1Department of Chemistry, Kalinga University, Naya Raipur, Chhattisgarh, India.
2Department of Chemistry, JBD Arts and Science College, Katghora, Chhattisgarh, India.
Corresponding suthor email: : krishna28kalingauniversity@gmail.com
Article Publishing History
Received: 18/03/2022
Accepted After Revision: 25/05/2022
In this paper, we have used a removal technique of fluoride from groundwater in Korba district, Chhattisgarh, using thermally activated neem (Azadirachta indica) leaves as adsorbents. For this purpose, we collected the groundwater sample in January–March 2021. The Ion-Selective Electrode (ISE) technique was used to assess the fluoride concentration in groundwater samples. Neem leaves were efficient at removing fluoride in this study. Fluoride has a split personality in the human system, having a damaging impact when fluoride concentration is more than 1.5 mg/L, causing dental and skeletal fluorosis, and a positive effect when concentration is less than 1.0 mg/L, causing caries preclusion, and health promotion. This small project provides the outcomes of a study on neem leaf powder for water defluoridation.
The analysis here discusses the applicability of inexpensive leaf adsorbents for successfully remediating fluoride contaminated water: contact time, pH, and adsorbent concentration all influence fluoride ion sorption effectiveness. The effects of treated leaf powder on pH, adsorbent dose, and contact time with aqueous solutions containing 2.28–10.04 mg/L fluoride ions were investigated. Fluoride adsorption is most substantial at pH 2. Fluoride removal diminishes dramatically when the pH exceeds 2. At adsorbent doses of 10 g/L, the necessary time for fluoride ion adsorption equilibrium is 120 minutes, and the highest removal efficiency attained was 85%, during that amount of adsorbent was 12 g/L. This research also discusses fluoride’s adsorption isotherm and kinetics by activated neem leaf powder.
Adsorption, Azadirachta Indica, Bioadsorbent, Defluoridation, Removal Efficiency.
Kashyap K. K, Ghosh M. K, Shahi S, Diwaker K. Defluoridation of Groundwater with the Help of Azadirachta indica leaves as Bioadsorbent in Korba, Chhattisgarh, India. Biosc.Biotech.Res.Comm. 2022;15(2).
Kashyap K.K, Ghosh M.K, Shahi S, Diwaker K. Defluoridation of Groundwater with the Help of Azadirachta indica leaves as Bioadsorbent in Korba, Chhattisgarh, India. Biosc.Biotech.Res.Comm. 2022;15(2). Available from: <a href=”https://bit.ly/3wOLz7h“>https://bit.ly/3wOLz7h</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Fluoride ions are negatively charged and are found in various minerals and may be in water and soil (Tolkou et al. 2021). Fluoride in ordinary water has developed into a severe anxiety in many parts of the world for humankind, and it should be within 0.6 – 1.5 mg/l (Patil et al. 2015; Prasad et al. 2021). India is one of the countries with many people affected by fluorosis due to drinking groundwater. Dental and skeletal fluorosis, neurotoxicity, endocrine impacts, and attention deficit hyperactivity disorder are among the side consequences of excessive fluoride exposure (Akafu et al. 2019; Kashyap and Ghosh 2021).
Because the difference between the intended and unwanted fluoride dose is so tiny, it’s critical to monitor and consider the quality of drinking water and, when required, eliminate excess fluoride from water to safeguard human health (Premathilaka and Liyanagedera 2019). The National Research Council of the United States evaluated EPA fluoride standards in (2006), They concluded that fluoride has direct and indirect effects on the brain, that excessive fluoride levels in water is also neurotoxic, for which a lot of analysis is required (Philippe Grandjean 2019).
Although groundwater provides protected potable water for billions of people worldwide, there are rare instances where pollution levels are too high, and groundwater cannot be utilized for potable purposes without treatment. Fluoride poisoning of drinking water is a significant environmental hazard that affects much of the world’s populace (Gronwall and Danert 2020; Kashyap and Ghosh 2021).
Fluoride concentrations in the water are so high in nations like India, Ghana, China, Pakistan, Bangladesh, and Tanzania (Yadav et al. 2019; Nde-Tchoupe 2019) that hundreds of thousands of people suffer from fluoride-related diseases. Fluoride concentrations in the water are so high that thousands of individuals are affected by fluoride-related conditions. Effective fluoride exclusion in these areas is a complex and fascinating undertaking due to chemical hurdles preventing fluoride from being successfully sorbed by most traditional adsorbents utilized in the drinking water industry (Rasool et al. 2018; Yadav et al. 2019). In addition, a lack of required infrastructure and technological know-how plays a crucial role. On the other hand, China and India are putting up significant effort to resolve the issue, and the situation is slowly improving (Wang et al. 2019; Kisku and Sahu 2019; Kashyap and Ghosh 2021).
Consequently, fluoride exclusion from drinking water has attracted a lot of interest in recent years, with many methods being explored in labs or used in the field (Yadav et al. 2018; Tolkou et al. 2019). Bio-remediation is the most common technology used to eradicate fluoride from drinking water. Adsorption methods are becoming more popular due to their ease of use and extensive selection of adsorbents. With varying degrees of success, research has been conducted on various types of low-cost adsorbents (Kanaujia et al. 2018; Kumar 2019; Yadav and Bhattacharya 2020).
Plant-based bioremediation to enhance water quality has become a hot topic of research. For defluoridation, several plant materials such as coconut shells, tamarind seeds, neem leaves, and neem bark have been utilized as adsorbents. In the treatment of water, activated carbon is frequently utilized. For the cleaning of polluted water, bioremediation is widely regarded as a cost-effective and environmentally beneficial solution. This research aims to scrutinize the usage of neem tree leaf powder for groundwater Defluoridation (Mihavo et al. 2021).
MATERIAL AND METHODS
The Korba district in Chhattisgarh’s coal-based thermal power center from 22°02″50″ to 23°01″20″ N and from 82°07″20′′ to 83°07″50″ E. The study area in different areas is full of natural possessions (Singha et al. 2019). Coal is the largest resource in the field of research. Talchir, Karaharbhari, Barakar, and Kamthi formations are characteristic of the Gondwana rock supergroup. The construction of the Talchir consists mainly of shale, sandstone, and a boulder bed. The formation of Karaharbhari is made up of subgraywacke, sandstone, and gallstone (Singha and Pasupuleti 2020). The appearance of Barakar includes arcosic sandstone, shale, and carbon seam. Kamthi formation consists of coarse ferruginous carbonated sandstone with extremely fine coal seams, whereas the CGC consists of undifferentiated granite and metamorphic litho units (Singha et al. 2019; Singha and Pasupuleti 2020).
Figure 1: Location of Korba district in Chhattisgarh (www.korbadistmapimage.com)
For the sampling of Groundwater and materials, borewells and hand pumps gathered field samples in various places around the Korba district. At least 1000 ml of each sample was acquired in cleaned plastic cans or bottles and kept at room temperature in the lab for analysis. Six field samples were obtained. For this study, groundwater samples were collected from the highly fluoridated zone of the Korba district. This study was conducted from January to March 2021. This device has the ISE and meter (Thermo Scientific, Orion Star A214), TISAB-III, and a fluoride standard of 100 mg/L. In the fluoride analysis, plastic goods were employed.
For the preparation of adsorbent, the leaves of the neem tree (Azadirachta indica) were exploited as a low-cost natural adsorbent. These were found in Naya Raipur, India, behind the Kalinga University Campus. The neem leaves were dried, crushed, and thoroughly washed with double distilled water. They were dried for 24 hours in a hot air oven at 60–90 °C, following which the dried material was crushed in a jaw crusher and screened at ASTM 50 m mesh. For the alkali treatment, a leaf biomass fine particle sample (40 gm) and 400 ml of 0.5 N NaOH was mixed in a 1000 ml round bottom conical flask. The liquid was progressively heated on the burner for 20-25 minutes after it began to boil. The treated biomass was rinsed with double distilled water until the color was gone entirely, and very clear water was generated.
RESULTS AND DISCUSSION
For this investigation, high fluoridated six groundwater samples were selected from Korba district regions for fluoride analysis of samples used the ISE method. The fluoride content in this region varied from 2.28 to 10.04 mg/L shown table
Table 1. Fluoride Concentration in selected groundwater samples of Korba district in mg/L
|
SN |
Sample spot and Sample code |
Fluoride Concentration in groundwater samples in mg/L |
| 1 | Tanera (S1) | 2.28 |
| 2 | Fulsar (S2) | 4.52 |
| 3 | Aamatikra (S3) | 6.0 |
| 4 | Aamatikra basti (S4) | 7.46 |
| 5 | Pondikala (S5) | 8.18 |
| 6 | Pondikala basti (S6) | 10.04 |
The successful use of the adsorption process necessitates developed low-cost, non-toxic, conveniently accessible, and locally available materials. Bioadsorbents meet these requirements. If the ideal circumstances were known, a better design and modelling process would be ushered in. From a kinetic standpoint, various essential factors, such as pH, contact duration, starting adsorbate concentration, and the adsorbent dose, were studied. Experiments like batch adsorption investigations were carried out (Birhanu et al. 2020). Experiments were conducted by agitating 100 ml of obtained groundwater samples containing F- at a speed of 200 strokes per minute while shaking 10 g/L of adsorbent. To maintain samples containing F- at the proper pH, 0.5 N HNO3 was added. At room temperature (27°C ± 0.5°C), all of the measurements were taken (Birhanu et al. 2020).
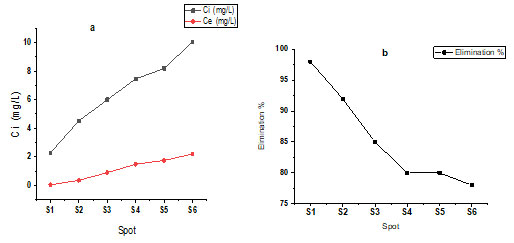
Effect of Initial Adsorbate Concentration: During the optimal time of contact, the rate of a purely adsorptive reaction varies in lockstep with the concentration of adsorbate. As the initial fluoride ion concentration grows, the capacity of the adsorbent materials is rapidly exhausted. The adsorption capability of treated biosorbents was studied methodically by increasing the starting concentration of F- from 2.28 to 10.04 mg/L.
Table 2. The significance of the initial adsorbate concentration, Operating order for the consequence of initial adsorbate concentration, Adsorbent dose – 10 g/L, Temperature was adjusted 27°C ± 0.5°C, Time of contact – 120 Minutes, Volume of sample – 100 ml, and pH 2.
| SN | Ci (mg/L) | Ce (mg/L) | Elimination% of F– |
| 1 | 2.28 | 0.04 | 98 |
| 2 | 4.52 | 0.36 | 92 |
| 3 | 6.0 | 0.90 | 85 |
| 4 | 7.46 | 1.49 | 80 |
| 5 | 8.18 | 1.75 | 80 |
| 6 | 10.04 | 2.20 | 78 |
The percent of elimination of fluoride ions varies through starting concentration at various initial pH levels. When the starting concentration of F– was raised from 2.28 to 10.04 mg/L with a constant sorbent dosage of 10 g/l at a pH of 2, treated bioadsorbents were shown to be reasonably active in decreasing fluoride ions from 98 to 78% shown in table 2 and figure 2 a and b (Philip et al. 2021).
Figure 2 : Effect of initial adsorbate concentration, b. Removal efficiency of F– from groundwater
Adsorption Isotherm model: To fit the investigational adsorption equilibrium data (Yuan et al. 2020; Philip et al. 2021) of fluoride on a bioadsorbent (neem leaves), the isotherm models of Langmuir, Freundlich, and Temkin were applied (Ali and Ismail 2021). These models are mathematically expressed as follows:
Langmuir isotherm:
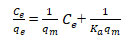
Where Ce is the equilibrium concentration of adsorbate (mg/L), qe denotes the quantity of adsorbed per gram of the adsorbent at equilibrium (mg/g), Ke represents the Langmuir isotherm constant (L/mg) and qm is the highest adsorption capacity (mg/g) of the adsorbent. As shown in Table 3 and figure 3 a.
Freundlich isotherm:
![]()
Where is is the Freundlich isotherm constant mg1-(1/n) Ll/n g-1, n indicates the adsorption intensity, (Priyantha and Kotabewatta 2019; Yuan et al. 2020). As shown in Table 3 and figure 3 b.
Temkin isotherm:
![]()
Where b is Temkin constant which is related to heat of sorption (J mol-1), (Altun et al. 2021) and KT is Temkin isotherm constant (Lg-1). As shown in Table 3 and figure 3 c.
Where is the equilibrium concentration of adsorbate (mg/L), denotes the quantity of adsorbed per gram of the adsorbent at equilibrium (mg/g), represents the Langmuir isotherm constant (L/mg) and is the highest adsorption capacity (mg/g) of the adsorbent. As shown in Table 3 and figure 3 a.
Freundlich isotherm:
Where is is the Freundlich isotherm constant mg1-(1/n) Ll/n g-1, n indicates the adsorption intensity, (Priyantha and Kotabewatta 2019; Yuan et al. 2020). As shown in Table 3 and figure 3 b.
Temkin isotherm:
Where b is Temkin constant which is related to heat of sorption (J mol-1), (Altun et al. 2021) and is Temkin isotherm constant (Lg-1). As shown in Table 3 and figure 3 c.
Figure 3: Langmuir isotherm studies plot not fitted for activated neem (Azadirachta indica) leaves as bioadsorbents for used defluoridation of groundwater. b. Freundlich isotherm studies plot fitted for activated neem (Azadirachta indica) leaves as bioadsorbents for used defluoridation of groundwater. c. Temkin isotherm studies plot not fitted for activated neem (Azadirachta indica) leaves as bioadsorbents for used defluoridation of groundwater (Ali and Ismail 2021).
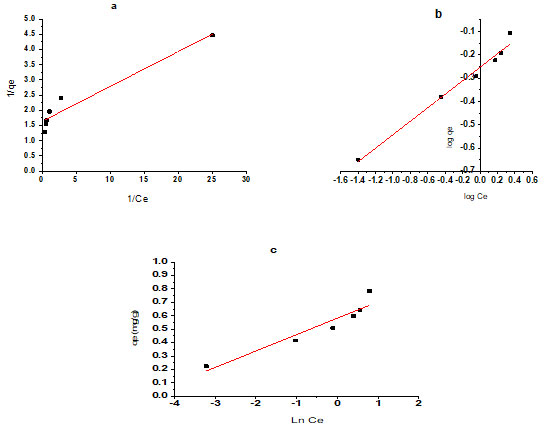
Table 3. In the case of bioadsorbent, a comparison of isotherm parameters from different isotherm models.
| Langmuir isotherm | Freundlich isotherm | Temkin isotherm | |||
| qm (mg/g) | 0.611139
|
Kf (mg1-(1/n) Ll/n g-1) | 0.55856
|
B (J mol-1) | 0.1222
|
| Ka (L/mg) | 14.22985
|
l/n | 0.28973
|
KT (Lg-1) | 117.0022
|
| R2 | 0.9196
|
R2 | 0.97551
|
R2 | 0.87558
|
Effect of contact time: The elimination of F– is shown to increase with increasing contact time duration to some extent. Increased contact time does not improve absorption due to fluoride ions on the accessible adsorption sites on the adsorbent material. According to early studies, fluoride ions appear to absorb quickly on the adsorbent material at their ideal pH values. Within the first hour of interaction with fluoride ions at a concentration of 10.04 mg/L and an adsorbent dose of 10.04 mg/L for treated bioadsorbents, 62% of the adsorption occurs. After this rapid adsorption, a more gradual approach to equilibrium occurs, with saturation happening in 1.5 to 3 hours. This contact time was used as the equilibrium period for further optimization of other parameters (Ali and Ismail 2021).
Table 4. Effect of contact time, Operating order for the consequence of contact time, Adsorbent dose – 10 g/L,
Temperature was adjusted 27°C ± 0.5°C, Ci = 10.04 mg/L, Volume of sample – 100 ml, and pH 2.
| Time
(min) |
Ci (mg/L) | Ce (mg/L) | Elimination % of F– |
| 30 | 10.04 | 4.61 | 54 |
| 60 | 10.04 | 3.81 | 62 |
| 90 | 10.04 | 2.71 | 73 |
| 120 | 10.04 | 2.20 | 78 |
| 150 | 10.04 | 2.20 | 78 |
| 180 | 10.04 | 2.20 | 78 |
Adsorption kinetic studies: Adsorption kinetics is a critical parameter that indicates adsorption efficiency. Figure 4 depicts the fluoride adsorption rate on the surface of adsorbents as a function of time (Siva kumar et al. 2019; Akinyeye et al. 2020). The pseudo-first-order rate (Lagergren 1898), equation is represented by follows:
![]()
Where qt and qe are the total of fluoride adsorbed (mg/g) at contact time t and at equilibrium respectively and k1 is the pseudo-first-order rate constant (min-1). Pseudo-first-order rate constant k1 and the equilibrium adsorption capacity qe were determined from the slope and intercept of the plots of log( qe – qe) against time are shown in figure 4a and table 5 along with the correlation coefficient (R2) (Ali and Ismail 2021).
Table 5. Virtual study of kinetic parameters in case of used bioadsorbent for defluoridation of groundwater.
| Pseudo-first-order | Pseudo-second-order | ||
| qe (mg/g) | 653.43953
|
qe (mg/g) | 0.82540
|
| K1 (min-1) | -0.00142
|
K2 (g/mg/min.) | 0.056069
|
| R2 | 0.73822
|
R2 | 0.98862
|
The rate-determining step is the pseudo-second-order kinetic model, which may be represented as:
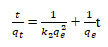
Where k2 denotes the adsorption rate, qt is the total amount of fluoride adsorbed at any given time, and qe denotes the equilibrium adsorption capacity. The slope and intercept of the plot of t/qt against t (Wolowicz and Wawrzkiewicz, 2021) displayed in figure 4 b, as well as the kinetic data in table 5.
Figure 4: Pseudo-first-order kinetic studies for activated neem (Azadirachta indica) leaves as bioadsorbents for used defluoridation of groundwater b. Pseudo-second-order kinetic studies fitted for activated neem (Azadirachta indica) leaves as bioadsorbents for used defluoridation of groundwater.
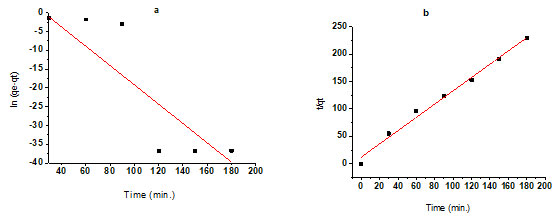
Effect of pH: The adsorption process is influenced by the pH of the aqueous solution (Mia and Jha, 2020). The effect of H+ concentration was studied at pH values 2, 4, 6, and 8. This was attuned by mixing 0.5N HNO3 for 120 minutes with 100 ml of a standard fluoride solution containing 10.04 mg/L fluoride and a dose of 10 g/L treated bioadsorbent. With increasing the pH of the resolution, we noticed a reduction in the amount of fluoride ion removal. At pH 2, this was found to be 78%, while at pH 4, it was found to be 75%. As a result, more research was done at these pH levels. In treated adsorbents, the entitlement of adsorption varies almost linearly between 2.0 and 8.0, achieving a maximum clearance at pH 2.0 after 120 minutes of contact time (Ali and Ismail 2021).
Table 6. Table for effect of pH, Operating order for the consequence of pH, Adsorbent dose – 10 g/L, Temperature
was adjusted 27°C ± 0.5°C, Time of contact – 120 Minutes, and Volume of sample – 100 ml,
| pH
|
Ci (mg/L) | Ce (mg/L)
|
Elimination % of F– |
| 2 | 10.04 | 2.20 | 78 |
| 4 | 10.04 | 2.51 | 75 |
| 6 | 10.04 | 3.31 | 67 |
| 8 | 10.04 | 4.01 | 60 |
Higher hydrogen ion concentrations at lower pH values may have neutralized negative charges on the surface of the treated bioadsorbents in this example. This makes it simpler for negatively charged fluoride ions to diffuse onto the increased active surface of the modified bioadsorbent.
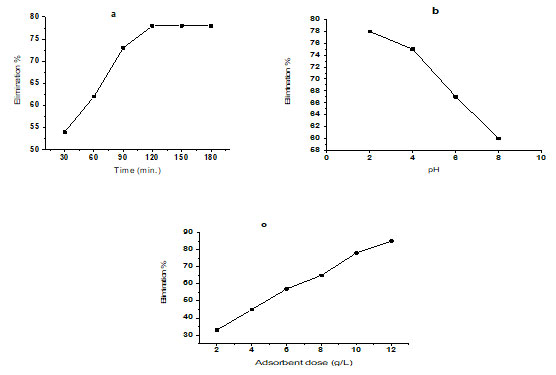
Effect of adsorbent dosage: The elimination of fluoride ions appears to augment when the quantity of adsorbent is increased. The starting fluoride ion concentration in all of these experiments was set at 10.04 mg/L. The adsorbent dosage in an aqueous solution was adjusted between 2 and 12 g/l at their optimum pH levels. Table 7 and figure 5c. demonstrate that treated bioadsorbent was efficient for 33 – 85% removal of fluoride ions, with maximum exclusion of 85 % recorded at 12 g/L, at a room temperature of 27°C ± 0.5°C (Ali and Ismail 2021).
Table 7. Table for effect of adsorbent dosage, Operating order for consequence of adsorbent measure, Effect of contact time, Operating order for the consequence of contact time, Temperature was adjusted 27°C ± 0.5°C, Ci = 10.04 mg/L, Volume of sample – 100 ml, and pH2.
| Adsorbent dose (g/L) | Ci (mg/L) | Ce (mg/L) | Elimination %
of F– |
| 2 | 10.04 | 6.62 | 33 |
| 4 | 10.04 | 5.52 | 45 |
| 6 | 10.04 | 4.31 | 57 |
| 8 | 10.04 | 3.51 | 65 |
|
10 |
10.04 | 2.20 | 78 |
| 12 | 10.04 | 1.50 | 85 |
Figure 5: The effect of contact time relations on F– subtraction, b. The effect of pH on F–
subtraction, c. Effect of adsorbent dosage on the subtraction of F–.
Contact duration, pH, and adsorbent concentration all affect the performance of neem leaf adsorbents in successfully remediating tainted fluoride water (Patel and Gupta, 2020; Pandey et al. 2020).
CONCLUSIONS
The findings of the present study used Azadirachta indica leaves as bioadsorbents to eliminate fluoride from groundwater samples from Korba district of Chhattisgarh. Fluoride in this region varied from 2.28 to 10.04 mg/L. As per the result analysis, we saw that contact duration, pH, and adsorbent concentration influence the effectiveness of low-cost leaf adsorbents in successfully remediating fluoride-polluted water. Maximum fluoride elimination was 78% at pH 2 and up to 85% at 12 g of the adsorbent dose. In the future, this technique of removing fluoride from highly fluoridated water will give positive results and be cheap and available everywhere.
Conflict of Interests: Authors declare no conflict of interests to disclose.
ACKNOWLEDGEMENTS
This study was financially supported, and lab facilities were provided by research grants from HOD in the Department of Chemistry, Kalinga University, New Raipur, India and the Principal of JBD Arts and Science College, Katghora, India. This research was fundamental and would have remained incomplete without the above institutes and their support.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Akafu T, Chimdi A, and Gomoro K (2019). Removal of Fluoride from Drinking Water by Sorption Using Diatomite Modified with Aluminum Hydroxide, Journal of Analytical Methods in Chemistry, Volume 2019, ID 4831926, 11 pages.
Akinyeye OJ, Ibigbami TB, Odeja OQ, et al. (2020). Evaluation of kinetics and equilibrium studies of biosorption potentials of bamboo stem biomass for removal of Lead (II) and Cadmium (II) ions from aqueous solution, African Journal of Pure and Applied Chem., 14(2): 24-41.
Ali ZTA, and Ismail ZZ (2021). Experimental and modeling study of water defluoridation using waste granular brick in a continuous up-flow fixed bed, Environ. Eng. Res., 26(2): 1990506.
Altun, T., Ecevit, H., Kar, Y., et al. (2021). Adsorption of Cr(VI) onto cross-linked chitosan-almond shell biochars: equilibrium, kinetic, and thermodynamic studies, J. Anal. Sci. Techno. 12:38
Birhanu, Y., Leta, S., and Adam, G. (2020). Removal of chromium from synthetic wastewater by adsorption onto Ethiopian low-cost Odaracha adsorbent, Applied Water Science, 10:227.
Chavali, R., Gunda, N. S. K., Naicker, S., et al. (2015). Rapid detection of fluoride in potable water using a novel fluorogenic compound 7-O-tert-butyldiphenylsilyl-4-methylcoumarin, Anal. Chem. Res., 6: 26–31.
Grandjean, P. (2019). Developmental fluoride neurotoxicity: an updated review, Environmental Health, 18: 110.
Gronwall, J., and Danert, K. (2020). Regarding Groundwater and Drinking Water Access through A Human Rights Lens: Self-Supply as A Norm., Water, 12: 419.
Kashyap, K.K., and Ghosh, M.K. (2021). Water Quality Index (WQI) for assessment of groundwater quality around gevra coalfields area, Chhattisgarh, J. App. Chem., 10(2):199-211.
Kanaujia, S., Singh, S., and Singh, B. (2018). Comparative studies of fluoride from groundwater by calcium corbonate, activated alumina and activated Punica grantum ash, Asian J. Chem., 30(4):753-758.
Kisku, G.C., and Sahu, P., (2019). Fluoride Contamination and Health Effects: An Indian Scenario, Environ. Concerns Sustain. Dev., 213–233.
Mihayo, D., Vegi, M. R., and Vuai, S. A. H. (2021). Defluoridation of Aqueous Solution Using Thermally Activated Biosorbents Prepared from Adansonia digitata Fruit Pericarp, Adsorption Science & Technology, Volume 2021, ID 5574900, 16 pages.
Miya, K. S., and Jha, V. K. (2020). Determination of Fluoride in Various Samples Using a Fluoride Selective Electrode, Journal of Analytical Sciences, Methods and Instrumentation, 10: 97-103.
Nde-Tchoupe, A.I., Tepong-Tsinde, R., Lufingo, M., et al. (2019). White Teeth and Healthy Skeletons for All: The Path to Universal Fluoride-Free Drinking Water in Tanzania, Water, 11: 131.
Ouardi, M.E., Qourzal, S., Alahiane, S., et al. (2015). Effective Removal of Nitrates Ions from Aqueous Solution Using New Clay as Potential Low-Cost Adsorbent, Journal of Encap. Adsorp. Sci. 5(4), Article ID:61668,13 pages.
Pandey, P., Khan, F., Ahmad, V., et al. (2020). Combined efficacy Azadirachta indica and Moringa oleifera leaves extract as potential coagulant in ground water treatment, SN Applied Sciences, 2:1300.
Patel, V., and Gupta, Dr. S. (2020). Removal of fluoride from groundwater using low cost natural adsorbents: a review, International Research Journal of Engineering and Technology, 07(03):4363-4368.
Patil, R. N., Nagarnaik, P. B., and Agrawal, D. K. (2015). Removal of fluoride from water by using bio-adsorbents: A state of art, International journal of pure and applied research in Eng. and Tech., 3(9): 272-79.
Philip, K., Jacob, R., and Gopalakrishnan, J. (2021). Characterization of Cassava Root Husk Powder: Equilibrium, Kinetic and Modeling Studies as Bioadsorbent for Copper(II) and Lead(II), J.Enca. Adsorp. Sci., 11: 69-86.
Prasad, K. S. B., Hussain, P J., and Kumar, P. B. (2021). Defluorination of groundwater by low-cost adsorbents. International Conference on Sustainable Systems and Structures (ICSSS 2019), IOP Conf. Series, Materials Science and Engineering, 1025: 012033.
Premathilaka, R. W., and Liyanagedera, N. D. (2019). Fluoride in Drinking Water and Nanotechnological Approaches for Eliminating Excess Fluoride, J. Nanotechnol, 1–15.
Priyantyha, N. and Kottabewatta, P.A. (2019). Biosorption of heavy metal ions on peel of Artocarpus nobilis fruit: 1—Ni(II) sorption under static and dynamic conditions, Applied Water Science, 9, 37.
Rasool, A., Farooqi, A., Xiao, T., et al. (2018). A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation, Environ. Geochem. Health, 40: 1265–1281.
Kumar, P.S., Suganya, S., Srinivas, S., et al. (2019). Treatment of fluoride-contaminated water A review, Env. Chem. Lett., 17: 1707-1726.
Singha, S. S., and Pasupuleti, S. (2020). Hydrogeochemical modeling based approach for evaluation of groundwater suitability for irrigational use in Korba district, Chhattisgarh, Central India, SN Applied Sciences, 2: 1551.
Singha, S. S., Pasupuleti, S., Singha, S., et al. (2019). A GIS‑based modifed DRASTIC approach for geospatial modeling of groundwater vulnerability and pollution risk mapping in Korba district, Central India, Environ. Earth Sci., 78: 628.
Kumar, N.S., Asif, M., Poulose, A.M., et al. (2019). Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass, Processes 2019, 7: 757
Tolkou, A K., Manousi, N., Zachariadis, G. A. et al. (2021). Recently Developed Adsorbing Materials for Fluoride Removal from Water and Fluoride Analytical Determination Techniques: A Review, Sustainability, 13: 7061.
Tolkou, A.K., Mitrakas, M., Katsoyiannis, I.A., et al. (2019). Fluoride removal from water by composite Al/Fe/Si/Mg pre-polymerized coagulants: Characterization and application, Chemosphere 231: 528–537.
Wang, H., He, H., Wang, H., et al. (2019). Trends of fluoride control in China, Environ. Earth Scien., 78: 580.
Wołowicz,A., and Wawrzkiewicz, M., (2021). Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method, Processes, 9(2): 285.
Yadav, A. K., and Bhattacharya, S. (2020) A review of emerging adsorbents for defluoridation of groundwater, J. Water Proc. Eng., 36: 101365.
Yadav, K.K., Gupta, N., Kumar, V., et al. (2018). A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability, Environ. Int, 111: 80–108.
Yadav, K.K., Kumar, S., Pham, Q.B., et al. (2019). Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review, Ecotoxicol. Environ. Saf., 182: 109362.
Yuan, G., Jhao, B., and Chu, K.H. (2020). Adsorption of fluoride by porous adsorbents: Estimating pore diffusion coefficients from batch kinetic data, Environ. Eng. Res. 25(5): 645-651.
Zhu, W., Liu, J., and Li, M. (2014). Fundamental Studies of Novel Zwitterionic Hybrid Membranes: Kinetic Model and Mechanism Insights into Strontium Removal, the Scientific World Journal Vol. 2014, Article ID 485820, 7 pages.


