacteriocin were identified using spot on lawn method in which Listeria monocytogenes MTCC657 was used as test organism. Listeria monocytogenes (MTCC657) were obtained from Microbial Type Culture Collection, Chandigarh, India. The cultures were maintained in MRS Agar (HiMedia) and Nutrient Agar (HiMedia) respectively. The isolate was identified based on morphological, biochemical and molecular (16S rRNA sequencing). For molecular identification the bacterial DNA was amplified with universal primers and send to Eurofins Scientific, Bangalore, (India) for genus identification. The sequence after identification were submitted to GenBank, NCBI.
Bacteriocin purification: The crude extract harvested from the isolate were purified using Amberlite XAD-16(HiMedia) adsorption by column chromatography. 5g of Amberlite XAD-16 were soaked in 50% isopropanol and stored at 4°C overnight. This was then washed repeatedly with distilled water to remove isopropanol. The cleaned Amberlite was added to 250mL of heat inactivated cell free supernatant of the isolate and incubated for 4h in a shaking incubator. This was then transferred to a chromatographic column for elution. The matrix was washed with 20mL of distilled water and 20mL of 40% Ethanol. The extract were eluted with 20mL of 70% isopropanol and followed by washing with 20mL of absolute ethanol. The eluted extract was evaporated to half its volume.
Nisin: 100mg of Nisin (HiMedia) was solubilized in 10mL of 0.02N HCL to give the concentration of 104 IU/mL (40 IU=1g). The solution was sterilized by filtration through 0.45mm filters and was stored at -4°C. (Mahdavi et al, 2007)
Biofilm formation studies: The objective of this study is to investigate the biofilm forming ability of Listeria monocytogenes MTCC657 on abiotic surfaces such as stainless steel, glass and aluminum foil at different temperatures using microtiter plate assay in order to evaluate the reduction potential of Nisin and GRDS6. A parallel study was undertaken to determine the minimal biofilm eradication concentration (MBEC) of GRDS6 and Nisin (standard) against Listeria monocytogenes MTCC657 biofilm using Calgary Biofilm Device (CBD®).
Tube method: Listeria monocytogenes MTCC657 isolates were tested for biofilm formation by standard method (Christensen et al 1982). 5mL of Tryptic Soy Broth (TSB) in test tubes were inoculated with a loop full of microorganisms from overnight culture plates and incubated for 48h at 37°C. After incubation the contents were decanted and washed with Phosphate buffered saline (PBS pH 7.3) and kept for drying at room temperature. Then the tubes were stained with crystal violet solution (4%) and rotated for uniform staining and contents were decanted. The tubes were placed upside down for draining. The biofilm formation was observed when a visible film lined wall and bottom of the tubes.
Microtiter Plate Method (Christensen et al, 1985):The test organism were inoculated from overnight cultured Nutrient agar plates into 5mL of TSB broth and incubated 18h at 30° C and transferred (125 µL) to 5mL growth medium (TSB). Then the culture were vortexed for 1min and a volume of 100µL was transferred into the wells of a 96 well microtiter plate. Plates were covered with a tightly fitting lid and incubated at 30° C for 24h, 48h and 72h. Each plate included 12 control wells comprising of 100µL of un-inoculated growth medium. After incubation, the cultures were removed and cell densities were determined by measuring turbidity at 595nm. The plates were then washed three times with sterile distilled water to remove loosely bounded bacteria and dried at 30° C for 3min. 100µL of 95% alcohol was added to each well to destain the biofilm and the concentration of crystal violet was determined by measuring optical density (OD) at 595nm.
Biofilm formation on different substrates: Overnight culture of the test organism was grown in 100mL conical flask containing TSB at 30° C for 24h. One un-inoculated conical flask with growth medium kept for incubation as control. The abiotic substrates were washed with detergent, rinsed with sterile distilled water and air dried and placed into hot air oven at 75° C for 30min. After 24h incubation, sterile substrates were aseptically added to the grown broth culture and incubated at 30° C for 24h, 48h and 72h. At the end of each incubation period, a set of substrates were removed aseptically from the broth culture for biofilm quantification using Crystal violet binding assay (CVB) (Stepanovic et al, 2004). The substrates were then washed with 5mL of deionized water and the adherent bacteria were fixed with 250mL of Methanol. Each substrate was stained with crystal violet for 15min and the excess stain were removed under running tap water and air dried. The dye bound to the adherent cells were resolubilized with 2.5mL of 33% glacial acetic acid. The resolubilized liquid of each substrate were collected and absorbance was measured at 595nm in which un-inoculated control was set as blank using spectrophotometer (HACH) (Pawar et al 2005).
Biofilm eradication studies on Listeria monocytogenes MTCC 657 using GRD S6 and Nisin Microtiter Plate Method: The microtiter plate method was slightly modified in which abiotic substrates (polystyrene, glass and aluminum foil) were added into sterile 96 well microtiter plate and filled with 200 µL of bacterial culture. Un-inoculated broth was used as negative control. The plates were covered and incubated at different temperatures ranging 45°C, 30°C, 4°C and -20°C for 72h. After incubation the substrates were transferred into fresh and sterile microtiter plate and washed three times with 250µL of sterile phosphate saline. The plates were shaken vigorously to remove non-adherent bacteria and added 200µL of different concentration of GRD S6 and Nisin to each well except control well and incubated for 1h. After incubation, the bacteriocins were removed and washed 5 times with sterile distilled water to remove loosely adherent bacteria, Nisin and GRD S6 (Mahdavi et al, 2007). The plates and substrates were stained for 5min with 200µL of 2% crystal violet per well. Excess stain was rinsed off and air dried. The dye bound to the adherent cells were resolubilized with 160µL of 33% glacial acetic acid and incubated for 15min at 30°C. The optical density was measured at 595nm using an ELISA reader. The reduction percentage of biofilm were calculated using the following formula (Harvey et al 2007, Stepanovic et al, 2000)

Calgary Biofilm Device® Method: Listeria monocytogenes MTCC657 biofilms were grown at different temperatures such as 45°C, 30°C, 4°C and -20°C to determine the biofilm eradication potential using Nisin and GRDS6 at different concentrations.
Step 1: Growing Listeria biofilm: The indicator strain inoculum were prepared (Harvey et al, 2007) and 200µL each were added to all the wells in microtiter plate closed with lid containing polystyrene pegs and incubated for 72h at 45°C, 30°C, 4°C and -20°C.
Step 2: Preparation of challenge plate: After incubation of 72h, a new microtiter plate was taken and two fold dilutions of GRDS6 and Nisin were prepared in TSB broth. Biofilm pegs were washed three times with sterile PBS. The pegs were then immersed in a fresh microtiter plate containing two fold dilutions of bacteriocins and incubated at 45°C, 30°C, 4°C and -20°C for 24h.
Step 3: Neutralization and recovery: To a new sterile microtiter plate, add 200µL of freshly prepared TSB broth to all the wells. The pegs from challenge plate were removed and rinsed twice with PBS. The pegs were then immersed into a new microtiter plate (recovery plate) and sonicated for 24h to disrupt the biofilm from the surface of the pegs. 200µL of neutralizing agent (Proteinase K) were added to the challenge plate and kept for 24h incubation.
Step 4: Determination of MIC and MBEC (Allan et al, 2011): To determine MIC values, the turbidity in the wells of the challenge plate was read at 650nm using ELISA reader. The MIC (OD650 < 0.1) is defined as the minimum concentration of bacteriocin that inhibits growth of the organism. To determine the MBEC values, the turbidity in the wells of recovery plate was read at 650nm. The MBEC is defined as the (OD650 < 0.1) minimum concentration of bacteriocin that inhibits growth of the organism and evidence of biofilm eradication.
Statistical Analysis: All results were presented in mean ± standard deviation (Mean±SD). A one- way analysis of variance (ANOVA) was used to determine significant difference between mean for each surface and strains. Statistical significance were evaluated at P<0.05. Graphs were plotted using Microsoft Excel (Adetunji et al, 2011).
RESULTS AND DISCUSSION
Screening of bacteriocin producing bacteria: Toddy was selected as a source of bacteriocin producing bacteria. From more than 30 colonies screened for the antibacterial activity against Listeria monocytogenes MTCC657, one colony were found to produce inhibitory zone and designated as GRDS6. The pure culture were maintained on MRS agar. The culture were found to be gram positive cocci by Gram’s staining. The isolate showed negative results for catalase test, bile esculin hydrolysis and found to be Vancomycin resistant. The strain were next subjected to 16S rRNA sequencing and phylogenetic analysis. Based on 16S rRNA phylogeny, the bacterial strain showed 98% similarity with Paenibacillus polymyxa. The sequence was submitted to GenBank, NCBI and the accession number MH113816 was obtained.
Production and purification of GRDS6 bacteriocin: Anti-listerial bacteriocin production was carried out using production medium (TGE and Tween 80), which supported maximum bacteriocin production. Extraction of anti-listerial bacteriocin was done using Amberlite XAD-16 absorption, which is a non-ionic macro-reticular resin that absorbs and release ionic species through hydrophobic interactions (Zenguo et al, 2007). The anti listerial bacteriocin will selectively adhere to the amberlite resin which is applied to the crude cell free supernatant. This bounded bacteriocin were eluted from XAD-16 with 70% isopropyl alcohol by column chromatography method and the fraction was evaporated to half the volume and stored in 4° C.
Biofilm formation studies: Primary studies to find out the biofilm formation ability of Listeria monocytogenes MTCC657 was carried out by tube method and microtiter plate method.
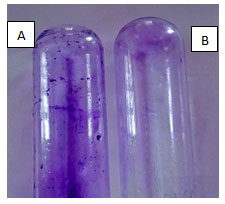
Tube method: The tube method involves the adherence of Listeria monocytogenes MTCC657 on the sides if the test tube through visual assessment. In our study, Listeria monocytogenes MTCC657 showed positive result i.e. adherence of cells after crystal violet staining (Fig.1). This indicates that Listeria monocytogenes MTCC657 is a strong biofilm producer. Studies shows that there are different strategies employed by microorganisms to produce biofilm and to understand the pathway. Saitou et al, (2009) reported that scientists has discovered that biofilm producing bacteria secrete certain chemicals that protect them from disinfectants and anti-microbial agents.
Figure 1: The biofilm formation by Listeria monocytogenes MTCC657 by tube method, A- Test strain with crystal violet staining indicates biofilm formation, B- Control
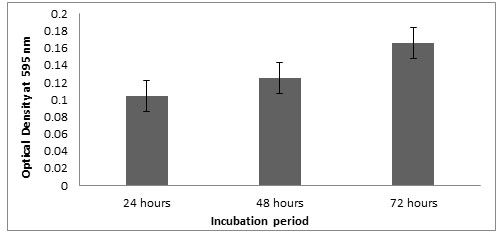
Microtitre plate method: Optical density of the Listeria monocytogenes MTCC657 biofilm grown at 24, 48 and 72 h were observed at 595nm. Results showed that the strain was able to form biofilm at 24h itself. The optical density at 595nm ranged from 0.103 to 0.165 (Fig.2) after 72h. In all the three incubations, the biofilm formation seems to be increasing. In our study, there is an increase in biofilm formation with extension of incubation period (Adetunji et al, 2008). According to Foualdynezhad et al, (2013) biofilm formation as a complicated process influenced by factors such as time, temperature and growth medium.
Figure 2: Biofilm formation at different incubation period in crystal violet binding assay
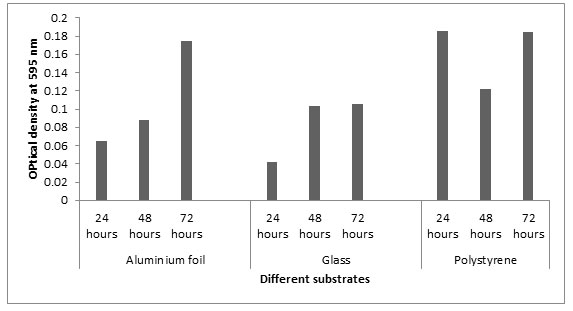
Biofilm formation on different substrates: In this study, we selected three abiotic surfaces such as polystyrene, glass and aluminum foil for different incubation periods. These substrates were selected because they are commonly used in food processing and preservation industry. The OD values at 595nm at 24 h showed that Listeria monocytogenes MTCC657 formed more biofilm on polystyrene (0.185) followed by aluminum foil (0.064) and glass (0.042). Similar results were observed in 48h and 72h. From our results, it is clear that Listeria monocytogenes MTCC657 has a high capacity of biofilm formation on these substrates. The absorbance values of biofilm adherence of Listeria monocytogenes MTCC657 showed that polystyrene maintained higher biofilm formation than aluminum foil and glass (Fig.3). According to Treese et al, (2006), this may be due to the surface hydrophobicity in polystyrene and other physico-chemical properties compared to other surfaces.
Figure 3: Biofilm formation on different substrates at different incubation periods
Biofilm eradication studies on Listeria monocytogenes MTCC657 using different bacteriocins
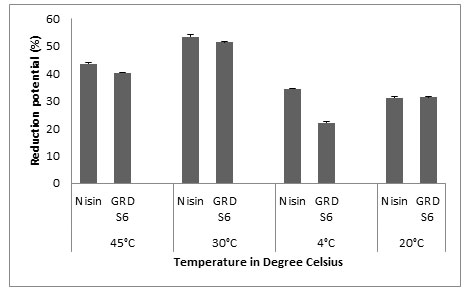
Microtiter Plate Method: The main objective of our study was to evaluate the biofilm forming ability of Listeria monocytogenes MTCC657 on three different substrates commonly used in food industries (polystyrene, glass and aluminum foil) at different temperatures (24h, 48h and 72h) and to assess the biofilm eradication potential of Nisin and GRDS6 bacteriocin. All the values were expressed as Mean±SD. The results shows that GRDS6 bacteriocin has similar activity of Nisin. For polystyrene, the reduction potential (%) of GRDS6 were 40.26±0.071 (45°C), 51.47±0.025 (30°C), 22.17±0.035 (4°C) and 31.65±0.165 (-20°C) respectively. And for Nisin the results were 43.63±0.298 (45°C), 53.40±0.721 (30°C), 34.40±0.224 (4°C) and 31.06±0.539 (-20°C). The activity of GRDS6 was equivalent with the activity of Nisin on biofilm of Listeria monocytogenes MTCC657 grown on polystyrene (Fig.4).
Figure 4: Y- axis represents the reduction potential (%) of L. monocytogenes MTCC 657 and X – axis represents the temperatures (°C)
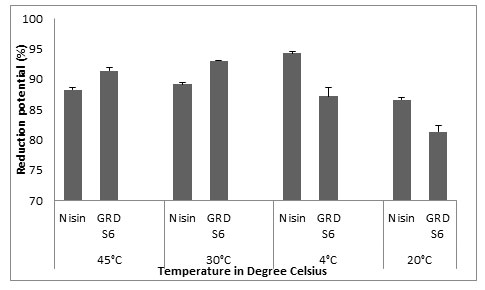
The results we got for biofilm eradication by Nisin on glass were 88.27±0.375, 89.14±0.349, 94.3±0.349 and 86.6±0.388 and for GRDS6, the values were 91±0.558, 93.04±0.142, 87.20±1.395 and 81.36±1.029 for 45°C, 30°C, 4°C and -20°C. From this result we can relate the biofilm eradication potential of GRDS6 to Nisin (Fig.5).
Figure 5: Y- axis represents the reduction potential (%) of L. monocytogenes MTCC 657 and X – axis represents the temperatures (°C)
For aluminum foil the results obtained as follows, 41.31±0.072 and 38.5±0.465 for 45°C, 42.28±0.149 and 36.59±1.518 for 30°C, 40.34±0.149 and 36.55±0.203 for 4°C, 41.84±0.084 and 17.46±0.204 for -20°C for GRDS6 and Nisin respectively (Fig.6). According to this result the reduction potential of GRDS6 and Nisin were relatable.
Figure 6: Y- axis represents the reduction potential (%) of L. monocytogenes MTCC 657 and X – axis represents the temperatures (°C)
Nisin was used as standard bacteriocin in our study as it is a proven bacteriocin and is commercially available. Nisin-containing packaging materials have potential applications to prevent growth of many foodborne pathogenic bacteria and spores of Clostridium botulinum and Listeria monocytogenes (Ray et al, 1992). It has been shown before that Listeria. monocyogenes will adhere to a wide variety of surfaces (Mafu et al, 1990). Many techniques have been developed to study bacterial attachment, some of which are based on counting the number of detached cells from the surface, such as standard plate counts and sonication (Green et al, 1993).
2Calgary Biofim Decice®: The results shows that the GRDS6 bacteriocin and Nisin are able to inhibit the biofilm formed by Listeria monocytogenes MTCC657 irrespective of the temperatures. The results are depicted in the table as follows (Table.1, 2, 3, 4).
Table 1. Susceptibility of Listeria monocytogenes MTCC 657 for Nisin and GRDS6 at 45°C
| Conc.of bacteriocin µg/mL | MIC | MBEC | ||
| Nisin | GRDS6 | Nisin | GRDS6 | |
| 1280 | 0.073±0.003 | 0.076±0.001 | 0.072±0.002 | 0.067±0.001 |
| 640 | 0.075±0.003 | 0.073±0.002 | 0.076±0.001 | 0.067±0.003 |
| 320 | 0.077±0001 | 0073±0.004 | 0.081±0.003 | 0.065±0.001 |
| 160 | 0.081±0.002 | 0.074±0.009 | 0.087±0.001 | 0.066±0.001a |
| 80 | 0.084±0.002 | 0.076±0.001 | 0.089±0.002 | 0.068±0.002 |
| 40 | 0.086±0.001 | 0.078±0.002a | 0.093±0.001 | 0.072±0.001 |
| 20 | 0.089±0.002 | 0.082±0.002a | 0.095±0.003 | 0.075±0.002 |
| 10 | 0.091±0.001 | 0.082±0.001a | 0.098±0.001 | 0.082±0.002 |
The given values are the optical density of L. monocytogenes MTCC 657 biofilm obtained by measuring the turbidity at 650nm on Elisa plate reader and expressed as Mean ± SD at significance p < 0.05. a – the significant relationship between Nisin and GRDS6
In 45°C, the biofilm formation is optimum and the biofilm eradication potential is low. Our results shows that, higher concentration of bacteriocin is needed for the eradication of biofilm for higher temperature (Table.1). There are studies which explains that the formation of biofilm by Listeria monocytogenes will increase with the time of incubation at higher temperature (Lee et al, 2003).
Table 2. Susceptibility of Listeria monocytogenes MTCC 657 for Nisin and GRDS6 at 30°C
| Conc.of bacteriocin µg/mL | MIC | MBEC | ||
| Nisin | GRDS6 | Nisin | GRDS6 | |
| 1280 | 0.061±0.002 | 0.064±0.001 | 0.057±0.002 | 0.063±0.001 |
| 640 | 0.065±0.001 | 0.064±0.002 | 0.056±0.002 | 0.063±0.002a |
| 320 | 0.066±0.002 | 0.065±0.004 | 0.059±0.001 | 0.062±0.002a |
| 160 | 0.071±0.001 | 0.067±0.002 | 0.061±0.003 | 0.067±0.004a |
| 80 | 0.073±0.001 | 0.071±0.002a | 0.065±0.002 | 0.070±0.001 |
| 40 | 0.078±0.003 | 0.073±0.001a | 0.067±0.003 | 0.073±0.001 |
| 20 | 0.081±0.001 | 0.074±0.002a | 0.071±0.002 | 0.076±0.002 |
| 10 | 0.082±0.002 | 0.080±0.001a | 0.072±0.001 | 0.077±0.001 |
The given values are the optical density of L. monocytogenes MTCC 657 biofilm obtained by measuring the turbidity at 650nm on Elisa plate reader and expressed as Mean ± SD at significance p < 0.05. a – the significant relationship between Nisin and GRDS6
At 30°C, based on statistical data Listeria monocytogenes MTCC657 showed significance difference (except 10 µg, 20µg/mL, 40 µg/mL and 80 µg/mL) among the activity of bacteriocins (Table.2) and therefore the activity of Nisin and GRDS6 bacteriocins are relatable.
Table 3: Susceptibility of Listeria monocytogenes MTCC 657 for Nisin and GRDS6 at 4°C
| Conc.of bacteriocin µg/mL | MIC | MBEC | ||
| Nisin | GRDS6 | Nisin | GRDS6 | |
| 1280 | 0.071±0.002 | 0.061±0.001 | 0.056±0.001 | 0.060±0.001 |
| 640 | 0.076±0.002 | 0.062±0.001 | 0.058±0.001 | 0.061±0.001 |
| 320 | 0.081±0.001 | 0.072±0.002 | 0.061±0.002 | 0.065±0.002 |
| 160 | 0.085±0.003 | 0.071±0.001 | 0.063±0.001 | 0.073±0.001a |
| 80 | 0.089±0.002 | 0.076±0.001a | 0.067±0.003 | 0.074±0.001 |
| 40 | 0.092±0.001 | 0.080±0.001a | 0.071±0.001 | 0.076±0.001 |
| 20 | 0.094±0.003 | 0.084±0.002a | 0.076±0.001 | 0.081±0.001 |
| 10 | 0.095±0.002 | 0.089±0.002 | 0.079±0.002 | 0.085±0.001 |
The given values are the optical density of L. monocytogenes MTCC 657 biofilm obtained by measuring the turbidity at 650nm on Elisa plate reader and expressed as Mean ± SD at significance p < 0.05. a – the significant relationship between Nisin and GRDS6
Biofilm formation were observed at 4°C and the statistical results of MIC showed significant difference among GRDS6 and Nisin. In the case of MBEC, no significance difference were observed (except 160µg/mL).
Table 4. Susceptibility of Listeria monocytogenes MTCC 657 for Nisin and GRDS6 at -20°C
| Conc. of bacteriocin µg/mL | MIC | MBEC | ||
| Nisin | GRDS6 | Nisin | GRDS6 | |
| 1280 | 0.053±0.001 | 0.050±0.002 | 0.056±0.001 | 0.062±0.001 |
| 640 | 0.055±0.003 | 0.058±0.001 | 0.059±0.002 | 0.061±0.003 |
| 320 | 0.061±0.002 | 0.055±0.001 | 0.061±0.001 | 0.064±0.001 |
| 160 | 0.063±0.001 | 0.061±0.001 | 0.063±0.002 | 0.065±0.001 |
| 80 | 0.069±0.002 | 0.065±0.003 | 0.065±0.003 | 0.067±0.002 |
| 40 | 0.071±0.002 | 0.069±0.001 | 0.068±0.001 | 0.069±0.003 |
| 20 | 0.076±0.003 | 0.071±0.001 | 0.072±0.002 | 0.072±0.001 |
| 10 | 0.081±0.002 | 0.070±0.002 | 0.078±0.001 | 0.074±0.001 |
The given values are the optical density of L. monocytogenes MTCC 657 biofilm obtained by measuring the turbidity at 650nm on Elisa plate reader and expressed as Mean ± SD at significance p < 0.05
The biofilm formation and eradication at -20°C was observed. The MIC values showed no significane difference. MBEC also showed the same results. From this study we can conclude that GRDS6 bacteriocin is similar to Nisin. According to Hanene et al, (2013), the starvation and cold stress enhance the biofilm formation, surface hydrophobicity and modify the membrane lipopeptides of Listeria monocytogenes. The assessment of the anti-biofilm activities of antimicrobial agents is generally based on interference with quorum sensing, inhibition of adhesion, enhancement of dispersion, and various experimental and promising alternatives, such as the use of biofilm-specific antibodies, bacteriophage-based treatments, and the species-specific control of biofilms (Chen et al, 2011).
This study shows that the GRDS6 bacteriocin is able to inhibit the biofilm formed by Listeria monocytogenes MTCC657 under different temperatures and different incubation periods. The activity of GRDS6 bacteriocin is similar to activity of Nisin. So we recommend to use this bacteriocin in the food preservation industry at higher (45°C) to freezing temperature (-20°C). In our study, toddy was used as a source for isolation of bacteriocin producing bacteria. The isolate we assayed for its bacteriocin production. Listeria monocytogenes MTCC657 is able to produce biofilm which helps the microorganism to survive and grow for an extended period of time. Adhesive ability and biofilm formation ability of Listeria monocytogenes MTCC657 on solid substrates (polystyrene, glass and aluminum foil) at different temperatures was determined and found to be higher on polystyrene at 72 h followed aluminum foil and glass. The biofilm eradication potential of GRDS6 bacteriocin and Nisin were evaluated by microtiter plate method and CBD® assay. Based on our results the activity of GRDS6 bacteriocin was similar to Nisin. The incubation temperature doesn’t influence the activity of the bacteriocin, so we recommend this bacteriocin in food storage industry.
ACKNOWLEDGMENT
We acknowledge the Management of GRD institutions for providing the research funds and high class infrastructure to complete the research.
Conflicts of Interest: The authors declare no conflicts of interest.
REFERENCES
Adetunji, V. O., & Adegoke, G. O. (2008). Formation of biofilm by strains of Listeria monocytogenes isolated from soft cheese’wara’and its processing environment. African Journal of Biotechnology, 7(16).
Adetunji, V. O., & Isola, T. O. (2011). Crystal violet binding assay for assessment of biofilm formation by Listeria monocytogenes and Listeria spp on wood, steel and glass surfaces. Global Veterinaria, 6(1), 6-10.
Allan, N. D., Omar, A., Harding, M. W., & Olson, M. E. (2011). A rapid, high-throughput method for culturing, characterizing and biocide efficacy testing of both planktonic cells and biofilms. Science against microbial pathogens: communicating current research and technological advances. Edition: Microbiology Book Series ed: Formatex, Editors: Mendez-Vilas A, 864-71.
Ash, C., Priest, F. G., & Collins, M. D. (1993). Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Antonie van Leeuwenhoek, 64(3-4), 253-260.
Banerjee, M., Copp, J., Vuga, D., Marino, M., Chapman, T., Van Der Geer, P., & Ghosh, P. (2004). GW domains of the Listeria monocytogenes invasion protein InlB are required for potentiation of Met activation. Molecular microbiology, 52(1), 257-271.
Barbuddhe, S. B., Malik, S. V. S., Kumar, J. A., Kalorey, D. R., & Chakraborty, T. (2012). Epidemiology and risk management of listeriosis in India. International journal of food microbiology, 154(3), 113-118.
Berry, E. D., Liewen, M. B., Mandigo, R. W., & Hutkins, R. W. (1990). Inhibition of Listeria monocytogenes by bacteriocin-producing Pediococcus during the manufacture of fermented semidry sausage. Journal of Food Protection, 53(3), 194-197.
Briandet, Romain, Valerie Leriche, Brigitte Carpentier, and Marie-Noelle Bellon-Fontaine. Effects of the growth procedure on the surface hydrophobicity of Listeria monocytogenes cells and their adhesion to stainless steel. Journal of food protection 62, no. 9 (1999): 994-998.
Budde, B. B., & Jakobsen, M. (2000). Real-time measurements of the interaction between single cells of Listeria monocytogenes and nisin on a solid surface. Appl. Environ. Microbiol., 66(8), 3586-3591.
Chen, Y. P., Zhang, P., Guo, J. S., Fang, F., Gao, X., & Li, C. (2013). Functional groups characteristics of EPS in biofilm growing on different carriers. Chemosphere, 92(6), 633-638.
Christiansen, L. N., Tompkin, R. B., Shaparis, A. B., Kueper, T. V., Johnston, R. W., Kautter, D. A., & Kolari, O. J. (1974). Effect of sodium nitrite on toxin production by Clostridium botulinum in bacon. Appl. Environ. Microbiol., 27(4), 733-737.
Colak, H., Hampikyan, H., Ulusoy, B., & Bingol, E. B. (2007). Presence of Listeria monocytogenes in Turkish style fermented sausage (sucuk). Food Control, 18(1), 30-32.
Cornelius, A. J., Hudson, J. A., & Wong, T. L. (2008). Enumeration and growth of naturally occurring Listeria spp. in unpackaged ham. Food microbiology, 25(2), 407-412.
de Souza, V. M., Franceschini, S. A., Martinez, R. C., Ratti, R. P., & De Martinis, E. C. (2008). Survey of Listeria spp. in matched clinical, food and refrigerator samples at home level in Brazil. Food Control, 19(10), 1011-1013.
De Witt, W., & Helinski, D. R. (1965). Characterization of colicinogenic factor E, from a non-induced and mitomycin C-induced. Proteus, 692-703.
Di Bonaventura, G., Piccolomini, R., Paludi, D., D’orio, V., Vergara, A., Conter, M., & Ianieri, A. (2008). Influence of temperature on biofilm formation by Listeria monocytogenes on various food‐contact surfaces: relationship with motility and cell surface hydrophobicity. Journal of applied microbiology, 104(6), 1552-1561.
Donlan, R. M., & Costerton, J. W. (2002). Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews, 15(2), 167-193.
Dusane, D. H., Pawar, V. S., Nancharaiah, Y. V., Venugopalan, V. P., Kumar, A. R., & Zinjarde, S. S. (2011). Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling, 27(6), 645-654.
Frank, J. F., & Koffi, R. A. (1990). Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. Journal of food protection, 53(7), 550-554.
Frank, J. F., & Koffi, R. A. (1990). Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. Journal of food protection, 53(7), 550-554.
Gandhi, M., & Chikindas, M. L. (2007). Listeria: a foodborne pathogen that knows how to survive. International journal of food microbiology, 113(1), 1-15.
Grady, E. N., MacDonald, J., Liu, L., Richman, A., & Yuan, Z. C. (2016). Current knowledge and perspectives of Paenibacillus: a review. Microbial cell factories, 15(1), 203.
Gupta, A., Gopal, M., Thomas, G. V., Manikandan, V., Gajewski, J., Thomas, G., … & Gupta, R. (2014). Whole genome sequencing and analysis of plant growth promoting bacteria isolated from the rhizosphere of plantation crops coconut, cocoa and arecanut. PLoS One, 9(8).
Gurgu LG., FI Bucur, Daniela Borda, Elena-Alexandra Alexa, Corina Neagu and Anca Ioana Nicolau (2019): Biofilms Formed by Pathogens in Food and Food Processing Environments Intech Open November 13th 2019 DOI: 10.5772/intechopen.90176
Harvey, J., Keenan, K. P., & Gilmour, A. (2007). Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiology, 24(4), 380-392.
Jung, D. S., Bodyfelt, F. W., & Daeschel, M. A. (1992). Influence of Fat and Emulsifiers on the Efficacy of Nisin in Inhibiting Listeria monocytogenes in Fluid Milk1. Journal of Dairy Science, 75(2), 387-393.
Mafu, A. A., Roy, D., Goulet, J., & Magny, P. (1990). Attachment of Listeria monocytogenes to stainless steel, glass, polypropylene, and rubber surfaces after short contact times. Journal of Food Protection, 53(9), 742-746.
Mahdavi, M., Jalali, M., & Kermanshahi, R. K. (2009). The effect of nisin on biofilm forming foodborne bacteria using microtiter plate method. Research in Pharmaceutical Sciences, 2(2), 113-118.
Martínez-Cuesta, M. C., Kok, J., Herranz, E., Peláez, C., Requena, T., & Buist, G. (2000). Requirement of autolytic activity for bacteriocin-induced lysis. Appl. Environ. Microbiol. 66(8), 3174-3179.
Mc Carthy, S. A., Motes, M. L., & Mc Phearson, R. M. (1990). Recovery of heat-stressed Listeria monocytogenes from experimentally and naturally contaminated shrimp. Journal of food protection, 53(1), 22-25.
McClure, P. J., Beaumont, A. L., Sutherland, J. P., & Roberts, T. A. (1997). Predictive modelling of growth of Listeria monocytogenes the effects on growth of NaCl, pH, storage temperature and NaNO2. International journal of food microbiology, 34(3), 221-232.
Parish, M. E., & Higgins, D. P. (1989). Extinction Of Listeria monocytogenes In Single‐Strength Orange Juice: Comparison Of Methods For Detection In Mixed Populations 1. Journal of Food Safety, 9(4), 267-277.
Rammelsberg, M., & Radler, F. (1990). Antibacterial polypeptides of Lactobacillus species. Journal of Applied Bacteriology, 69(2), 177-184.
Ray, B. (1992). Nisin of Lactococcus lactis ssp. lactis as a food biopreservative. Food preservatives of microbial origin, 207-264.
Ryser, E. T., Arimi, S. M., & Donnelly, C. W. (1997). Effects of pH on distribution of Listeria ribotypes in corn, hay, and grass silage. Appl. Environ. Microbiol., 63(9), 3695-3697.
Saitou, K., Furuhata, K., Kawakami, Y., & Fukuyama, M. (2009). Biofilm formation abilities and disinfectant-resistance of Pseudomonas aeruginosa isolated from cockroaches captured in hospitals. Biocontrol science, 14(2), 65-68.
Shivangi S, Palanisamy Bruntha Devi, Kessavane Ragul & Prathapkumar Halady Shetty (2020) Probiotic Potential of Bacillus Strains Isolated from an Acidic Fermented Food Idli Probiotics and Antimicrobial Proteins (2020) April 7 2020 Springer On Line First
Stepanović, S., Ćirković, I., Ranin, L., & S vabić‐Vlahović, M. (2004). Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Letters in applied microbiology, 38(5), 428-432.
Stepanović, S., Vuković, D., Dakić, I., Savić, B., & Švabić-Vlahović, M. (2000). A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Journal of microbiological methods, 40(2), 175-179.
Yoshiyama, M., & Kimura, K. (2009). Bacteria in the gut of Japanese honeybee, Apis cerana japonica, and their antagonistic effect against Paenibacillus larvae, the causal agent of American foulbrood. Journal of Invertebrate Pathology, 102(2), 91-96.
Zhu, X., Zhao, Y., Sun, Y., & Gu, Q. (2014). Purification and characterisation of plantaricin ZJ008, a novel bacteriocin against Staphylococcus spp. from Lactobacillus plantarum ZJ008. Food chemistry, 165, 216-223.