1Division of Biochemistry and Molecular Biology, Department of Biomedical Sciences,
Faculty of Medicine, Universitas Padjadjaran, Sumedang, Indonesia, 45363
2Research Center for Medical Genetics, Faculty of Medicine,
Universitas Padjadjaran, Bandung, Indonesia, 40161
3Faculty of Medicine, Universitas Padjadjaran, Sumedang, Indonesia, 45363
4Division of Epidemiology, Department of Public Health, Faculty of Medicine,
Universitas Padjadjaran, Bandung, Indonesia, 40161
5Cell Culture Laboratory, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia, 40161
6Division of Parasitology, Department of Biomedical Sciences, Faculty of
Medicine, Universitas Padjadajaran, Sumedang, Indonesia, 45363
7Division of Pharmacology and Therapy, Department of Biomedical Sciences,
Faculty of Medicine, Universitas Padjadjaran, Sumedang, Indonesia, 45363
Corresponding author email: fuji@unpad.ac.id
Article Publishing History
Received: 11/07/2020
Accepted After Revision: 20/09/2020
For the past few decades, medicinal plants have been inspected to have an anti-obesity effect, including Aloe vera. Various of study has been conducted but shows different results regarding the effect of Aloe vera in lipid accumulation. Aloe vera have showed to be an inhibitory effect in adipogenesis by downregulation of PPAR expression which can suppressed lipid accumulation. This study aims to examine the effect of Aloe vera ethanol extract on lipid accumulation during adipocyte differentiation using 3T3-L1 cell line. Four sets of 3T3-L1 preadipocytes along with the Aloe vera ethanol extract treatments (0 ppm, 10 ppm, 20 ppm, and 40 ppm) were prepared. Adipogenic differentiation cocktail consisting of 0.5 mM isobutylmethylxanthine (IBMX), 0.25 μM dexamethasone, and 1 μg/mL insulin were given to each well on day 0. Mediums were replaced every two days. On day 12, the wells were stained with Oil Red O, and the red-stained lipid droplets were observed under the microscope. Macroscopically, the red stain showed almost the same amount of stain within treatments.
The control group and 20 ppm group showed a slight increase in lipid accumulation compared to 10 pp and 40 ppm gorup when observed under the microscope with 40x and 100x magnification. The lipid accumulation were then measured using a spectrophotometer at a wavelength of 550 nm for quantification. The addition of Aloe vera ethanol extract in 3T3-L1 preadipocytes showed no significant differences in lipid accumulation (p>0.05), although it showed a decrease in the lipid absorbance value. In conclusion, the addition of Aloe vera did not reduce the lipid accumulation in 3T3-L1 cell differentiation.
3T3-L1 Cell Line, Adipocyte Differentiation, Aloe Vera, Lipid Accumulation.
Ariyanto E. F, Baharuddin A. N, Sujatmiko B, Wikayani T. P, Qomarilla N, Berbudi A, Rohmawaty E. Effect of Aloe vera Ethanol Extract in Lipid Accumulation During Adipocyte Differentiation Using 3t3-L1 Cell Line. Biosc.Biotech.Res.Comm. 2020;13(3).
Ariyanto E. F, Baharuddin A. N, Sujatmiko B, Wikayani T. P, Qomarilla N, Berbudi A, Rohmawaty E. Effect of Aloe vera Ethanol Extract in Lipid Accumulation During Adipocyte Differentiation Using 3t3-L1 Cell Line. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2Pj0Wjj
Copyright © Ariyanto et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Obesity is becoming one of Indonesia’s major health issues, and is associated with an elevated mortality risk of cardiovascular diseases (Harbuwono et al, 2018; Jiang et al, 2013). Studies on a vast range of medicinal plants have been investigated and reported to be useful in treating obesity (Hasani-Ranjbar et al, 2013). The researches are focused on searching for herbal plants that can prevent excess body fat accumulation (Misawa et al, 2012). These supplements modify the weight regulation of the human body by altering appetite, metabolism, or absorption of calories (Chandrasekaran et al, 2012).
For thousands of years, Aloe vera has been broadly applied as a medicinal plant to overcome various health problems, including obesity (Christaki and Florou-Paneri, 2016; Rajeswari et al, 2012). A previous study examined the effect of Aloe-emodin, an anthraquinone compound isolated from Aloe vera leaf, on 3T3-L1 preadipocyte and resulted in suppressed lipid accumulation during the adipocyte differentiation using 3T3-L1 preadipocyte (Anand et al, 2010). The suppressed PPARγ expression is thought to be the reason (Anand et al, 2010). Adipocyte differentiation was also nearly blocked with 20 μM Aloe vera in a study using human mesenchymal stem cells (Subash-babu and Alshatwi, 2012). Whereas in other studies, the administration of dietary aloe QDM complex in obese mice lowered the body weight and suppressed the expression of PPARγ which then averts the differentiation of adipocyte (Shin et al, 2012).
A recent study showed that oral administration of isolated phytosterols from Aloe vera significantly reduced visceral fat weights than obese control in Zucker diabetic fatty rats (Misawa et al, 2012). The antiobesity mechanism in the study suggested that the ingestion of isolated phytosterols of Aloe vera suppressed the expression of gluconeogenic and lipogenic enzymes, and enzymes related to glycolysis and lipolysis were elevated. In addition to that, transcriptional factors were also significantly decreased in which can ultimately inhibit adipocyte differentiation (Misawa et al, 2012). However, in other studies, Aloe vera supplementation showed an insignificant reduction in body weight gain in high-fat diet (HFD) mice (Pothuraju et al, 2016).
Nevertheless, the effect of Aloe vera extract on adipocyte differentiation has not been fully understood.Dysregulation and dysfunctional of adipocyte plays a critical role in the etiopathogenesis of obesity (Unamuno et al, 2018). Adipocytes are formed by the process of preadipocytes proliferation and differentiation (Moreno-Navarrete and Fernández-Real, 2012). Parameters used to assess adipocyte differentiation are lipid accumulation and the increased expression of specific adipocyte genes (Moreno-Navarrete and Fernández-Real, 2012; Ruiz-Ojeda et al, 2016).
As obesity is becoming a major health issues, researchers were also challeged to study vast range of medicinal plants to overcome it, this includes Aloe vera. Further studies of the effect of Aloe vera ethanol extracts on adipocyte differentiation need to be carried out. Studies using the adipocyte differentiation system is one way that can be done to examine the effects of Aloe vera on the development of obesity. This study aims to investigate the effect of Aloe vera ethanol extract on adipocyte differentiation, mainly in lipid accumulation, using 3T3-L1 cell lines.
MATERIAL AND METHODS
Research design:This is an experimental analytical qualitative and quantitative study. The qualitative study focused on the morphology of the Oil Red O stained lipid accumulation, whereas the quantitative analysis was carried out to validate the qualitative observation. The objective of this study is to observe the effect of Aloe vera ethanol extract in inhibiting lipid accumulation during 3T3-L1 preadipocytes differentiation.
Aloe vera ethanol extraction: Aloe vera leaves were purchased from a local supermarket in Bandung. Leaves were washed, peeled, and dried. The dried Aloe vera were then blended using an electric blender and boiled with ethanol 95% and later filtered. The filtrate was concentrated, obtaining thick gel-like extract of Aloe vera. Before use, the particles were diluted with dimethyl sulfoxide (DMSO) solution to create an extract with a concentration of 10 ppm, 20 ppm, and 40 ppm.
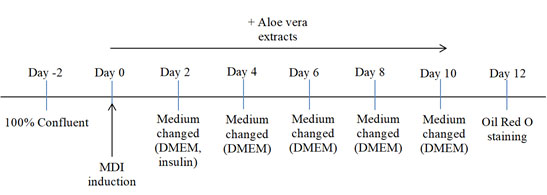
Cell culture and differentiation: The protocol used in this analysis was based on previously published study (Ariyanto et al, 2019). This study was conducted at Cell Culture Laboratory, Faculty of Medicine, Universitas Padjadjaran, Indonesia. Cell culture was the first step in performing this experiment. 3T3-L1 cell lines were cultured in Dulbecco’s Modified Edge’s Medium (DMEM), obtained from Sigma-Aldrich, containing 10% Fetal Bovine Serum (FBS) and stored at 37˚C, 5% CO. The cells were left to grow for 48 hours, or until it has reached 100% confluent. Lastly, cells were incubated as a confluent culture for another 48 hours (Figure 1).
Figure 1: Cell culture and differentiation protocol. MDI: differentiation cocktail consisting of 0.5 mM isobutylmethylxanthine (IBMX), 0.25 μM dexamethasone, and 1 μg/mL insulin; Insulin: 1 μg/mL insulin-containing DMEM.
The induction of adipocyte differentiation started right after the incubation process and was marked as day 0. In this experiment, four different mediums were prepared in a twelve-well plate: medium control (0 ppm), medium with 10 ppm Aloe vera ethanol extract, medium with 20 ppm Aloe vera ethanol extract, and medium with 40 ppm Aloe vera ethanol extract. The MDI (0.5 mM IBMX, 0.25 μM dexamethasone, 1 μg/mL insulin), obtained from Sigma-Aldrich, cocktail differentiation were also set.
On day 0, wells containing 3T3-L1 cell lines were given MDI induction with different extract treatments depending on the dosages. On day 2, the medium of the wells were exchanged DMEM and insulin mediums. As in day 4, the wells were replaced with DMEM only. The medium was continuously changed throughout the experiment every two days until mature adipocyte were obtained. Samples on day 12 were then stained using Oil red O.
Oil Red O staining: Upon completion of the differentiation process, the cells were washed twice with PBS and fixed for 20 minutes with formaldehyde 4%. Isopropanol 60% were given to wash the cells before staining. Oil Red O solution was used to stain the cells for 15 minutes. The wells were rewashed with Isopropanol 60%. Cells were then observed under the microscope. Red stain indicates lipid accumulation.
Quantification: Isopropanol 98% were added to the cells to elute the stain of Oil Red O. Oil Red O absorbance values were determined using a spectrophotometer at wavelength of 550 nm.
Statistical analysis: GraphPad Prism version 8.3 for Windows (GraphPad software, Inc. San Diego, CA) was used to perform statistical analysis. The data obtained were expressed as mean ± standard deviation (SD). Differences between separate treatment variations were analyzed using one-way ANOVA with Tukey’s posthoc test. Differences were considered statistically significant if the value of p < 0.05.
RESULTS AND DISCUSSION
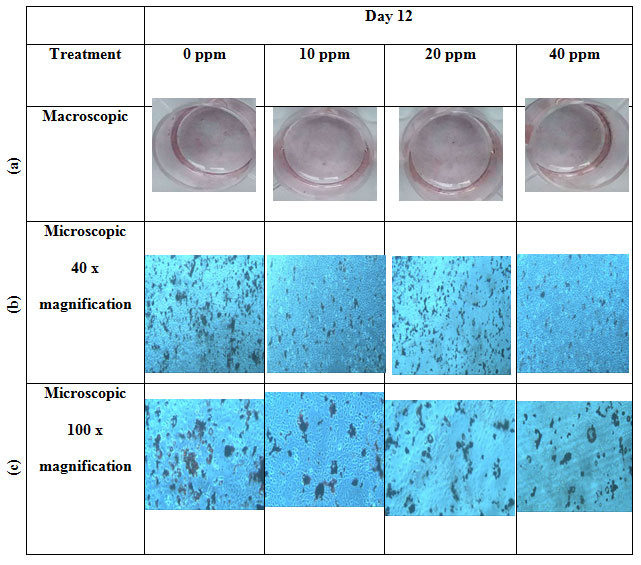
Qualitative analysis of lipid accumulation: Lipid accumulation on each well was observed macroscopically by the naked eye and microscopically under the microscope with 40x and 100x magnification. Macroscopically, the red stain showed the amount of the lipid droplet formation (Figure 2-a). The red stain within each treatment showed almost the same amount of stain, which indicates no significant differences between treatments. Lipid accumulation observed under the microscope with 40x and 100x magnification showed that the control group and 20 ppm group has a slight increase in lipid accumulation compared to 10 ppm and 40 ppm group (Figure 2b-c). Quantification of lipid droplets formation was then counted to confirm the following observation.
Figure 2: Macroscopic and microscopic features of Oil Red O stained on 3T3-L1 cells with four different treatments: 0 ppm, 10 ppm, 20 ppm, 40 ppm: (a) macroscopic images, (b) microscopic 40x magnification images, and (c) microscopic 100x magnification images.
Quantitative analysis of lipid accumulation: At the wavelength of 550 nm, the control group turned out to have the highest lipid absorbance value compare to the other groups. However, the higher the extracts given did not show a constant decline in the lipid absorbance value. 10 ppm group showed the lowest lipid absorbance value, whereas 40 ppm group and 20 ppm group became the second and third lowest lipid absorbance value, respectively. Statistically, the outcome of this analysis showed no significant difference between groups, even though the administration of Aloe vera ethanol extract showed a decrease in lipid absorbance value (p>0.05) (Figure 3).
Figure 3: Quantification analyses of lipid droplets accumulation. Oil Red O stains were eluted with isopropanol 98%. Lipid absorbance values were measured using a spectrophotometer at wavelength 550 nm. (p>0.05).
This research investigated the effect of Aloe vera ethanol extract on lipid accumulation using 3T3-L1 preadipocytes cell line. The purpose of this study is to investigate whether Aloe vera ethanol extract supplementation in 3T3-L1 cell lines can suppress lipid acculumation during adipocyte differentiation. Adipocyte differentiation process requires a complex algorithm of various adipogenic gene expression (Lowe et al, 2011). Cooperative manner of peroxisome proliferator-activated receptor-gamma (PPARγ) and CCAT/enhancer-binding proteins (C/EBPs) gene expression plays a vital role in adipogenesis, which together forms mature adipocyte phenotype (Moseti et al, 2016). In addition to that, sterol regulatory element-binding protein (SREBP) has the ability to induce the expression of PPARγ, which also makes it a key determinant of adipocyte fate (Ruiz-Ojeda et al, 2016; Moseti et al, 2016). Understanding of these processes leads us to a better comprehension of courses that involves the inhibition of adipogenesis.
Various studies on Aloe vera effects have been performed. An earlier study reported an insignificant reduction in body weight gain between HFD mice and HFD mice supplemented with Aloe vera (Pothuraju et al, 2016). In another study, Aloe vera supplementation towards obese mice also exhibited no difference compared to HFD obese mice (Shin et al, 2011). Similarly, Aloe vera also did not present a significant difference in body weight reduction in HFD mice in a reasearched carried out by Chihara et al (Chihara et al, 2013).
In addition to that, there are also plenty of studies that exhibit a significant weight loss with the administration of Aloe vera. A study showed a decrease in body weight and visceral fat weight in Aloe vera gel powder given obese mice compared with controlled obese mice (Misawa et al, 2012). Other prior studies have also shown that oral administration of isolated phytosterols from Aloe vera may reduce visceral fat weights significantly compared to obese control in ZDF rats (Misawa et al, 2012). SREBP1, a pro-adipogenic, were markedly decreased in the study, which ultimately inhibits adipocyte differentiation (Misawa et al, 2012). As in other studies, the administration of dietary aloe QDM complex in obese mice lowered the body weight and suppressed the expression of PPARγ which then averts the differentiation of adipocyte (Shin et al, 2012).
Beside testing Aloe vera on animals, Aloe vera has also been experimented on cell cultures. Commercial botanical products, including Aloe vera, were investigated fin the differentiation of 3T3-L1 adipocytes for effects on lipogenic activity (Babish et al, 2010). Surprisingly, the study showed the capability of Aloe vera in increasing lipid accumulation (Babish et al, 2010). Moreover, the resulting research is contradicts the study conducted by Anand et al. which examines the effect of Aloe-emodin, an anthraquinone compound isolated from Aloe vera leaf, on 3T3-L1 preadipocyte (Anand et al, 2010). Lipid accumulation was suppressed during the adipocyte differentiation using 3T3-L1 preadipocyte (Anand et al, 2010). Furthermore, adipocyte differentiation was also nearly blocked with 20 μM Aloe vera using human mesenchymal stem cells (Subash-babu and Alshatwi, 2012). Downregulation of PPARγ expression in the study is likely to cause an inhibitory effect in adipogenesis (Anand et al, 2010; Subash-babu and Alshatwi, 2012).
This study, however, did not show a statistically significant difference among treatments. The results also showed that the reduction in lipid absorbance value reduction was not in a consistent form in line with the increase of Aloe vera ethanol extract concentration. The 10 ppm group showed the lowest lipid absorbance value, whereas the 20 ppm group showed the highest lipid absorbance value among samples with Aloe vera treatments.The outcomes of previous studies and the current study showed some differences. These differences might occur due to several reasons. First, the Aloe vera used were obtained from different places. Second, the subjects used were different from one another. Some experiments assessed Aloe vera’s effect on mice while th others evaluated the effect on cell lines. In vivo and in vitro studies have a different characteristic, which might also differs the result. Third, the mice and cell lines were obtained from different areas and were not homogenous species. This might result in different outcomes with the addition of Aloe vera to different subjects.
This is the first experiment in Cell Culture Laboratory, Faculty of Medicine, Universitas Padjadjaran, which uses Aloe vera. Therefore, in conducting this study, we started by using a small amount of Aloe vera ethanol extracts concentration. This result might suggest that the concentrations used were not the optimum concentrations needed showing an inconsistent decline in the lipid accumulation. Further studies on protocol optimization in using Aloe vera ethanol extract on 3T3-L1 cell lines, and exploring the master regulatory gene of adipogenesis (PPARγ, C/EBP, SREBP) are strongly recommended for the forthcoming experiment.
CONCLUSION
In this study, the addition of Aloe vera ethanol extracts during 3T3-L1 adipocyte differentiation showed a decrease in lipid absorbance value even though statistically showed no significant differences (p>0.05). As this is the first study which uses Aloe vera ethanol extract on 3T3-L1 adipocyte differentiation, this study can serves as a guide for further studies in this field.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Dr. rer. nat. Afiat Berbudi, dr., M.Kes. for the kind gift of 3T3-L1 preadipocytes and to Universitas Padjadjaran for the research fund.
Conflict of Interest: The authors declared no conflict of interest in this study.
Funding Source: This research was funded by Universitas Padjadjaran Research Grant, No. 3855/UNG.C/LT/2019 for EFA.
REFERENCES
Anand S, Muthusamy VS, Sujatha S, Sangeetha KN, Raja RB, Sudhagar S, Devi NP and Lakshmi BS, (2010). Aloe emodin glycosides stimulates glucose transport and glycogen storage through PI3K dependent mechanism in L6 myotubes and inhibits adipocyte differentiation in 3T3L1 adipocytes. FEBS Lett. 584, 3170.
Ariyanto EF, Odaiyappan NALS, Lidyana L, Wikayani TP, Qomarilla N, Wira DW and Triatin RD, (2019). Seeding cell number required for optimal lipid accumulation during adipocyte differentiation using 3T3-L1 cell line. WNOFNS. 25, 220.
Babish JG, Pacioretty LM, Bland JS, Minich DM, Hu J and Tripp ML, (2010). Antidiabetic screening of commercial botanical products in 3T3-L1 adipocytes and db/db mice. J Med Food. 13, 535.
Chandrasekaran CV, Vijayalakshmi MA, Prakash K, Bansal VS, Meenakshi J and Amit A, (2012). Review article: herbal approach for obesity management. Am J Plant Sci. 3, 2003.
Chihara T, Shimpo K, Beppu H, Tomatsu A, Kaneko K, Tanaka M, Yamada M, Abe F and Sonoda S, (2013). Reduction of intestinal polyp formation in min mice fed a high-fat diet with Aloe vera gel extract. Asian Pac J Cancer Prev. 14, 4435.
Christaki EV and Florou-Paneri PC, (2016). Aloe vera : a plant for many uses. J Food Agric Env. 8, 245.
Harbuwono DS, Pramono LA, Yunir E and Subekti I, (2018). Obesity and central obesity in Indonesia: evidence from a national health survey. Med J Indones. 27, 114.
Hasani-Ranjbar S, Jouyandeh Z and Abdollahi M, (2013). A systematic review of anti-obesity medicinal plants – an update. J Diabetes Metab Disord. 12, 28.
Jiang J, Ahn J, Huang W-Y and Hayes RB, (2013). Association of obesity with cardiovascular diseases mortality in the PLCO trial. Prev Med. 57, 60.
Lowe CE, O’Rahilly S and Rochford JJ, (2011). Adipogenesis at a glance. J Cell Sci. 124, 2681.
Misawa E, Tanaka M, Nabeshima K, Nomaguchi K, Yamada M, Toida T and Iwatsuki K, (2012). Administration of dried Aloe vera gel powder reduced body fat mass in diet-induced obesity (DIO) rats. J Nutr Sci Vitaminol. 58, 195.
Misawa E, Tanaka M, Nomaguchi K, Nabeshima K, Yamada M, Toida T and Iwatsuki K, (2012). Oral ingestion of Aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in zucker diabetic fatty rats. J Agric Food Chem. 60, 2799.
Moreno-Navarrete JM and Fernández-Real, (2012) Adipocyte differentiation in Adipose Tissue Biology. Symonds M.E., ed., Springer-Verlag, New York.
Moseti D, Regassa A and Kim WK, (2016). Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci. 17, 124.
Pothuraju R, Sharma RK, Rather SA, and Singh S, (2016). Comparative evaluation of anti-obesity effect of Aloe vera and Gymnema sylvestre supplementation in high-fat diet fed C57BL/6J mice. J Intercult Ethnopharmacol. 5, 403.
Rajeswari R, Umadevi M, Rahale CS, Pushpa R, Selvavenkadesh S, Kumar KPS and Bhowmik D, (2012). Aloe vera: the miracle plant its medicinal and traditional uses in India. J Pharmacogn Phytochem. 1, 118.
Ruiz-Ojeda FJ, Rupérez AI, Gomez-Llorente C, Gil A and Aguilera CM, (2016). Cell models and their application for studying adipogenic differentiation in relation to obesity: a review. Int J Mol Sci. 17, 1040.
Shin E, Shim KS, Kong H, Lee S, Shin S, Kwon J, Jo TH, Park YI, Lee CK and Kim K, (2011). Dietary Aloe improves insulin sensitivity via the suppression of obesity-induced inflammation in obese mice. Immune Netw. 11, 59.
Shin S, Kim S, Oh HE, Kong H, Shin E, Do SG, Jo TH, Park YI, Lee CK and Kim K, (2012). Dietary Aloe QDM complex reduces obesity-induced insulin resistance and adipogenesis in obese mice fed a high-fat diet. Immune Netw. 12, 96.
Subash-babu P and Alshatwi AA, (2012). Aloe-emodin inhibits adipocyte differentiation and maturation during in vitro human mesenchymal stem cell adipogenesis. J Biochem Mol Toxicol. 26, 291.
Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G and Catalán V, (2018). Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 48, e12997.