Institute of Pharmaceutical Research, GLA University, Mathura, Uttar Pradesh, India.
Corresponding author email: raghav.mishra@gla.ac.in
Article Publishing History
Received: 25/09/2021
Accepted After Revision: 21/12/2021
Due to the complexity of gastric emptying, as well as its considerable variability, the in vivo efficacy of drug delivery devices cannot be predicted. When it pertains to drugs with an absorption window in the upper small intestine, a controlled drug delivery system with a longer residence period in the stomach may be of considerable practical significance. Recent developments have shown that floating microspheres are particularly well suited for mixing sustained and delayed releases to achieve a variety of release models with a minimal risk of dumping. The aim of present investigation is to develop and analyze the floating microspheres of amethopterin, which after oral administration could increase the gastric residence time and enhance the bioavailability of the drug by sustained release and minimize the dose dependent side effects as well as improves patient compliance. Floating microspheres of ethyl cellulose, Polyvinyl alcohol and polyvinyl pyrrolidone-K90 were formulated by emulsification solvent evaporation technique.
The various parameters of prepared microspheres were studied for SEM, flow properties, buoyancy, yield, percent drug loading, in vitro dissolution studies, stability in different pH and FTIR studies. Microspheres prepared with different concentrations of polymers were spherical in shape with smooth surface. The size of microspheres was in range of 256.02 µm and 362.84 µm. Good drug entrapment and buoyancy were observed for formulation F2. The in vitro drug release after 6h was found to be in range from 58.15% to 96.28%. It was established that the newly created floating microspheres of Amethopterin provide an appropriate and practical solution for the sustained release of medication over a longer period of time, resulting in increased oral bioavailability, effectiveness, as well as better patient compliance.
Amethopterin, Antineoplastic, Buoyancy, Floating Microspheres.
Mishra R. Development and Optimization of Floating Microspheres in Amethopterin. Biosc.Biotech.Res.Comm. 2021;14(4).
Mishra R. Development and Optimization of Floating Microspheres in Amethopterin. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/31RVMnH“>https://bit.ly/31RVMnH</a>
Copyright © Mishra This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Oral route of administration is the most convenient and widely used method of drug administration, and the development of stomach specific oral controlled- release delivery systems is a challenging job due to the variation of pH in different segments of gastrointestinal tract, the fluctuation in gastric residence time and the difficulty in localizing an oral delivery system in a selected region of the gastrointestinal tract. Rapid gastrointestinal transit can prevent the absorption of complete drug in the absorption zone and reduce the efficacy of administered dose since the majority of drugs are absorbed in stomach or upper part of small intestine.
Polymers are generally employed in the development of floating microspheres. A number of different substances have been investigated for the preparation of floating microspheres; these materials include polymers of natural origin or synthetic origin and also semisynthetic substances. Floating microspheres can be prepared by using both hydrophilic and hydrophobic polymers. The concept of floating or porous microspheres can also be utilized to minimize the irritant effect of weakly acidic drugs on stomach by avoiding direct contact with the mucosa and providing a mean of getting low dosage for prolonged periods (Bulgarelli et al. 2000; Davoudi et al. 2013; Prakash et al. 2015; Srikar et al. 2018; Mishra et al. 2020; Birajdar et al. 2021; Kumar et al. 2021).
Amethopterin synonym Methotrexate (MTX) (Figure 1) is an antineoplastic agent whose mechanism is similar to alkylating agents. It is a highly toxic drug with a very low therapeutic index. It causes toxicities like stomatitis, gingivitis, glossitis, ulceration, and bleeding of the mucous membrane when given orally and hematological effects like leucopenia, thrombocytopenia, anemia, hemorrhage from various sites in single-dose intravenous administrations, and also some hepatic toxicities by administering as conventional dosage forms. Sustained and targeted delivery of amethopterin will reduce these toxicities considerably by maintaining a low and constant level of drug in the blood. Therefore, floating microspheres have emerged as an efficient means of prolonging gastric residence time, targeting stomach mucosa, and enhancing the bioavailability.
Floating microspheres remain buoyant due to lower density than the gastric and intestinal fluids. They are not subjected to ‘all or nothing’ gastric emptying nature of single unit system and releases the drug in a controlled fashion. In the current research, emulsification solvent evaporation including EC, PVP-K90, and PVA polymers were used to form hollow microspheres containing Amethopterin. (Chen et al. 2000; Singh et al. 2000; Shishu et al. 2007; Garg et al. 2008; Brunton et al. 2011; Rathor et al. 2011; Abbas et al. 2020; Kumal et al. 2020; Tomar et al. 2021; Thakur et al. 2021; Kharb et al. 2021).
MATERIAL AND METHODS
Amethopterin was obtained as a gift sample (Naprod Life Science Mumbai) whereas ethyl cellulose (EC), polyvinyl pyrrolidone K-90 (PVP K-90) and polyvinyl alcohol (PVA) were obtained from Merck India, Mumbai. Other chemicals used were of analytical grade. Floating microspheres containing amethopterin were-prepared by emulsification solvent evaporation technique using EC, PVP-K90 and PVA polymers for microspheres (Soppimath et al. 2001) (Table 1). Amethopterin (100 mg) was weighed accurately and dissolved in 8 mL ethyl alcohol, followed by the addition of 2 mL isopropyl alcohol and 5 mL methylene dichloride.
The polymer solution was slowly introduced into 100 mL of 1% polyvinyl alcohol aqueous solution while stirring at 250 rpm using a mechanical stirrer equipped with a 3-blade propeller. The solution was stirred for 10 minutes and microspheres were collected by filtration. The floating microspheres were collected by decantation, while the non-floating microspheres were discarded along with any polymer precipitates. The microspheres were dried in an oven at 50℃ for 2hours, weighed and then stored in a desiccator at room temperature for further use.
The developed floating microspheres were evaluation considering following parameters:
- Surface Morphology: The prepared microspheres were fixed on a brass stub using double sided adhesive tape and then made electrically conductive by coating, in vacuum, with a thin layer of gold for 30 minutes and then examined by scanning electron microscope (SEM) operated at 10kV (JOEL-JXA840A electron probe micro-analyser, Japan) (Jain et al. 2005) (Figure 2(A) and 2(B))
Flow Properties: Flow properties were determined in terms of Carr’s index (CI) using the following formulae:
CI = 𝝆t-𝝆b / 𝝆t
where,
𝝆t = Tapped density and 𝝆b = Bulk density
The angle of repose [ϴ] of the microsphere, which measures the resistance to flow, was determined by fixed funnel method, using the following equation:
tan ϴ = h/r
where,
h = height of the pile and r = radius of the cone formed after making the microspheres flow through glass funnel from a fixed height (Kale et al. 2007).
The particle size of floating microspheres was performed with the help of optical microscope for randomly selected sample for all formulations (Table 2).
- Buoyancy test for microspheres: In triplicates, microspheres (100mg) were dispersed in solution composed of HCl (300 mL, pH 1.2 at 37℃) containing Tween 20 (0.02% w/v) to simulate gastric conditions (Patel et al. 2011, Raveendra et al. 2012).
The use of 0.02% Tween 20 was to account for the wetting effect of the natural surface-active agents, such as phospholipids in the gastrointestinal tract (GIT) (Jain et al. 2009).
The mixture was stirred on magnetic stirrer at 100 rpm and 37±0.5℃. After 12hours, the floating particles were separated by filtration. The sinking particles were separated by filtration. Both particle types were weighed after drying at 40℃ overnight. The buoyancy was determined by the weight ratio of the floating particles to the sum of floating and sinking particles. Percent buoyancy of formulations is shown in Table 3.
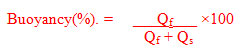
where,
Qf = Weight of floating microspheres and Qs = Weight of settled microspheres
Yield of Microspheres: The prepared microspheres were collected and weighed. The actual weight of obtained microspheres divided by the total amount of all drug and polymers solid material that was used for the preparation of all the microspheres (Patel et al. 2006). Yield of microspheres is shown in Table 3.

Percent Drug Loading: Amethopterin content in the floating microspheres was estimated by a UV-Visible Spectrophotometer (Lambda 25, Perkin Elmer,US) method based on the measurement of absorbance at 303 nm in distilled water (Semalty et al. 2007). Microspheres equivalent to 100 mg were weighed and added in 100 ml of distilled water. The volumetric flask was stirred continuously for 24 hours on a magnetic stirrer. At the end of 24 h sample was withdrawn, diluted suitably and measured spectrophotometrically at 303 nm for drug content. Quantitative estimation of amethopterin was calculated by using equation obtained by Linear regression analysis of calibration curve in distilled water. The drug loading in microspheres was estimated using the formula:
Drug Loading (L) = (Qm / Wm) ×100
where,
Wm is the weight of microspheres and Qm is the quantity of drug present in Wm of microspheres (Semalty et al. 2008).
Percent drug loading of various formulations is shown in the Table 3.
- In vitro dissolution studies: In vitro dissolution studies were performed using USP XXIII, Type-II (paddle) dissolution apparatus. The accurately weighed sample (50 mg) of formulations from F1 to F6 were dropped individually into 500 mL of phosphate buffer (pH 7.2) maintained at a temperature of 37±0.5℃ and stirred at a speed of 50 rpm. At different time intervals, 1mL aliquot of the sample was withdrawn and the volume was replaced with an equivalent amount of buffered dissolution medium kept at 37℃. The collected samples were filtered and diluted with 9mL of phosphate buffer and analyzed at ƛmax 303nm using a UV- Visible spectrophotometer (Lambda 25, Perkin Elmer,US) against the buffer taken as blank. (Figure 3) Percent cumulative drug release from floating microspheres was calculated using Beer- Lambert’s Equation. The drug release was calculated using various models and shown in Table 3 (Patel et al. 2005).
- Stability of microsphere at different gastric pH: Floating microspheres are low density systems that have sufficient buoyancy to float over gastric contents and remain in stomach for prolonged period where they are exposed to different pH and different enzymatic conditions which can influence their physiochemical properties and drug release behavior and can alter their physiochemical properties and drug release behavior and can alter their stability characteristics. To test this hypothesis, drug loaded microspheres were subjected to different pH media where they encountered different ionic strengths and enzymatic conditions and the change in their properties was elucidated by counter checking their particle size. The pH dependent stability studies were carried out in following media:
- pH 1.1: 12 mL HCl (32%) with 1188 mL H2
- pH 3.5: 150 mL solution (10.5g citric acid + 100mL NaOH of 1M + 395.5 mL H2O) with 100mL HCl.
- Simulated Gastric Fluid (SGF): 0.2% NaCl, Pepsin 0.7% HCl with pH 1.2.
10 mL of simulated fluid were added to 10mg of microspheres. The samples were analyzed after a period of 12hours in each of the above media. The above time intervals were selected for the study based on expected formulation residence time in stomach. Particle size was determined on the preset time periods (Kalaria et al. 2009). The results are recorded in Table 4.
IR Spectroscopic Studies: Drug-Polymer interaction was studied by FT-IR spectroscopy (Shimadzu Affinity I, FT-IR spectrophotometer). Samples were prepared by triturating 10 % of the drug or microspheres with 95% of KBr in glass pestle-mortar. The IR spectra of the drug and the microspheres were recorded, the identical peaks of the drug and drug with polymers concluded that neither the polymer nor the method of preparation has any significant effect on the drug stability (Jain et al. 2006; Kaushik et al. 2010).
RESULTS AND DISCUSSION
The floating microspheres of amethopterin were prepared by emulsion solvent evaporation method using ethyl cellulose, polyvinyl pyrrolidone and polyvinyl alcohol (Saravanan et al. 2011; Ahmadi et al. 2020; Ramadan et al. 2020; Maddibovina et al. 2020).
Figure 1: Amethopterin
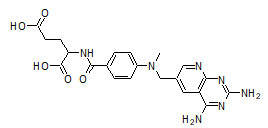
Table 1. Composition of floating microspheres
| Ingredient | F1 | F2 | F3 | F4 | F5 | F6 |
| Drug | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| EC | 0.5 | 1.0 | 1.5 | 0.5 | 1.0 | 1.0 |
| PVP-K90 | – | – | – | 1.0 | 0.5 | 1.5 |
| PVA | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
EC: Ethylcellulose; PVP K-90: Polyvinyl pyrrolidone K-90; PVA: Polyvinyl alcohol.
The prepared floating microspheres were evaluated for different physicochemical tests such as particle size, true density, flow properties, drug content, in vitro float ability and in vitro drug release studies (Mahor et al. 2020).
Table 2. Flow Properties of floating microspheres
| Formulation code | Angle of Repose [ϴ] | Carr’s Index [%] | Average Particle size [µm] |
| F1 | 28.35±0.670 | 18.32±0.364 | 342.32±3.240 |
| F2 | 24.64±0.502 | 15.25±0.342 | 362.84±2.452 |
| F3 | 23.54±0.341 | 12.54±0.322 | 356.42±0.2465 |
| F4 | 21.65±0.422 | 17.53±0.232 | 260.56±1.8602 |
| F5 | 24.52±0.438 | 13.66±0.522 | 256.02±2.5446 |
| F6 | 22.61±0.246 | 12.42±0.426 | 276.24±2.6773 |
Results are shown as Mean ± Standard Deviation, (n=3)
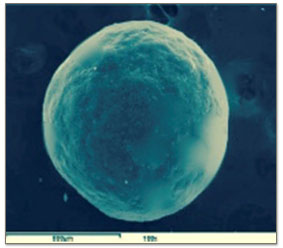
Scanning electron microscopy showed that F1, F2, F4, F5, F6 formulation produced spherical microspheres compared to F3. The scanning electron microscopy confirmed the hollow nature of microspheres with pores on the surface of floating microspheres, which imparted floating properties to the prepared floating microspheres.
Figure 2(A): The cluster of microspheres
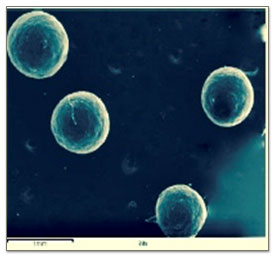
Figure 2(B): A single microsphere
To estimate flow properties of prepared floating microspheres, micromeritic properties like particle size and true density were determined. The densities of floating microspheres were found to be less then density of gastric fluid, therefore tended to float over gastric fluid. So, the prepared microspheres combine the advantages of multiple unit systems and good floating properties. However, like all floating systems their efficacy is dependent on the presence of enough liquid in the stomach, requiring frequent drinking of water. Particle size analysis of different formulation was done by optical microscopy. The average particle size for microspheres was in the range between 256.02µm and 362.84µm. The average particle size of microspheres was found to be increasing with the increase in concentration of polymer (Ramadan et al. 2020).
Drug content in F1, F2, F3, F4, F5 and F6 formulation were estimated by UV spectrophotometric method. Percent loading efficiency were found in the range of 42.62% to 95.36%. Formulation F4 containing ethyl cellulose (0.5%) and polyvinyl pyrrolidone (1%) showed maximum loading of drug up to 95.36%. The rank order of percent loading was found to be as followed F4>F1>F6>F2>F3>F5. In vitro drug release studies of all the formulations were performed in phosphate buffer of pH 7.2 at 303 nm.
Table 3. Release parameters of floating microspheres
| Formulation code | Drug release after 6 h | % Drug loading | % Buoyancy | %Yield |
| F1 | 71.81% ±1.28 | 84.36±0.36 | 96.28±1.48 | 78.96±2.64 |
| F2 | 92.71% ±2.36 | 58.84±0.82 | 94.36±1.86 | 95.38±0.38 |
| F3 | 80.32% ±1.10 | 53.92±1.46 | 90.62±1.54 | 82.56±1.64 |
| F4 | 68.22% ±1.46 | 95.36±2.36 | 60.26±2.62 | 70.48±1.74 |
| F5 | 67.57% ±1.48 | 42.62±1.68 | 54.56±2.84 | 76.32±1.38 |
| F6 | 58.15% ±1.02 | 76.38±1.72 | 68.38±1.36 | 82.93±1.76 |
Results are shown as Mean ± Standard Deviation, (n=3).
Figure 3: The in vitro drug release profile
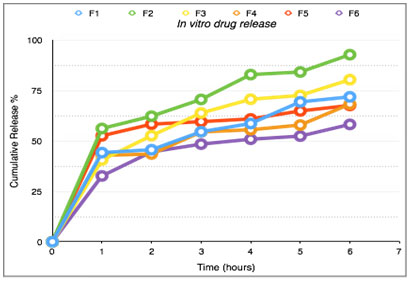
Significant difference was observed in the release pattern of amethopterin floating microspheres EC, PVA and PVP. It was found that the drug release from the formulations were distinguishably different for the different polymers used in the formulations. The rank order of drug release after 6 hours was found to be 92.71, 80.32, 71.81, 68.22, 67.57, 58.15 percent of formulation F2, F3, F1, F4, F5, F6 respectively. Formulation F2 containing ethyl cellulose (1%) showed the maximum release after 6 hours. Stability studies of microspheres at pH 1.1, 3.5 and SGF were conducted and found to be stable (Yang et al. 2021). FT-IR studies showed that there was no interaction between the drug and polymers.
Table 4. Stability Studies of floating microspheres
| Medium | Initial Size | Final Size |
| pH1.1 | 256.02±2.5446 | 258.12±2.5446 |
| pH3.5 | 256.02±2.5446 | 260.42±2.5446 |
| SGF | 256.02±2.5446 | 258.68±2.5446 |
Results are shown as Mean±Standard Deviation, (n=3).
CONCLUSION
The current Amethopterin formulation research was carried out in an effort to develop a floating drug delivery system composed of a floating multiple-unit system. By emulsion solvent evaporation, novel floating hollow microspheres were effectively developed for the sustained and stomach-specific action of Amethopterin. As a result of its low density, this multiparticulate drug delivery method demonstrated excellent flotation characteristics. From in vitro drug release studies, it can be concluded that, by changing the ratio of polymer and solvent, drug release can be controlled. The results of all the physiochemical tests of all formulations were found to be satisfactory.
In vitro float ability studies revealed that most of the microspheres (54.56 % to 96.28 %) were floatable. The in vitro drug release was found to be in range of 58.15 % to 92.71 % at the end of 6 hours. The proposed solution, which combines good buoyant ability with an appropriate drug release pattern, may be beneficial in terms of improved Amethopterin bioavailability. The findings of the present study presented that these floating microspheres can be selected for the development of gastro retentive drug delivery system of Amethopterin for potential therapeutic use.
ACKNOWLEDGEMENTS
The authors received no direct funding for this research. Moreover, authors acknowledge with gratitude the Department of Pharmacy, GLA University, Mathura, Uttar Pradesh for providing the necessary infrastructure and facilities to conduct this research work.
Conflict of interests: Authors declare no conflict of interests to disclose.
REFERENCES
Abbas, A. K., and Alhamdany, A. T. (2020). Floating Microspheres of Enalapril Maleate as a Developed Controlled Release Dosage Form: Investigation of the Effect of an Ionotropic Gelation Technique. Turkish Journal of Pharmaceutical Sciences, 17(2), 159.
Ahmadi, P., Jahanban-Esfahlan, A. and Ahmadi, A. (2020). Development of Ethyl Cellulose-based Formulations: A Perspective on the Novel Technical Methods. Food Reviews International, 1-48.
Birajdar, A. A., Deshmukh, M. T. and Shete, R. V. (2021). A Review on Gastro-Retentive Floating Microspheres. Journal of Drug Delivery and Therapeutics, 11(1), 131-138.
Brunton, L. L., Chabner, B. A. and Knollmann, B. C. (2011) In Antineoplastic agents: Goodman and Gilman’s. The pharmacological basis of therapeutics, Mc Graw Hill. New York Pages 1243-1247.
Bulgarelli, E., Forni, F. and Bernabei, M. T. (2000). Effect of Matrix Composition and Process Conditions on Casein-Gelatin Beads Floating Properties. International Journal of Pharmaceutics, 198(2), 157-15.
Chen, J. and Park, K. (2000). Synthesis of fast-swelling, super porous sucrose hydrogels. Carbohydrate Polymers, 41(3), 259-268.
Davoudi, E. T., Noordin, M. I., Kadivar, A., et al. (2013). Preparation and Characterization of a Gastric Floating Dosage Form of Capecitabine. BioMed Research International, 2013, 495319.
Garg, R. and Gupta, G. (2008). Progress in controlled gastro retentive delivery systems. Journal of Pharmaceutical Research, 7(3), 1055-1066.
Jain, S. K. and Gupta, A. (2009). Development of glucose 43/01 beads of metformin hydrochloride for floating delivery, AAPS Pharm Sci Tech, 10, 1128-1136.
Jain, S. K., Agarwal G. P. and Jain N. K. (2006). Evaluation of porous carries based floating orlistat microspheres for gastric delivery. AAPS PharmSciTech, 7(4), 2006, E54-E62.
Jain, S. K., Awasthi, A. M., Jain, N. K., et al. (2005). Calcium silicate-based microspheres of repaglinide for gastro-retentive floating drug delivery: Preparation and in-vitro characterization. Journal of Controlled Release, 107(2), 300-309.
Kalaria, D. R., Sharma, G., Beniwal, V. et al. (2009). Design of biodegradable nanoparticles for oral delivery of doxorubicin: In vivo pharmacokinetics and toxicity studies in rats. Pharmaceutical Research, 26(3), 492-501.
Kale, R. D. and Tayade, P. T. (2007). A multiple unit floating drug delivery system of piroxicam using eudragit polymer. International Journal of Pharmaceutical Science, 69(1), 120-123.
Kaushik, D., Sardana, S. and Mishra D. (2010). In vitro characterization and cytotoxicity analysis of 5-FU loaded chitosan microsphere for targeting colon cancer. International Journal of Pharmaceutical Education and Research, 44(3), 267-273.
Kharb, M., and Tanwar, Y. (2021). Development and statistical optimization of gastroretantive floating microspheres of pregabalin prepared by W/O/O multiple emulsion method. International Journal of Applied Pharmaceutics, 4(8), 199-206.
Kumal, V. B., Thapa, C., Ghimire, P., et al. (2020). Formulation and optimization of Enalapril Maleate-loaded floating microsphere using Box–Behnken design: In vitro study. Journal of Applied Pharmaceutical Science, 10(08), 095-104.
Kumar, A., and Bharkatiya, M. (2021). A Recent Update on Formulation and Development of Gastro-Retentive Drug Delivery Systems. International Journal of Pharmaceutical Sciences and Nanotechnology, 14(1), 5257-5270.
Maddiboyina, B., Hanumanaik, M. and Nakkala, R. K. (2020). Formulation and evaluation of gastro-retentive floating bilayer tablet for the treatment of hypertension. Heliyon, 6(11), e05459.
Mahor, S., Chandra, P., and Prasad, N. (2021). Design and in-vitro Evaluation of Float-adhesive Famotidine Microspheres by using Natural Polymers for Gastroretentive Properties. Indian Journal of Pharmaceutical Education and Research, 55(2), 407-417.
Mishra, R. and Mishra, A. (2020). An Overview on Floating Microsphere. International Journal of Pharmaceutical Research, 12(04), 377-386.
Patel, A., Ray, S. and Thakur, R. S. (2006). In vitro evaluation and optimization of controlled release floating drug delivery system of metformin hydrochloride. DARU, 14(2), 57-64.
Patel, J. K., Patel, R. P. and Patel, M. P. (2005). Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS PharmSciTech, 6(1), EE49-EE55.
Patel, K.., Jain, P. K., Baghel, R., et al. (2011). Preparation and in vitro evaluation of a micro balloon delivery system for domperidone. Pharma Letter. 3, 131-141.
Prakash, S., Bhandari, A., Mishra, R., et al. (2015). Development and optimization of floating microspheres of gliclazide. International Journal of Pharmaceutical Science and Research, 6(5), 807-817.
Ramadan, A. A., Elbakry, A. M. and Sarhan, H. A. (2020). Silymarin loaded floating polymer (s) microspheres: characterization, in-vitro/in-vivo evaluation. Pharmaceutical Development and Technology, 25(9), 1081-1089.
Rathor, S. and Ram, A. (2011). Porous microsphere of 5-FU. A tool for site specific drug delivery in gastric cancer. International Journal of Current Pharmaceutical Research, 3(1), 38-42.
Raveendra, M., Narayana, K. L., Reddy, C. S., et al. (2012). Formulation and in vitro evaluation of floating microspheres of timolol maleate. Journal of Pharmaceutical, Chemical and Biological Sciences, 3, 936-946.
Saravanan, M., and Anupama, B. (2011). Development and evaluation of ethylcellulose floating microspheres loaded with ranitidine hydrochloride by novel solvent evaporation-matrix erosion method. Carbohydrate polymers, 85(3), 592-598.
Semalty, A. and Semalty, M. (2007). Preparation and characterisation of mucoadhesive microspheres of ciprofloxacin hydrochloride. Indian Drugs, 44, 368-372.
Semalty, M. and Semalty, A. (2008). Gastro-retentive floating microspheres of diltiazem hydrochloride. Pharma Buzz, 3(12), 24-28.
Shishu, Gupta, N. and Agarwal, N. (2007). Stomach specific drug delivery of 5-FU using floating alginate beads. AAPS PharmSciTech, 8(2), E143-E149.
Singh, B. N. and Kim, K. H. (2000). Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. Journal of Controlled Release, 63(3), 235-259.
Soppimath, K. S., Kulkarni, A. R. and Aminabhavi, T. M. (2001). Development of hollow microspheres as floating controlled-release systems for cardiovascular drugs: Preparation and release characteristics. Drug Development and Industrial Pharmacy, 27(6), 507-515.
Srikar, G. (2018). Floating microspheres: A prevailing trend in the development of gastroretentive drug delivery system. Asian Journal of Pharmaceutics, 12(04), 235-242.
Thakur, S., Ramya, K., Shah, D. K., et al. (2021). Floating Drug Delivery System. Journal of Drug Delivery and Therapeutics, 11(3-S), 125-130.
Tomar, A., Singh, A., Gupta, A., et al. (2021). Floating drug delivery system: A review. International Journal of Indigenous Herbs and Drugs, 6(1), 33-39.
Yang, L., Wang, S., Wang, R., et al. (2021). Floating chitosan-alginate microspheres loaded with chlorantraniliprole effectively control Chilo suppressalis (Walker) and Sesamia inferens (Walker) in rice fields. Science of The Total Environment, 783, 147088.


