1Department of Biotechnology, National College, (Autonomous), Tiruchchirappalli, Affiliated to Bharathidasan University, Tamil Nadu, India.
2Department of Biosciences, Integral University Lucknow, Uttar Pradesh India.
3Department of Biotechnology, B.N. College Of Engineering & Technology, Lucknow Uttar Pradesh, India
Corresponding author email: haseeb.biotech@gmai.com
Article Publishing History
Received: 17/01/2020
Accepted After Revision: 29/02/2020
In the current study cytotoxic and antibacterial activities of MDR bacteria S.aureus and E.coli mediated synthesized Silver Nanoparticles were determined. The Antibacterial activities of silver nanoparticles were determined against L. monocytogenes and S. abony. The cytotoxicity of silver nanoparticles was determined against SH-SY5Y cell lines. MTT assay and DAPI staining were used to determine the cytotoxic potential. DCFH-DA was used to determine ability of ROS generation of synthesized silver nanoparticles. The MIC values of MDR S.aureus mediated synthesized against L. monocytogenes and S. abony were found to be 36.22 µM and 35.65 µM respectively. Whereas MIC values of MDR E.coli mediated synthesized AgNPs against L. monocytogenes and S. abony were calculated to be 41.44 µM and 31.62 µM, respectively. Similarly, IC50 values of MDR S.aureus and MDR E.coli mediated synthesized AgNPs against SH-SY5Y cell line was reported to be 148.25 μM & 152.1 μM. DAPI results suggest that synthesized silver nanoparticles were able to case nuclear condensation in the treated cell. Qualitative ROS generation via DCFH-DA assay suggest that MDR bacteria S.aureus & E.coli mediated synthesized Silver Nanoparticles have the capacity to generate significant amount of Reactive Oxygen Species(ROS), the higher amount of ROS generation was observed on a concentration of 300 µM AgNPs concentration.
Antibacterial, Cytotoxic; MDR; SH-SY5Y; ROS.
Haseeb M, Khan M. S, Baker A, Imran M. M, Jaabir M. S. M. Cytotoxic and Antibacterial Activity Evaluation of MDR Bacteria Mediated Synthesized Silver Nanoparticles. Biosc.Biotech.Res.Comm. 2020;13(1).
Haseeb M, Khan M. S, Baker A, Imran M. M, Jaabir M. S. M. Cytotoxic and Antibacterial Activity Evaluation of MDR Bacteria Mediated Synthesized Silver Nanoparticles. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2uaHQ7Q
Copyright © Haseeb et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Increase in microbial and cancer resistance is on a continuous rise and has now become a global problem. The primary cause of this increased resistance is due to rapid misuse of antimicrobial and chemotherapeutic agents (Skladanowski et al., 2016). In order to address this fast rising problem, scientific fraternity is in continuous search of alternative treatment. Researchers are now concentrating more unconventional and alternative approaches such as use of antimicrobial peptides, nanoparticles and quorum quenching nanomaterials (Ma et al., 2018).The rapid emergence of nanotechnology and specifically nanoparticles has led to the discovery and recognition of nanoparticles as a potent antimicrobial and anticancer agent. Silver ions in particular have shown their extraordinary potential as antimicrobial and anti-inflammatory agent, and are being used widely since ancient times. Silver NPs (AgNPs), due to their unique characteristic properties and high therapeutic potential have found great application. The AgNPs are now used in diagnosis and probing of cancer (Alshaye et al., 2017), biomolecular detection (Karim et.al., 2018), catalysis (Burdusel et al., 2018), wound healing (Orlowski et al., 2018). The antimicrobial potential of these silver nanoparticles (AgNPs) against MDR strains of a variety of pathogenic bacteria and cytotoxic potential against cancer cells has been well established (Abalkhil et al., 2017; Huang et al., 2017).
The bactericidal potential of the AgNPs can be attributed to large surface area to volume ratio, a consequence of smaller size, which permits the AgNPs to interact closely with the bacterial membrane (Helmlinger et al., 2016). Several studies propose that silver nanoparticles disturb the function of their target cells by getting attached to the cell membrane surface (Yan et al., 2018). Other studies have suggested that silver nanoparticles could induce cell death in gram negative bacteria E. coli, by causing the formation of small ‘‘pores’’ in the bacterial cell wall, which increases the permeability and ultimately inactivates the respiratory chain and electron transport system.
In the present work the cytotoxic and antibacterial potential of previously synthesized silver nanoparticles from MDR S.aureus and E.coli was determined (Mohd Haseeb et al., 2019).
Through this study we have aimed to address the challenges associated with cancerous cells and bacterial infections. The synthesized silver nanoparticles were characterized by various techniques such as UV-Vis Spectroscopy, Zeta potential, Dynamic Light Scattering (DLS), Transmission Electron Microscopy (TEM), and Fourier Transformed Infra Red (FTIR) Spectroscopy (Mohd Haseeb et al; 2019). The synthesized silver nanoparticles were subsequently tested for their antibacterial activity against L. monocytogenes and S. abony. The cytotoxic activity and the ability of these silver nanoparticles to generate ROS was determined against SH-SY5Y
MATERIAL AND METHODS
All the Media and chemicals used in the experiment were purchased from Sigma Aldrich (St. Louis, USA) and HiMedia, India.
Antibacterial Activity:The antibacterial potential of MDR S.aureus and E.coli synthesized Silver nanoparticles was determined against .monocytogenes and S. abony. These bacteria (OD600-0.60) were allowed to grow in Nutrient broth (NB) for 24 hours then incubated for 24 hrs at 37ᵒC kept at 180 rpm in a rotary shaker incubator. 100 ml of cultured broth was used to prepare bacterial lawns. The antibacterial potential of MDR S. aureus and E.coli mediated synthesized silver nanoparticles was determined by method discussed elsewhere (Mohd. Haseeb et al., 2019). 25 milliliters of sterile Mueller Hinton Agar (MHA) medium was poured in sterilized petriplates to prepare culture plates. Sterilized cotton swab was used to swab the bacterial strains from culture plates. Three wells of 5 mm diameter each were made in every plate by using Sterilized gel puncture. One of the well from each plate was filled with sterilized dH2O (for control) and the other two were added with MDR S.aureus and MDR E.coli mediated synthesized AgNPs. The inoculated plates were incubated 24 hours at 37ᵒC temperature. Clear zone of inhibition around the wells were examined in order to determine the antibacterial potential of as synthesized AgNPs.
Minimum Inhibitory Concentration (Mic) of Agnps: MIC50 is the amount of drug needed to inhibit the bacterial growth to 50%. A flat-bottom, 96 well plates was employed to determine the MIC50 by broth dilution method. This enabled the inoculum to be prepared in distilled water allowing the determination of absorbance by UV- spectrophotometer and thereby eliminating the need of interference from colored media. Double diffusion method, was used to determine the MICs of the E.coli synthesized silver nanoparticles. The bacterial strains were grown till mid-log phase, culture harvesting was achieved by centrifugation, 1mM sodium Phosphate buffer (SPB) was used to wash the harvest at pH 7.4, and finally diluted to 2 × 105 CFU/ml in same buffer. AgNPs were serially diluted in desired concentrations by taking 90 microtitre (µL) of Mueller-Hinton broth in 96 well- microtitre plates. Bacterial suspension of 95×104 CFU/ well was taken as inoculum. After inoculation, wells were incubated for overnight at 37o C. The lowest concentration of silver nanoparticles inhibiting the bacterial growth was used to determine MIC50 of biosynthesized AgNPs.
Evaluation of Cytotoxic Potential Against Sh-Sy5y Cell Line: The synthesized silver nanoparticles were analyzed for their anticancer activities against SH–SY5Y Cell line. MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay was used to determine the Cytotoxic activity of synthesized AgNPs whereas DAPI (4’, 6-diamino-2-phenylindiole) toxicology assays was used to assess the nuclear degradation. In order to assess the same, the cells were treated with varying doses of silver nanoparticles. SH-SY5Y cells were grown in 96 well plates with each well having 1 X 104 cells, followed by 24 hours incubation in CO2 incubator. After a successful incubation period, silver nanoparticles of varying concentrations were used to treat the cells. The experiment was repeated in triplicate with untreated cells as the positive control and incubated for 48 hours. Spent medium was removed, followed by loading of wells with 50µl of MTT dye (5mg/ml in PBS) and freshly prepared culture media. Then the plate was incubated for 4 hours. Formazon crystals formed during the process were dissolved by adding 100µl of DMSO (Dimethyl Sulfoxide) and incubated for one hour. The reduced MTT was quantified by measuring optical density at 570 nm in ELISA reader
The Percentage inhibition of the cells was calculated using the formula
X = 100 − (Atest – Ablank) / ( Acontrol – Ablank) × 100
Where,
X- Percentage inhibition of cells Atest – absorbance of the test sample at 570 nm.
Ablank – absorbance of blank at 570 nm Acontrol – absorbance of the control sample at 570 nm The IC50 value was calculated by the obtained data.
Nuclear condensation in silver nanoparticles treated SH-SY5Y cells was determined by using nuclear fluorescent stain 4’, 6-diamino-2-phenylindiole (DAPI) dye. SH-SY5Y Cells were seeded in 96 well plates, treated with varying concentration silver nanoparticles were maintained in CO2 humidification incubator at 37 °C for 4 hrs. It was followed by careful removal of medium at appropriate times and washing the cells twice with saline phosphate buffer (SPB). The treated cells were washed again, stained with DAPI for 20 minutes at 37 °C, along with a permeabilizing buffer that allows the fixing of the stain into treated cells. Methanol and phosphate buffer saline was used to wash the stain respectively. Fluorescence microscope was used to visualize and capture the images of the stained cells and cells with fragmented and condensed nuclei were considered to be the dead cells.
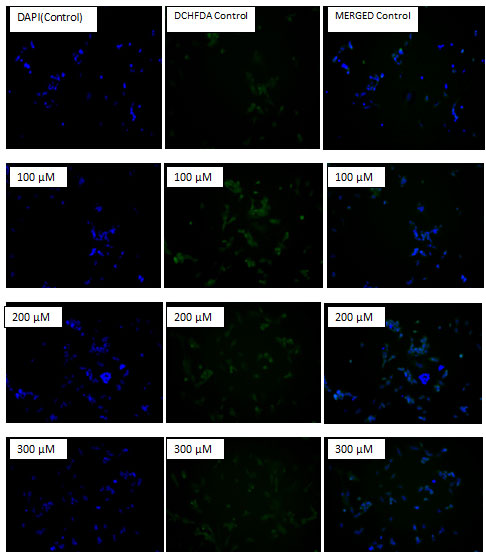
Assessment of Intracellular Ros (Reactive Oxygen Species) Generation:The intracellular reactive oxygen species (ROS) level was measured by DCFH-DA method as per standard protocol. It is based on ROS-induced formation of the highly fluorescent dye 2’7’- dicholorofluorescin diacetate (DCFH-DA). Shortly, Human neuroblastoma cell lines SH-SY5Y were seeded in a 12 well plate at a density of 5 x 104 per well and incubated for 24 hr at 37 ᵒC. After treatment with AgNPs (0, 50, 100, 200, and 300 µM) for 12 hrs, the cells were incubated with 10 µM DCFH-DA for 30 minutes at 37 ᵒC. Cells were washed to remove the excessive amount of DCFH-DA. Images were captured by using an inverted fluorescence microscope) Nikon ECLIPDE Ti-S, Japan).
RESULTS AND DISCUSSION
The antimicrobial potential of silver nanoparticles from MDR S.aureus and E.coli was determined against L. monocytogenes and Salmonella abony. The antibacterial potential of as synthesized silver nanoparticles were confirmed on the basis of the clear zone of inhibition observed around the area inoculated with NPs. Different concentration of silver NPs synthesized MDR S.aureus and MDR E.coli was used to calculate and determine the minimum inhibitory concentrations (MIC).
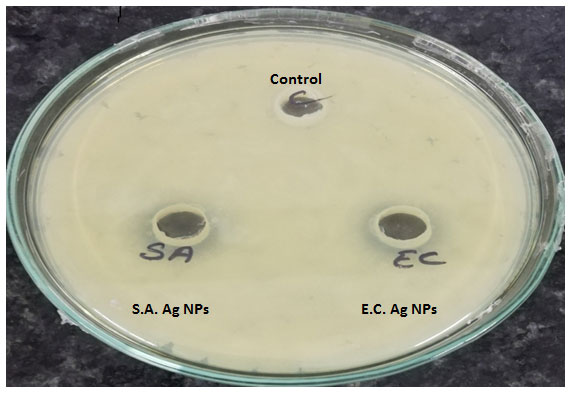
Figure 1: Plates showing the zone of inhibition of MDR S.aureus and E.coli mediated synthesized Silver NPs against- L. monocytogenes
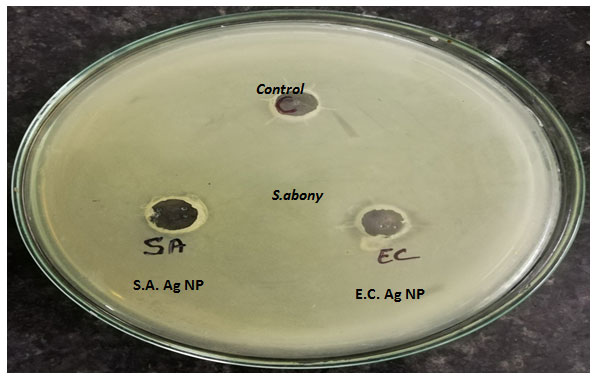
Figure 2:Plates showing the zone of inhibition of MDR S.aureus and E.coli mediated synthesized Silver NPs against- S.abony
The zone of inhibition of MDR S.aureus silver nanoparticles on L. monocytogenes and Salmonella abony was found to be 18 mm and 19 mm respectively. The zone of inhibition of MDR E.coli synthesized silver nanoparticles on L. monocytogenes and Salmonella abony was found to be 17 mm and 18 mm respectively. The MIC values of synthesized silver nanoparticles against treated bacteria are given in table 1.
The exact mechanism of action of Ag+ ions on microorganisms is not fully known. It is believed that the DNA of Ag+ treated microbial cells loses the ability of replication and protein inactivation occurs (Divya et.al. 2016). It has also been shown that silver ions cause protein denaturation by binding to functional groups on the protein. In a study involving E.coli and Metal oxide nanoparticles, treated bacterial cells were observed to have a significant increase in membrane permeability, making cells incapable of regulating the proper transport across the cell membrane and ultimately leading to cell death (Gomaa, 2017).
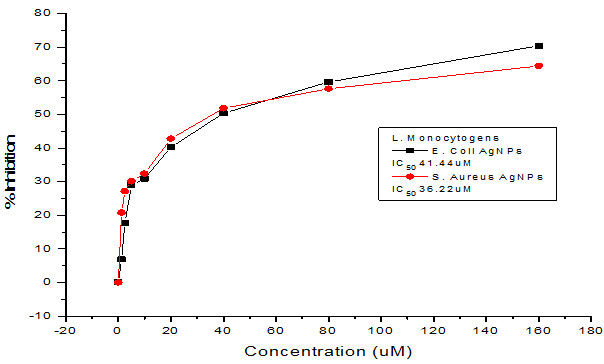
Figure 3: Curve showing the MIC of silver NPs against a) Normal strain of E.coli. b) MDR E.coli
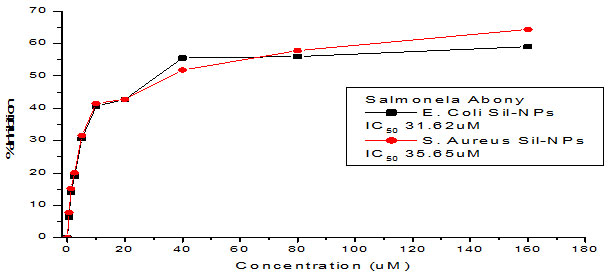
Figure 4: Curve showing the MIC of silver NPs against a) Normal strain of E.coli. b) MDR E.coli
Table 1: Representing MIC values of synthesized silver nanoparticles
| Name of sample | MIC (µM) | |
| L. monocytogenes | S. abony | |
| MDR S.aureus AgNPs | 36.22 µM | 35.65 µM |
| MDR E.coli AgNPs | 41.44 µM | 31.62 µM |
It has earlier been reported that antimicrobial potential is dependent upon dose and size, which makes gram-negative bacteria an obvious target as compared with gram positives bacteria AgNPs may interact directly with microbial cells, may inhibit the enzymes of respiratory chain and affect the permeability of protons and phosphates for example; by halting the transmembrane transfer of electrons, disrupting the cell envelope thereby making it susceptible to the ROS (Reactive Oxygen Species) (Rajeshkumar and Malarkodi, 2014). Additionally, Silver nanoparticles can cause damage to bacterial cells by permeating the cell and interacting with proteins, DNA and some other cell constituents containing sulfur and phosphorus (Nayak and Chitra, 2015). In a similar research experiment where E.coli and K. pneumonia were treated with AgNPs the highest antibacterial effect was recorded on E.coli with a zone of inhibition of 13 mm and the lowest was examined on K. pneumonia with a zone of inhibition of 7mm (Feng et.al., 2000).
Cytotoxicity Of Mdr E.Coli Synthesized Silver Nanoparticles: The synthesized silver nanoparticles forms MDR E.coli were assessed for their cytotoxic potential on HCT SH-SY5Y neuroblastoma cancer cell line. The cell viability or cytotoxic activity was assessed via MTT assay and nuclear fragmentation was studied via DAPI staining.
The vey aim of cell viability assays is to determine the cellular response of any toxic material that has the potential to influence the metabolic activity of the target cells.
MTT analysis is used to analyse the mitochondrial succinate dehydrogenase activity as metabolically active viable cells have the capacity to convert the MTT to purple color formazon crystal which gives maximum absorbance near 570 nm. The cellular mechanism behind this reduction of MTT probably involves NADH or a similar reducing agent from which electrons are transferred to tetrazolium, which is then reduced to formazon. The dead cells lose the ability to reduce the MTT into formazon, so the formation of colored product decreases with decrease in cell viability hence the intensity of colored product which is directly proportional to number of viable cells in culture, is measured to determine the level of apoptosis (Präbst et al., 2017).
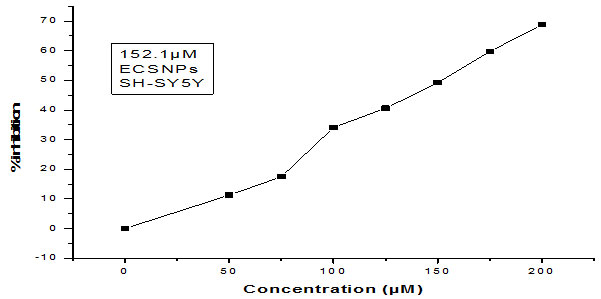
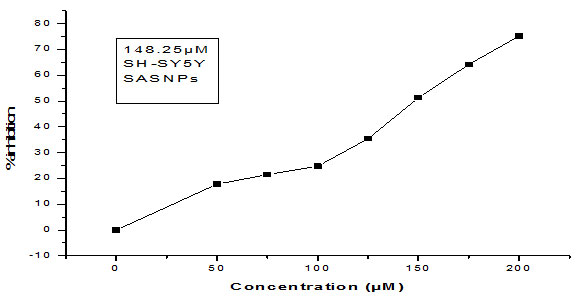
Here in our study, the cytotoxicity of silver nanoparticles in human neuroblastoma cancer cells SH-SY5Y was determined by treating the cells with varying dose of ( 10 µM to 300 µM) for 24 hr and 48 hr respectively, followed by MTT reduction assay. After a successful incubation period of 24 hr, 50% reduction in cell viability was noticed. The IC50 values were found to be 148.25 μM & 152.1 μM for MDR S.aureus and MDR E.coli mediated synthesized Silver nanoparticles. Almost same patterns of results with strong cytotoxic effects were observed after 48 hrs of treatment period, proving silver nanoparticles to be more cytotoxic and of anti-proliferative nature. Earlier studies have predicted similar findings in which the cytotoxic effects of silver nanoparticles have been reported (Singh et al., 2018). The cytotoxic efficacy of silver nanoparticles in affecting the survival of various cell types by disturbing the mitochondrial activity, structure and metabolism have been also predicted by several types of research (Suliman et al., 2015). In several previous studies, smaller sized Ag-nanoparticles have been shown to have more toxicity than the NPs with a larger size (Gurunathan et al., 2015). It has also been demonstrated that the type of capping agent dictates the potency of silver nanoparticles (Patra et.al., 2018).
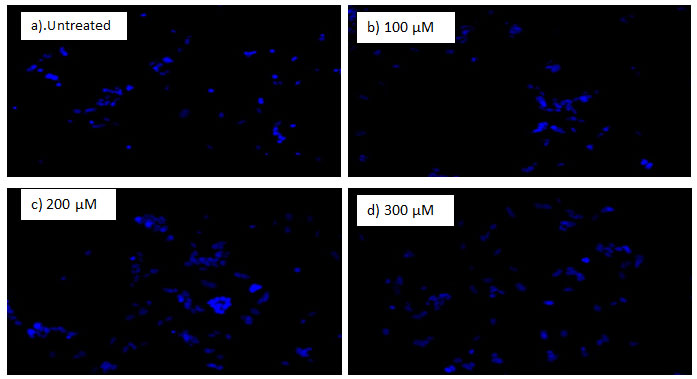
In order to determine the apoptotic cell death induced by as synthesized silver NPs, DAPI nuclear staining method was used. The apoptotic cells are characterized by cell shrinkage extensive blabbing in the plasma membrane, and condensation of chromatin and formation of apoptotic bodies from separated cellular fragments (Sheikh et al., 2018)
Figure 5: Showing the IC50 of MDR E.coli mediated synthesized AgNPs against human neuroblastoma cell lines SH-SY5Y.
Figure 6: Graph showing the IC50 of S.aureus mediated synthesized AgNPs against Human neuroblastoma cell lines SH-SY5Y.
Figure 7: DAPI Images of SH-SY5Y (taken at a magnification of 40x): a) Control cells, on left and b), c) & d) Cells treated with S.aureus mediated synthesized AgNPs, on right.
Figure 8: DAPI Images of SH-SY5Y (taken at a magnification of 40x): a) Control cells, on left and b), c) & d) Cells treated with E.coli mediated synthesized AgNPs, on right.
The cells treated with AgNPs, were stained with DAPI, a DNA staining dye. DAPI is a fluorescent staining dye which binds preferentially to the AT-rich regions of double stranded DNA. When DAPI binds DNA its fluorescence intensity increases around 20 folds as compared with unbound DAPI (Baharara et.al., 2018). The intensity of stain is less in the viable cells as the membrane permeability is intact, DAPI fails to pass through but when cell permeability is compromised due to apoptosis; DAPI enters inside the cell with ease and a strong blue fluorescence is produced as it binds to DNA (Baharara et.al., 2018).
After treatment with AgNPs for 24 h, significant nuclear changes in SH-SY5Y neuroblastoma cancer cells were observed (Fig. 7 & 8). As apparent from pictures of DAPI staining, AgNPs induced nuclear condensation and fragmentation in treated cancer cells in a dose dependent manner whereas the control cells exhibited normal cell morphology (Fig. 7 & 8). The apoptotic effect of silver nanoparticles can be attributed to excessive ROS generation which leads to DNA and protein damage (Foldbjerg et al., 2009; Gurunathan et al., 2018). The findings of our current research are in agreement with previously published reports that exposure of cells to NPs induces apoptotic cell death ( Kishore et al., 2018; Mohammed et al., 2018). The results were evident that AgNPs induced apoptosis in neuroblastoma cells in a time dependent and dose-dependent manner.
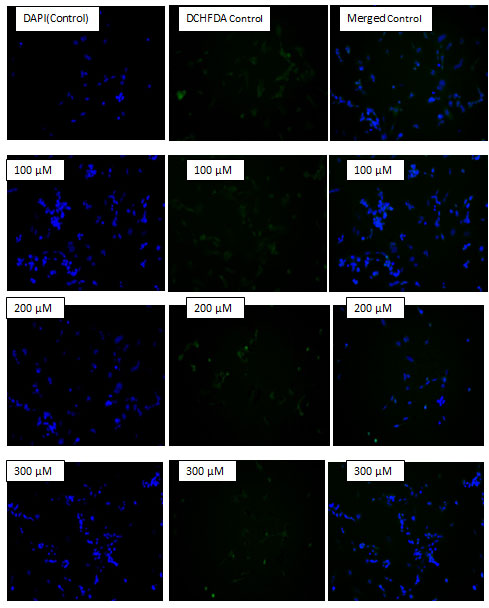
The ability to generate ROS by AgNPs from MDR strains of E.coli and S.aureus was analyzed in human neuroblastoma cell lines SH-SY5Y. It was measured by the H2DCF-DA assay. After 24 h of exposure, increasing concentrations of AgNPs significantly increased ROS levels at various concentrations used in this study. In general, nanoparticles are known to kill cancer cells by producing ROS. Ag ions play an important role in catalyzing ROS production in the presence of oxygen species and AgNPs can induce oxidative stress in a variety of cellular systems by generating ROS, including in human cancer cells. To examine the effect of MDR S. aureus and E.coli mediated synthesized AgNPs on ROS generation, human neuroblastoma cell lines SH-SY5Y were treated with various concentrations (0, 50, 100, 200, and 300 µM) of AgNPs for 24 h, and then, ROS generation was measured in an H2DCF-DA assay. After 24 h of exposure, increasing concentrations of AgNPs significantly increased ROS levels at various concentrations used in this study. ROS levels generated in response to AgNPs treatment were significantly higher than those in untreated cells.
Figure 9: Effect of MDR E.coli mediated synthesized AgNPs on reactive oxygen species (ROS) generation. SH-SY5Y cells were treated with or without AgNPs for 24 h, and ROS generation was measured using DCFH-DA.
Figure 10: Effect of MDR S.aureus mediated synthesized AgNPs on reactive oxygen species (ROS) generation. SH-SY5Y cells were treated with or without AgNPs for 24 h, and ROS generation was measured using DCFH-DA.At all tested concentrations, the most pronounced effects were observed at higher concentrations, showing up to 2–3-fold higher ROS levels (Fig- 9 & 10).
Marked differences were evident in the production of ROS between treated and untreated control cells. Treated cells emitted bright fluorescence and displayed deformed morphologies because of the loss of integrity of plasma membrane due to ROS generation. The cytotoxicity effects may have been due to the induction of oxidative stress and apoptosis upon ROS overproduction.
Cancer cells generate high levels of ROS that leads to a state of increased basal oxidative stress. Since this state of oxidative stress makes cancer cells vulnerable to agents that further augment ROS levels, the use of pro-oxidant agents is emerging as an exciting strategy to selectively target tumor cells (Cordero et al., 2012). However, the induction of ROS formation plays an important role in the chemotherapeutic activity of several anticancer drugs and a large number of anticancer compounds (Fruehauf et al., 2007; Trachootham et al., 2009). Our data for the qualitative analysis of ROS generation suggested that AgNPs induced ROS-mediated induction of apoptosis in SH-SY5Y cells in a concentration- dependent manner.
CONCLUSION
In the present work, the cytotoxic and antibacterial activities of MDR S.aureus and E.coli mediated synthesized silver nanoparticles (AgNPs) were determined. The MIC values of MDR S.aureus mediated synthesized silver nanoparticles against L. monocytogenes and S. abony were found to be 36.22 µM and 35.65 µM respectively. Whereas MIC values of MDR E.coli mediated synthesized AgNPs against L. monocytogenes and S. abony were calculated to be 41.44 µM and 31.62 µM, respectively. Similarly, IC50 values of MDR S.aureus and MDR E.coli mediated synthesized AgNPs against SH-SY5Y cell line was reported to 148.25 μM & 152.1 μM. DCFH-DA results show that silver nanoparticles are capable of generating large amount of ROS in their target cells; the ROS generation was recorded to be increasing on the increase of silver nanoparticle concentration. It was for the first time that MDR bacteria mediated synthesized were tested for their cytotoxicity against SH-SY5Y cell lines. Further research on various other cell lines is inevitable to establish these nanoparticles as a potent anticancer agent, also, being of MDR bacteria origin; these nanoparticles must also be assessed for any kind of associated antigenic challenge in animal models in order to determine the safety of these synthesized Silver nanoparticles.
ACKNOWLEDGMENT
The authors acknowledge the support of Faculty and staff of Department of Biotechnology National College (Autonomous), Tiruchirapalli, Tamilnadu, India, for providing all the necessary facilities to carry out the current research work.
REFERENCES
Abalkhil, Tarad Abdulaziz, Sulaiman Ali Alharbi, Saleh Hussein Salmen, and Milton Wainwright: (2017). Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotecnology & Biotechnological Equipment 31, no. 2: 411-417.
Alshaye, Najla A., Mudawi M. Elobeid, Dalal HM Alkhalifah, and Afrah E: (2017). Mohammed. Characterization of Biogenic Silver Nanoparticles by Salvadora persica leaves extract and Its Application Against Some MDR Pathogens E. coli and S. aureus. Res. J. Microbiol 12: 74-81.
Baharara, Javad, Tayebe Ramezani, Nasrein Hosseini, and Marzieh Mousavi: (2018). Silver Nanoparticles Synthesized Coating with Zataria multiflora Leaves Extract Induced Apoptosis in HeLa Cells Through p53 Activation.” Iranian journal of pharmaceutical research: IJPR 17, no. 2: 627.
Burdușel, Alexandra-Cristina, Oana Gherasim, Alexandru Mihai Grumezescu, Laurențiu Mogoantă, Anton Ficai, and Ecaterina Andronescu: (2018). Biomedical applications of silver nanoparticles: An up-to-date overview.” Nanomaterials 8, no. 9: 681.
Divya, Koilparambil, Liya C. Kurian, Smitha Vijayan, and Jisha Manakulam Shaikmoideen: (2016). Green synthesis of silver nanoparticles by Escherichia coli: Analysis of antibacterial activity. Journal of Water and Environmental Nanotechnology1, no. 1: 63-74.
Feng, Qing Ling, Jian Wu, G. Q. Chen, F. Z. Cui, T. N. Kim, and J. O. Kim: (2000). A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of biomedical materials research 52, no. 4: 662-668.
Foldbjerg, Rasmus, Ping Olesen, Mads Hougaard, Duy Anh Dang, Hans Jürgen Hoffmann, and Herman Autrup: (2009). PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicology letters 190, no. 2: 156-162.
Fruehauf, John P., and Frank L. Meyskens: (2007). Reactive oxygen species: a breath of life or death?. Clinical Cancer Research 13, no. 3: 789-794.
Gomaa, Eman Zakaria: (2017). Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. The Journal of general and applied microbiology 63, no. 1: 36-43.
Gurunathan, Sangiliyandi, Jae-Kyo Jing, Jae Woong Han, Xi-Feng Zhang, Jung Hyun Park, and Jin-Hoi Kim: (2015). Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells.” Nanoscale research letters 10, no. 1: 35.
Gurunathan, Sangiliyandi, Muhammad Qasim, Chanhyeok Park, Hyunjin Yoo, Jin-Hoi Kim, and Kwonho Hong: (2018). Cytotoxic Potential and Molecular Pathway Analysis of Silver Nanoparticles in Human Colon Cancer Cells HCT116. International journal of molecular sciences 19, no. 8: 2269.
Haseeb, Mohd, Mohd Sajid Khan, Abu Baker, Imran Khan, Iram Wahid, and MS Mohamed Jaabir. “Anticancer and antibacterial potential of MDR Staphylococcus aureus mediated synthesized silver nanoparticles.” An International Peer Reviewed Open Access Journal For Rapid Publication: 26.
Helmlinger, J., C. Sengstock, C. Groß-Heitfeld, C. Mayer, T. A. Schildhauer, M. Köller, and M. Epple: (2016). Silver nanoparticles with different size and shape: equal cytotoxicity, but different antibacterial effects. RSC advances 6, no. 22: 18490-18501.
Huang, Yu, Chao-Qiang Fan, Hui Dong, Su-Min Wang, Xiao-Chao Yang, and Shi-Ming Yang: (2017). Current applications and future prospects of nanomaterials in tumor therapy. International journal of nanomedicine 12: 1815.
Karim, Md N., Samuel R. Anderson, Sanjay Singh, Rajesh Ramanathan, and Vipul Bansal: (2018). Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosensors and Bioelectronics 110: 8-15.
Kishore, Medikondu, Abeer Talib Abdulqader, Hanan Shihab Ahmad, and Y. Hanumantharao: (2018). Anticancer and antibacterial potential of green silver nanoparticles synthesized from Maytenus senegalensis (L.) leaf extract and their characterization. Drug Invention Today 10, no. 4.
Ma, Zhi-Ping, Yu Song, Zhong-Hua Cai, Zhi-Jun Lin, Guang-Hui Lin, Yan Wang, and Jin Zhou: (2018). Anti-quorum sensing activities of selected coral symbiotic bacterial extracts from the South China Sea.” Frontiers in cellular and infection microbiology 8: 144.
Martin-Cordero, Carmen, Antonio Jose Leon-Gonzalez, Jose Manuel Calderon-Montano, Estefania Burgos-Moron, and Miguel Lopez-Lazaro: (2012). Pro-oxidant natural products as anticancer agents. Current drug targets 13, no. 8: 1006-1028.
Mohammed, Afrah, Alaa Al-Qahtani, Amal Al-Mutairi, Bashayir Al-Shamri, and Kawther Aabed: (2018) Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 8, no. 6: 382.
Nayak, B. K., and N. Chitra. Anima Nanda: (2015). Comparative antibiogram analysis of AgNPs synthesized from two Alternaria spp. with amoxicillin antibiotics. J. Chem. Pharm. Res 7: 727-731.
Orlowski, P., Zmigrodzka, M., Tomaszewska, E., Ranoszek-Soliwoda, K., Czupryn, M., Antos-Bielska, M., Szemraj, J., Celichowski, G., Grobelny, J. and Krzyzowska, M.: 2018. Tannic acid-modified silver nanoparticles for wound healing: the importance of size. International journal of nanomedicine, 13, p.991.
Orlowski, P., Zmigrodzka, M., Tomaszewska, E., Ranoszek-Soliwoda, K., Czupryn, M., Antos-Bielska, M., Szemraj, J., Celichowski, G., Grobelny, J. and Krzyzowska, M: 2018. Tannic acid-modified silver nanoparticles for wound healing: the importance of size. International journal of nanomedicine, 13, p.991.
Präbst, K., Engelhardt, H., Ringgeler, S. and Hübner, H., (2017). Basic colorimetric proliferation assays: MTT, WST, and resazurin. Cell Viability Assays: Methods and Protocols, pp.1-17.
Rajeshkumar, S., and C. Malarkodi: (2014). In vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioinorganic chemistry and applications 2014.
Sheikh, E., Bhatt, M.B. and Tripathi, M., 2018. Bio-based synthesised and characterized monodispersed Curcuma longa silver nanoparticles induces targeted anticancer activity in breast cancer cells. Pharmacognosy Magazine, 14(57), p.340.
Singh, H., Du, J., Singh, P. and Yi, T.H., (2018). Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artificial cells, nanomedicine, and biotechnology, 46(6), pp.1163-1170.
Składanowski, M., M. Wypij, D. Laskowski, P. Golińska, H. Dahm, and M. Rai: (2017). Silver and gold nanoparticles synthesized from Streptomyces sp. isolated from acid forest soil with special reference to its antibacterial activity against pathogens.” Journal of Cluster Science 28, no. 1: 59-79.
Suliman Y, Al Omar, Daoud Ali, Saud Alarifi, Abdul Halim Harrath, Lamjed Mansour, and Saleh Hamad Alwasel: (2015). Evaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic effect of silver nanoparticles in human lung epithelial cells. Environmental toxicology 30, no. 2: 149-160
Trachootham, Dunyaporn, Jerome Alexandre, and Peng Huang. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach Cell Tech 4 21 32-38
Yan, Xueting, Bin He, Lihong Liu, Guangbo Qu, Jianbo Shi, Ligang Hu, and Guibin Jiang: (2018). Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: proteomics approach. Metallomics 10, no. 4: 557-564.