1Department of Biotechnology, Hemchandracharya North Gujarat University, Patan, Gujarat, India
2,Dr.Indu Dayal Meshri College of Science and Technology, Patan, Gujarat, India
Corresponding author email: arpit3290@gmail.com
Article Publishing History
Received: 14/04/2020
Accepted After Revision: 14/06/2020
Measles virus phosphoprotein tetramerization domain contains ligand named Acetate ion, (acetate, C2H3O2). It was identified as a Drug Target protein. Its protein structure (PDB format) was downloaded from the Protein Data Bank. Ligand Acetate ion, (acetate, C2H3O2) was obtained for further analysis. Compounds were selected from available drug libraries such as PubChem, DrugBank, ChemSpider etc. physical and chemical properties were tabulated in order to compare similarity score. Furthermore they were screened and docked with Acetate ion, (acetate, C2H3O2) using PyRx-virtual screening software for Structure based drug design (SBDD). Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) properties were determined by using online tool Danish Quantitative Structure-Activity Relationship (QSAR) database. The Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) analysis and docking results revealed 13 compounds as potential leads in our dry lab experiments. These chemical compounds can be further investigated in wet lab experiments with a view to finding effective drugs against Measles.
Acetate ion, Absorption, Distribution, Metabolism, Excretion and Toxicity, ADMET, Measles, PyRx
Trivedi A, Trivedi S, Patel J, Dave S. Design and Prediction of ADMET Properties of Drugs for Measles Using In-Silico Approaches. Biosc.Biotech.Res.Comm. 2020;13(2).
Trivedi A, Trivedi S, Patel J, Dave S. Design and Prediction of ADMET Properties of Drugs for Measles Using In-Silico Approaches. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3aL1oip
Copyright © Trivedi et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Measles infection is occurred by a virus from the paramyxovirus family. These viruses are small parasitic microbes. Once you got infected, the virus attacked host cells and utilizes cellular components to complete its life cycle(Taylor et. al., 2002; Kayeet al., 2001). The respiratory tract is initially infected by the virus. However, it ultimately increases to other parts of the body through the blood stream. There are twenty four recognized genetic types of measles, although only six are at present circulating(Aickin, et al., 1994). Measles is spread through droplets from the mouth, nose or throat of infected persons. Initial symptoms can be observed after 10–12 days of infection. It includes bloodshot eyes, high fever, a runny nose, and tiny white spots on the inside of the mouth. A few days later, a rash expands, starting on the face and upper neck and slowly spreading downwards, (Naim, 2015).
Measles virus, that is a single-stranded, negative-sense RNA virus belongs to the Mononegavirales order which includes a number of human pathogens such as Ebola, Nipah, and Hendra viruses whose genome is encapsidated by multiple copies of the viral nucleoprotein (N). This N-RNA complex is the template for transcription and replication by the viral polymerase complex contains the large protein (L) that carries the RNA-dependent RNA polymerase activity and the phosphoprotein (P), the polymerase cofactor (Communieet al., 2013).The phosphoprotein of measles virus is a modular protein contains an essentially disordered N-terminal domain and of a C-terminal moiety (PCT) made up of alternating disordered and globular regions(Johansson et al.,2003).
In measles virus, during genome replication, synthesis of viral RNA and encapsidation by nucleoprotein are associated. Nucleoprotein also has the ability to self-assemble on cellular RNA to form nucleocapsid resembling particles in the absence of viral RNA and viral proteins. The association of phosphoprotein with the soluble, monomeric form of Nucleoprotein avoids it from binding to cellular RNA. The nucleoprotein – phosphoprotein (N-P) complex is the substrate employed by the polymerase to initiate encapsidation of genomic RNA. Nucleoprotein forms complexes with phosphoprotein and with the phosphoprotein – large protein (P-L) complex during transcription and, during replication too (Johansson et al.,2003).
Measles was an unavoidable disease during the human advancement with considerable level of morbidity and mortality. The seriousness of measles infection was to a great extent contained by the advancement of a live attenuated antibody that was brought into the vaccination programs. However, all efforts to eliminate the disease failed and continued to result in major deaths yearly. The expansion of molecular biology techniques permitted the rescue of measles virus from cDNA that facilitated vital insights into a variety of aspects of the biology of the virus and its pathogenesis. Consequently these technologies aided the development of novel vaccine candidates that stimulate immunity against measles and other pathogens. Depending on the promising prospective, the utilization of measles virus as a recombinant vaccine and a therapeutic vector is concentrated (Naim, 2015).
Measles is a viral infection that begins in the respiratory system. It stays a significant cause of death amongst young children all over the world, in spite of having, the accessibility of a safe and effective vaccine. According to the World Health Organization (WHO), there were almost 1,10,000 worldwide deaths happened due to measles in 2017 and majority of them were children under the age of 5. Measles was a predictable infection during the human development with considerable degree of morbidity and mortality. The severity of measles virus infection was mostly contained by the improvement of a live attenuated vaccine that was commenced into the Vaccination Plans. Still, all efforts to eliminate the disease are unsuccessful and it sustained to yearly result in significant deaths (Naim, 2015).
Vaccine of Measles has been found years ago. The disease was remained under control due to vaccination program for many years. Worldwide measles deaths have diminished by 84 percent worldwide as of late — from 550,100 deaths in 2000 to 89,780 out of 2016 — measles is as yet regular in many developing nations, especially in parts of Africa and Asia. However, the cases of measles are being reported in recent years. Undoubtedly the death ratio has been decreased still huge number of cases is reported. Measles is a very infectious viral illness. It remains a significant reason for death among youthful kids all around, in spite of the accessibility of a protected and successful antibody. An expected 7 million individuals were influenced by measles in 2016 (https://www.who.int/immunization/diseases/measles/en/, 2019).
Measles vaccine was replaced by mixing up measles, mumps, and rubella vaccine in 1988, with seekingthe elimination all three diseases from the UK. As the implementation of the vaccine, coverage has enhanced steadily with 93% of children now being vaccinated by 2 years of age. Though the overall reduction in the incidence of measles, outbreaks in older children have recently been reported, suggesting that a two dose strategy may be needed to make sure eradication(Brownet al., 1994).Evaluations of vaccination programs are generally based on the theory that vaccines have an impact only against specific diseases. This theory may not be true for measles vaccine. Current studies designate that vaccines may have significant non-specific effects as girls receiving high titre measles vaccines were established to have reduced long term survival compared with recipients of standard titre vaccines. Secondly, studies of standard titre measles vaccine have reported a better than expected reduction in mortality in areas with high mortality. Since these observations implies that measles immunization may have a non-specific, useful effect (Aaby et al., 1995).
To design the drug with the help of the computer is a specialized branch that uses computational approaches to create drug-receptor interactions (Bissantzet al., 2000).These types of methods are greatly dependent on the tools of bioinformatics, applications and databases. Many of the promising drugs fail in clinical trials after many years of research. The failure is due to toxicity or problems with metabolism. The ADMET properties of drugs are Absorption, Distribution, Metabolism, Excretion and Toxicity in other words bioavailability and bioactivity. However; these properties are typically measured in the wet lab, they can also be assumed with help of bioinformatics software and tools. The wide range of bioinformatics tools and web indexes (search engines) are available for this work (Bissantz et al., 2000; Iskaret al., 2011). Once potential lead molecules have been identified the next step is to find their structural and ADMET properties. This commonly consists of the arrangement of changes in accordance with the essential and secondary structure of the compound. The protocol can be enhanced by using software that explores related compounds to the lead candidate (Songet al., 2009).
MATERIAL AND Methods
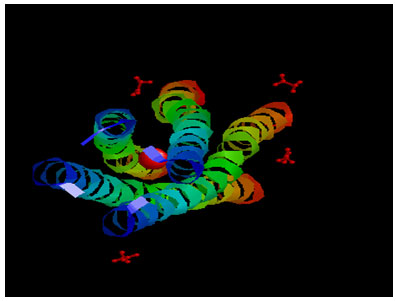
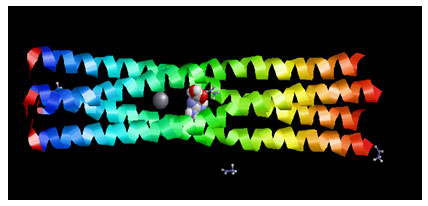
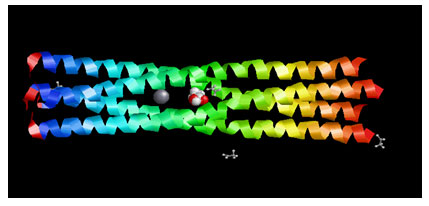
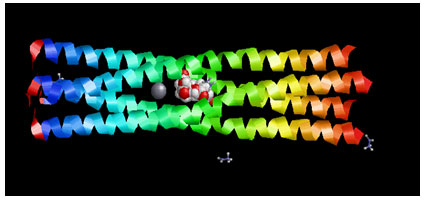
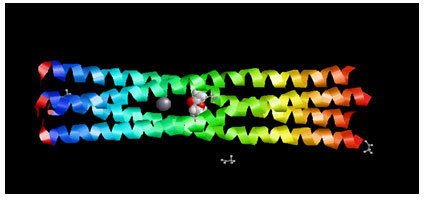
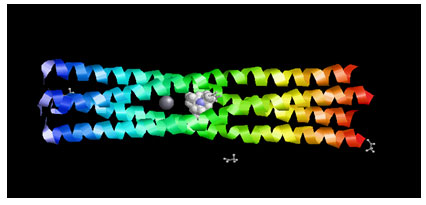
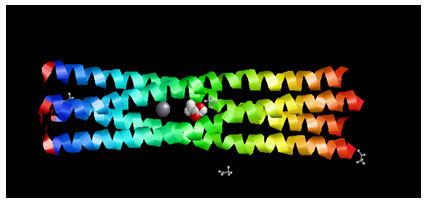
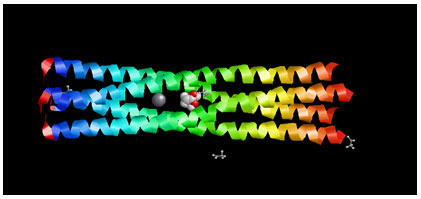
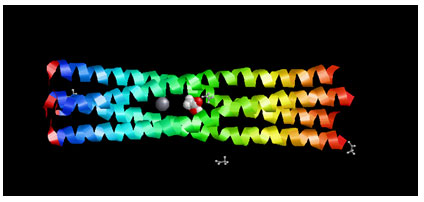
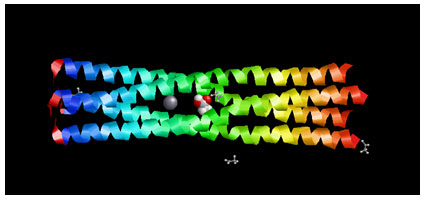
Target Identification: Phosphoprotein tetramerization domain of Measles Virus, which is responsible for the induced folding of the C-terminal Domain of the Nucleoprotein (figure 1) was taken as target protein(Johansson et al., 2003).
Figure 1 : Measles virus phosphoprotein tetramerization domain
The structure file of protein with PDB ID 4BHV was downloaded from Protein Data Bank. The experimental details are, Resolution[Å]: 2.1, R-Value: 0.232 (obs.) and R-Free:0.242.
Drug Library: Compounds having related structures and properties with Acetate ion, (acetate, C2H3O2) were obtained and screened using online chemical databaseswizPubChem, DrugBank, KEGG, ChemSpider etc.(Table 1) (Dias et al., 2008; Fernando et al., 2008).
Docking: Docking was performed on the structure filessuch as .sdf/.mol/.pdb of the compounds usingPyRx docking program with a view to obtaining binding energy(Table 2).
ADMET: Docked chemical substances were tested using QSAR database for ADMET properties such as Carcinogenicity, Lethal concentration, Mutagenicity, In vitro tests, Health End points etc. Thirteen compounds lastly considered as drug target candidates since they accomplish most of the characteristics(Table 3).
RESULTS AND DISCUSSION
Table 1. Physical and Chemical Properties of Chemical Compounds
| Drug
Accession Number |
Log P | State | pKa | Polarizability | Molecular
Weight (g/mol) |
Boiling
Point |
Melting
Point |
| CID 176 | -0.12 | Solid | 4.54 | 5.34 Å3 | 60.052 | 117.9 °C | 16.6 °C |
| CID 757 | -1 | Solid | 3.53 | 6.2 Å3 | 76.0514 | NA | 79.5 °C |
| CID 760 | -0.59 | Solid | 3.3 | 5.35 Å3 | 74.0355 | NA | 98 °C |
| CID 763 | NA | NA | NA | NA | 117.108 | NA | NA |
| CID6116 | 0.24 | Solid | 4.54 | 4.96 Å3 | 158.166 | NA | > 160 °C |
| CID 6144 | 5.01 | NA | NA | NA | 380.171 | NA | 300 deg C |
| CID 7997 | NA | NA | NA | NA | 102.133 | 214.9° F | -93 °C |
| CID 8471 | NA | liquid | NA | NA | 101.193 | 192.7° F | 174.5° F |
| CID 82741 | NA | NA | NA | NA | 133.19 | NA | NA |
| CID 101751 | NA | NA | NA | NA | 104.016 | NA | NA |
| CID 517045 | NA | NA | NA | NA | 82.034 | NA | 328°C |
| CID 3474584 | NA | NA | NA | NA | 65.984 | NA | NA |
| CID 6917684 | NA | NA | NA | NA | 472.5 | NA | NA |
Table 2. Showing Binding Energy of Chemical Compounds
| Drug Accession Number | Binding energy
kca/mol |
| CID176 | -5.01 |
| CID757 | -5.29 |
| CID760 | -5.14 |
| CID763 | -4.76 |
| CID6116 | -5.01 |
| CID6144 | -4.44 |
| CID7997 | -6.51 |
| CID8471 | -5.98 |
| CID82741 | -5.01 |
| CID101751 | -5.01 |
| CID517045 | -5.01 |
| CID3474584 | -5.01 |
| CID6917684 | -5.28 |
Table 3. Absorption, Distribution, Metabolism, Excretion and Toxicity Properties
| Drug Accession Number | Health End Point | Mutagenicity | In Vitro Tests | Carcinogenicity | ||
| Severe skin irritation
|
Skin sensitization | Respiratory sensitization | Ames test (Salmonella) | HGPRT | ||
| CID176 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID757 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID760 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID763 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID6116 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID6144 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID7997 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID8471 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID82741 | NEG | NEG | NEG | POS | NEG | NEG |
| CID101751 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID517045 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID3474584 | NEG | NEG | NEG | NEG | NEG | NEG |
| CID6917684 | NEG | NEG | POS | NEG | NEG | NEG |
( * POS = POSITIVE) (* NEG =NEGATIVE)
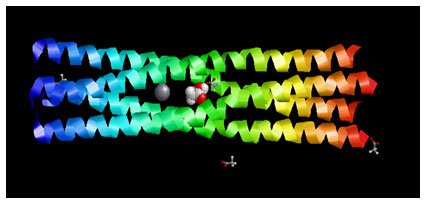
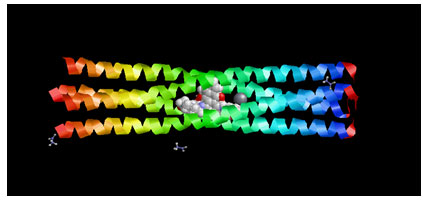
Figure 2: Showing the Docking Pose of Acetic acid (CID176) with Measles virus phosphoprotein tetramerization domain
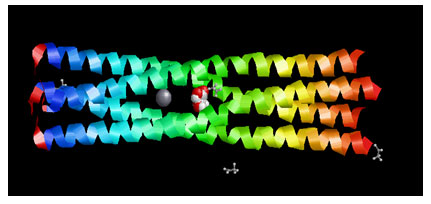
Figure 3: Showing the Docking Pose of Glycolic acid (CID757)with Measles virus phosphoprotein tetramerization domain
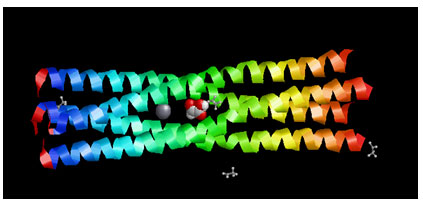
Figure 4: Showing the Docking Pose of Glyoxylic acid (CID760)with Measles virus phosphoprotein tetramerization domain
Figure 5: Showing the Docking Pose of Glycocyamine (CID763) with Measles virus phosphoprotein tetramerization domain
Figure 6: Showing the Docking Pose of Calcium acetate (CID6116)with Measles virus phosphoprotein tetramerization domain
Figure 7: Showing the Docking Pose of Sodium edetate (CID6144)with Measles virus phosphoprotein tetramerization domain
Figure 8: Showing the Docking Pose ofPropyl acetate (CID7997)with Measles virus phosphoprotein tetramerization domain
Figure 9: Showing the Docking Pose ofTRIETHYLAMINE (CID8471)with Measles virus phosphoprotein tetramerization domain
Figure 10: Showing the Docking Pose of Tetramethyl ammonium acetate (CID82741)with Measles virus phosphoprotein tetramerization domain
Figure 11: Showing the Docking Pose of Disodium ethene-1,1-diolate (CID101751)with Measles virus phosphoprotein tetramerization domain
Figure 12: Showing the Docking Pose of Sodium acetate (CID517045)with Measles virus phosphoprotein tetramerization domain
Figure 13: Showing the Docking Pose of Lithium acetate (CID3474584)with Measles virus phosphoprotein tetramerization domain
Figure 14: Showing the Docking Pose of Ametantrone acetate (CID6917684) with Measles virus phosphoprotein tetramerization domain
In the present study 80 chemical compounds were analyzed as drug target candidate for Measles virus phosphoprotein tetramerization domain. Physical and chemical properties including pKa value, Molecular weight(MW), State, Polarizibility, Melting and Boiling Point were obtained from DrugBank, PubChem, ChemSpider etc. online databases with a view to understanding the essentiality of the compound as a drug target(Table 1)(Dias et al., 2008).
pKa value of a weak acid varies between -2 to 12. pKa values more than twelve area unit are considered to be alkaline drug and lower than -2 area units are considered to be a strong acid. The ability of a molecule to be polar or non polar is indicated by Polarizability, which is reliant upon the charge distribution and affects the bond formation in a chemical compound.The standard range of binding energy is between -3 to -9 Kcal/mol.Docking results indicate that lower binding energy illustrate the higher affinity of the drug target candidate(Table 2)(Modiset al., 2003).
Each drug has to fulfill its ADMET properties such as Mutagenicity, Toxicity, In-vitro test and Carcinogenicity etc. in order to prove their potentiality as a drug. These properties are analyzed by confirmative tests such as Ames test, Food and Drug Administration’s Center for Drug Evaluation and Research (FDA- CDER) properties on Rat and Mouse,Skin irritation, Skin sensitization, Respiratory sensitization, Lethal Concentration, Lethal Dose in human, and Hypoxanthine-guannine phosphoribosyltransferase (HGPRT) test(Yang and Chen, 2004).Negative results of Skin sensitization, Skin irritation, Respiratory sensitization, Ames test and Hypoxanthine-guannine phosphoribosyl transferase (HGPRT) tests denote that the molecules can be taken into consideration for drug designing.
CID6917684 shows positive Respiratory sensitization; hence it requires modifications in the structure. The result of the Ames testproves that the drug is mutagenic(Wadood et al., 2013; Williamson, 2014). CID82741 compound requires structural modifications as it gives positive Ames test. CDER proprietary male rat, FDA cancer male rat, FDA cancer female rat, FDA cancer male mouse, CDER proprietary male mouse, FDA cancer female mouse, CDER proprietary female rat, -CDER proprietary female mouse determine Carcinogenicity(Wadood et al., 2013; Williamson, 2014).No compound indicates positive Carcinogenicity(Table 3).
Lethal dose (LD)is taken into consideration to notify the short term poisoning potential of the material. Lethal concentration (LC)denotes the concentration of the chemical. This toxicity is dependent upon Lethal concentration of the drug. If the range of Lethal concentration is 100 – 1000mg/L that shows moderate toxicity of compound and based on that concentration of Lethal dose is set as 0.5-5 gm/kg. If Lethal concentration range is 10 -100 mg/L then it shows high toxicity of the substance and therefore Lethal dose must be 5-50 mg /kg. If Lethal concentration ranges between 1000 – 10000 mg/L then it shows minute toxicity of the compounds and Lethal dose is set as 5 – 15 gm/ kg(Wadood et al., 2013; Williamson, 2014). Mentioned all thirteen compounds possese Lethal concentration range 100 – 1000 mg/L as a result they are sensibly toxic and their Lethal dose should be kept 0.5 – 5 gm/Kg.
We validate below chemical compounds as potential lead molecules for Measles virus phosphoprotein tetramerization domainafter assessing physical and chemical properties, docking and ADMET analysis of all the screened chemical compounds,. Acetic acid – (CID176), Glycolic acid – (CID757), Glyoxylic acid – (CID760), Glycocyamine – (CID763), Calcium acetate – (CID6116), Sodium edetate – (CID6144), Propyl acetate – (CID7997), TRIETHYLAMINE – (CID8471), Tetramethylammonium acetate – (CID82741), Disodium ethene-1,1-diolate – (CID101751), Sodium acetate – (CID517045), Lithium acetate – (CID3474584), Ametantrone acetate – (CID6917684).
Further we attempted to find out nature of the obtained compounds. These compounds include naturally occurring plant/ animal, metabolites or secondary metabolites, human metabolites, metabolite intermediates and synthetic compounds in order to understand the possible role of nutraceuticals intervention. Here none of the compounds are natural compounds. Acetic acid, glycolic acid, glyoxylic acid are intermediate metabolites, glycocyamine is a human metabolite whereas calcium acetate, sodium edetate, propyl acetate, triethylamine, tetramethylammonium acetate, sodium acetate , lithium acetate, ametantrone acetate disodium ethene-1,1-diolate are synthetic compounds. Glycocyamine plays a role as a human metabolite, involved in metabolic pathways of amino acids ( https://www.drugbank.ca/drugs ; https://pubchem.ncbi.nlm.nih.gov/).
CONCLUSION
Measles virus phosphoprotein tetramerization domain contains ligand named Acetate ion, (acetate, C2H3O2). It was considered as a Drug Target protein. Its protein structure (PDB format) was attained from the Protein Data Bank. Ligand Acetate ion, (acetate, C2H3O2) was considered for investigation. 80 compounds were selected from available online chemical libraries and they were screened and docked with Acetate ion, (acetate, C2H3O2) using PyRx virtual screening software. Upon ADMET analysis using QSAR database, 13 compounds were identified as potential leads. These thirteen chemical compounds can be further characterized and examined in wet lab for the development of new drug for Measles.
ACKNOWLEDGEMENT
This study was supported by Dr. Indu Dayal Meshri College of Science and Technology and the department of Biotechnology, Hemchandracharya North Gujarat University, Patan, Gujarat, India
REFERENCES
Aaby, P., Samb, B., Simondon, F., Seck, A. M. C., Knudsen, K. and Whittle, H. (1995). Non-specific beneficial effect of measles immunisation: Analysis of mortality studies from developing countries. Bmj, 311(7003): 481. https://doi.org/10.1136/bmj.311.7003.481.
Aickin, R., Hill, D. and Kemp, A. (1994). Measles immunisation in children with allergy to egg. Bmj, 309(6949): 223. https://doi.org/10.1136/bmj.309.6949.223.
Bissantz, C., Folkers, G. and Rognan, D. (2000). Protein-Based Virtual Screening of Chemical Databases . 1 . Evaluation of Different Docking / Scoring Combinations. J. Med. Chem., 43: 4759–4767.
Brown, D. W. G., Ramsay, M. E. B., Richards, A. F. and Miller, E. (1994). Salivary diagnosis of measles: A study of notified cases in the United Kingdom, 1991-3. Bmj, 308(6935): 1015. https://doi.org/10.1136/bmj.308.6935.1015.
Communie, G., Maurin, D., Blackledge, M. and Ruigrok, R. W. H. (2013). Structure of the Tetramerization Domain of Measles Virus. Journal of Virology, 87(12): 7166–7169. https://doi.org/10.1128/JVI.00487-13.
Dias, R. and de Azevedo Jr. W. (2008). Molecular Docking Algorithms. Current Drug Targets, 9(12): 1040–1047. https://doi.org/10.2174/138945008786949432.
Fernando, L., Macedo, S., Pauli, I., Caceres, R. A., Filgueira, W. and Jr, D. A. (2008). Drug-Binding Databases: 1092–1099.
Iskar, M., Zeller, G., Zhao, X., Noort, V., Van and Bork, P. (2011). Drug discovery in the age of systems biology : the rise of computational approaches for data integration. Current Opinion in Biotechnology, 23: 1–8. https://doi.org/10.1016/j.copbio.2011.11.010.
Johansson, K., Bourhis, J., Campanacci, V., Cambillau, C., Canard, B. and Longhi, S. (2003). Crystal Structure of the Measles Virus Phosphoprotein Domain Responsible for the Induced Folding of the C-terminal Domain of the Nucleoprotein . The Journal of Biological Chemistry, 278(45): 44567–44573. https://doi.org/10.1074/jbc.M308745200.
Kaye, J. A., Del Mar Melero-Montes, M. and Jick, H. (2001). Mumps, measles, and rubella vaccine and the incidence of autism recorded by general practitioners: A time trend analysis. British Medical Journal, 322(7284): 460–463. https://doi.org/10.1136/ewjm.174.6.387.
Modis, Y., Ogata, S., Clements, D. and Harrison, S. C. (2003). A ligand-binding pocket in the dengue virus envelope glycoprotein. Proceedings of the National Academy of Sciences of the United States of America, 100(12): 6986–6991. https://doi.org/10.1073/pnas.0832193100.
Naim, H. Y. (2015). Measles virus; informations. Human Vaccines and Immunotherapeutics, 11(1):21–26. https://doi.org/10.4161/hv.34298.
Song, C. M., Lim, S. J. and Tong, J. C. (2009). Recent advances in computer-aided drug design. Briefings in Bioinformatics, 10(5): 579–591. https://doi.org/10.1093/bib/bbp023.
Taylor, B., Miller, E., Lingam, R., Andrews, N., Simmons, A. and Stowe, J. (2002). Measles, mumps, and rubella vaccination and bowel problems or developmental regression in children with autism: Population study. BMJ, 324(7334): 393–396. https://doi.org/10.1136/bmj.324.7334.393.
Wadood, A., Ahmed, N., Shah, L., Ahmad, A., Hassan, H. and Shams, S. (2013). In-silico drug design : An approach which revolutionarised the drug discovery process In-silico drug design : An approach which revolutionarised the drug discovery process. OA Drug Design and Delivery, 1(1): 1–4. https://doi.org/10.13172/2054-4057-1-1-1119.
Williamson, E. M. (2014). Synergy : Interactions within Herbal Medicines Synergy and other interactions in phytomedicines. Phytomedicine, 8(5):401–409.
Yang, J. and Chen, C. (2004). GEMDOCK : A Generic Evolutionary Method for Molecular Docking. Proteins: Structure, Function, and Bioinformatics, 55: 288–304. https://doi.org/10.1002/prot.20035.
(https://www.who.int/immunization/diseases/measles/en/, 2019).
(https://www.drugbank.ca/drugs)
(https://pubchem.ncbi.nlm.nih.gov/)