1Department of Biotechnology, Reva University, Bengaluru, India
2Department of Mechanical Engineering, Indian Institute of Science, Bengaluru, India
3 College of Sericulture, UAS. Chinthamani, India
Corresponding author email: shilpa171182@gmail.com
Article Publishing History
Received: 17/10/2019
Accepted After Revision: 18/12/2019
In the last few years, bioengineered tumor models for breast cancer has revitalized academia and industries with better tools to assess dynamic features such as cancer progression, invasion and metastasis. Previously, breast cancer biology has been studied predominantly by means of two-dimensional (2D) cell cultures, which were deficient in mimicking the tumor microenvironment (TME). To alleviate such limitations, three-dimensional (3D) tumor models have been proposed, which are cost-effective and reliably reproduce the complexity of the breast-cancer TME. We have developed two types of silk scaffolds, HFIP-based sponges and lyophilized, to mimic the TME. Silk fibroin, for fabricating the scaffold, was extracted from Bombyx since they are known to be biocompatible, tough, elastic, and biodegradable. We show that MDAMB-231 cells cultured in these scaffolds have altered proliferation, stemness, hypoxia and propensity to transition from an epithelial to a mesenchymal phenotype. This 3D breast cancer model can be a cost-effective alternative and could be used to study the molecular mechanisms and impact of drugs.
Silk Fibroin Scaffold, 3D Cell culture, Tumor Microenvironment Analysis, Breast Cancer Cell Models.
Raju S. R, Gowda K.S. M, Jampani A. Comparison of MDAMB-231 Cells Cultured Under Different Conditions on 2D and 3D Silk Scaffolds. Biosc.Biotech.Res.Comm. 2019;12(4).
Raju S. R, Gowda K.S. M, Jampani A. Comparison of MDAMB-231 Cells Cultured Under Different Conditions on 2D and 3D Silk Scaffolds. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2sRpLKX
INTRODUCTION
The exponential rise in cancer and allied issues globally has revitalized academia and industries to explore and achieve efficient tools and methodologies for early and effective diagnosis. Amongst the major efforts, in-vitro tumor models as been appreciated as a potential tool for understanding cancer biology. It contributes towards the screening of efficient anti-cancer agents Chang (2008). In-depth exploration shows that majority of the in-vitro researches use monolayer culture (2D) for cancer cell development. Such conventional paradigms are also known as Cancer Cell Monolayer Cultures (CCMC). However, such approaches exhibited significantly high limitations due to lack of tumor-specific microenvironments Chang (2008), Vinci (2012), Erler (2009), Polonia-Alcala (2018). To overcome the drawbacks of 2D scientific community explores in vitro models to mimic the microenvironment augment their efficacy in preclinical trials. To achieve such objectives, the use of Three-Dimensional (3D) structure for optimal spatial growth of cells would be of paramount significance . The key differences in cell phenotypes and molecular signature when cells are cultured on 2D and 3D structures has led to the proliferation of 3D cell-based platforms in biosciences. 3D cell culture platforms typically vary as per material’s characteristics, processing mechanisms, and their structures. For example, the key 3D cell culture platforms are reconstituted ECM, synthetic hydrogels, porous polymer scaffolds, and nano-topography, (Belli (2018) and Kolenda (2018).
Previous and recent studies, Ravi (2015), Lee (2007), Edmondson (2014), Wang (2018), Bai (2019) have shown that the numerous 3D cell mechanisms, including spheroid, hydrogel or scaffold-based cultures, can enable environmental cues in the same way as observed in physiological or pathological tissue. Though, 3D cell characteristics can be easily reproduced across the different solutions of the 3D cell-based platforms, it is inevitable to define 3D cell-based platforms as complementary or integrated tool to perform cancer microenvironment analysis. Varied illustrations of the accessible 3D cell culture platforms can be visualized as a methodology with the potential to inculcate in-vitro conditions self-assembly, perfusion, and co-culture with other cells.
Several studies also have suggested that tumors should be viewed as a well-structured pathological organ, (Egeblad 2010) consisting different types of cells like fibroblasts, endothelial cells, immune cells or adipocytes Hanahan (2012) rather than a mass of unrestrained proliferating cells. This alternate viewpoint broadens the horizon for in vitro models by co-culturing of cells with varied origins. Early research had revealed that co-culture methods show significant changes in different biological mechanisms like epithelial to mesenchymal transition, metastasis, neo-angiogenesis, fibroblast’s transformation into Cancer- Associated Fibroblasts (CAFs), (Kim 2015, Angelucci 2012, Sethi 2015, Esendagli 2014 and Sung 2013). Similarly, co-culture mechanisms have exhibited the transformation of macrophages into Tumor-Associated Macrophages (TAMs), ( Kim 2015, Angelucci 2012, Sethi 2015, Esendagli 2014 and Sung 2013, Belli (2018), Kolenda (2018). However, there exists large scope for understanding the pathology of tumor microenvironment, which can be vital for the development of new and effective cancer therapies.
Considering all this, in our work, the emphasis is made on the development of a 3D breast cancer model by employing a natural silk scaffold. Silk fibroin fibres have been employed in medicine since a long time, especially as surgical sutures. However, its significance as a biomaterial has increased many-folds in the last few years due to the development of silk-based 3D scaffolds for cell culture, (Kearns 2008, Altman 2003 and Jastrzebska 2015). Considering its robustness especially in terms of biocompatibility, biodegradability and the self-assembly, it has been applied in numerous bio-engineering purposes including the development of cartilage and bone tissues Sundelacruz (2009), Yodmuang (2015). In the last few years, tumor models for hepatocarcinoma Kundu (2013), and mammary adenocarcinoma Talukdar (2011), osteosarcoma Tan (2011) have been developed using silk scaffolds. Few researches and allied only models which have used three-dimensionality of the tissue as well as heterotypic interactions between cells Chiew (2017), Onion (2016), Amann (2017) and Valdez (2017) Belli (2018), Kolenda (2018).
In this study, we have cultured cells with three different conditions each on 2D, plain silk layers and 3D silk scaffolds as mentioned below. MDAMB-231 cells as control, Conditioned Media- MDAMB-231 cells are cultured in media taken from flasks containing 3T3 cells. This media is called conditioned media as it contains the proteins and other factors secreted by 3T3 cells, which could influence MDAMB-231 cell growth like in co-culture conditions3T3-MDAMB-231- In this condition 3T3 cells are cultured/grown on the scaffolds for 24 hr, after 24 hr 3T3 cells are removed using 2mM EDTA (EDTA helps in cell detachment from the surface but the ECM secreted by the cells stay intact on the cultured surface). MDAMB-231 cells are then cultured on these ECM-coated scaffolds.
In order to facilitate 3D architecture, we used natural silk which was extracted from the cocoons of Bombyx mori. Standard protocols were followed for extracting silk protein Fibroin from Bombyx mori cocoons and making scaffolds out of them. We developed two types of scaffolds; HFIP-based sponges and lyophilized scaffolds. In our work, we enhanced scaffold production mechanism along with cell seeding, long-term 3D cell culture and cell detachment mechanism. We have characterised our model by measuring the swelling and by using microscopy, cell proliferation assay and gene expression analysis. In this study, the consideration of the genetic modification after culturing cells under different conditions, gave efficient cell production along with better cells separation. Such labeling helped making optimal assessment of their reciprocal interactions by means of gene expression (patterns) analysis.
MATERIAL AND METHODS
We have developed two types of silk scaffold. HFIP-based sponges and lyophilized scaffolds which were obtained by extracting silk fibroin from silkworm Bombyx mori. Silk fibroin is biocompatible, tough, elastic, and exhibit better mechanical properties and biodegradability with tunable degradation rates. Unlike classical 2D culturing, we employed 3D culturing. Breast cancer cells were seeded on this naturally-derived biomaterial matrix for mimicking of the TME while assuring sufficient crosstalk amongst cancer cells and stroma. In this study, the morphological and proliferation properties of breast cancer cells grown on these scaffolds were evaluated. The detailed discussion of the materials and methods used in this study is given in the sub-sequent sections.
Silk-Scaffold Development
We first extracted silk-protein fibroin from Bombyx mori cocoons. To extract silk-protein fibroin from Bombyx mori cocoons, we followed the standard protocols proposed in Danielle (2011). We first degummed worm-free, clean cocoons, with predefined weight, by boiling them in 0.02M Na2CO3solution for 40 minutes. Once degummed, the fiber mesh obtained was washed three times for 20 minutes individually and then air-dried overnight under aseptic conditions. The dried silk fibers, we weighed and dissolved using 12% w/v silk/9.3M Lithidum bromide (LiBr) and maintained at 700C for four hours. To perform further dialysis the dissolved silk solution was added with LiBr into a dialyze membrane which was prepared before pouring the silk Libr mixture.The dialysis membrane was prepared as the standard protocol proposed by Rosenberg (1996). In this method, the required length of dialysis membrane was cut and boiled in 2%Na2CO3 +1mM EDTA for 10 minutes which was later washed thoroughly. Silk with LiBr mixture was poured to the dialysis membrane and was processed for 48 hours against Millipore water. Noticeably, during the dialysis process, the water being used was changed at a defined interval (1, 3, 6, 12 and subsequently every 12 h). After dialysis for 48 h, the solution inside the dialysis membrane was transferred into a falcon, which was centrifuged twice at 9000 rpm at 40C for 20 mins. The clear supernatant was collected and stored at 4°C in fresh falcons while the debris was discarded. Before making scaffolds, we calculated the percentage as well as weight of silk in the sample. In our experiment, the percentage of silk was calculated by adding 0.5 ml of the solution on aluminum foil, which was kept for drying at the temperature of 600C.Before adding silk solution with aluminum foil, it (i.e., aluminum foil) was weighted and recorded. Thus, obtaining dried silk with foil we weighted it and recorded. To calculate the weight of silk we used following equation.
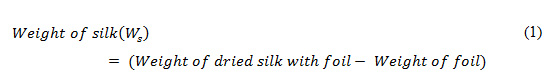
Thus, obtaining the weight of silk, we calculated the percentage of silk using (2).
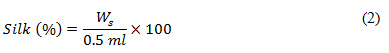
In (2), 0.5 ml signifies the volume of silk solution considered for analysis.
Making of SFs
As already stated, in this study two distinct types of scaffolds were developed; HFIP based sponges and lyophilized scaffolds. To prepare lyophilized scaffolds 1ml of 4% silk fibroin (per well) was added in a 24 well plate and lyophilized for 24 hours. On contrary, to prepare HFIP based sponges (i.e., scaffolds), 7% w/v lyophilized silk/HFIP solution was prepared where silk fibroin was dissolved completely in the HFIP solution. During silk dissolving phase, NaCl was sieved for uniform sized 250-425 . 3.4 gm of NaCl was spread in 35mm dish. This process was repeated for as many scaffolds as required. To obtain a uniform layer the 35 mm dish with NaCl was tapped gently, followed by adding 1 ml of silk/HFIP solution per well using a syringe in the fume hood. Subsequently, dish was wrapped with parafilm and left in the chemical hood for 1-2 days for the silk/HFIP solution to penetrate through the salt. Once the salt appeared wet i.e., the silk solution reached the bottom of the dish. The dish was left open in chemical hood for HFIP to evaporate for a day, which was then followed by adding 1ml of methanol to each container and sealed with parafilm and left for a day. Excess of methanol was removed from the sample. The containers containing samples were transferred to a two liter beaker with Millipore water and left for salt to dissolve. The water was changed 2-3 times per day and was continued for 3 days for salt leaching. Once the salt dissolved, the scaffolds were separated from the dishes and were collected in a falcon and stored in Millipore water at 40C until used. To prepare plain silk sheets (as control), 1ml of silk/HFIP solution was poured in 35 mm dish was left open in chemical hood for HFIP to evaporate for a day, which was then followed by adding 1ml of methanol to each container. The prepared sample was left for a day. Excess of ethanol was removed from the silk sheets and was dried.
Characterization of the developed Scaffolds
Once the scaffolds were ready for further assessment, we performed in depth characterization in terms of swelling property, water-uptake property, mechanical characterization, Scanning Electron Microscopy (SEM) analysis etc. A brief of the characterization methods applied in this study to assess efficacy of the developed 3D breast cancer model using Silk Fibroin Scaffold.
Swelling property of the scaffolds
To examine swelling property of the developed scaffolds, they were hydrated for 24 hr in ionized water and Phosphate buffer saline (PBF) separately and each scaffold was incubated at room temperature and 37°C. After 24 hr, the scaffolds were assessed for its swelling percentile, which was obtained using following equation (3).
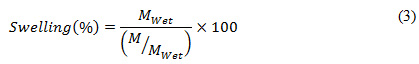
In above equation (3), the variable signifies the weight of scaffold after 24 hours of soaking in water or PBS, while refers the dry weight before soaking.
Water-uptake property of the scaffolds
Quantification of water uptake of the scaffolds were obtained by soaking scaffolds in ionized water and PBS seperately for 24 hours at room temperature, followed by drying at the 50°C. To calculate water-uptake property, we used the following equation (4):
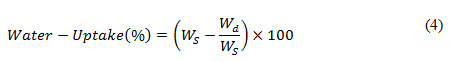
In (4), the variables Ws and Wd signifies the weights of the wet scaffolds and the dried scaffolds, respectively.
Mechanical Properties of the Scaffolds
To perform mechanical characterization of the developed SFs, we used Dynamic Mechanical Analysis (DMA) method. Typically, DMA is applied to assess elastic properties (bending, elasticity, tensile strength). DMA analysis was obtained for both the scaffolds i.e., HFIP based sponges as well as 4% lyophilized scaffold.
Cell culture
In this process, MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle’s medium—high glucose (DMEM, Sigma–Aldrich) which was supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS) (Sigma-Aldrich, North American origin) and antibiotics penicillin streptomycin powder (Hi Media) 0.4 µg /ml final concentration. The cells were cultured at 37°C, where the relative humidity was maintained at 95% with 5% CO2. In our experiment, we used cells from an 80% confluent dish. Silk scaffolds and plain sheets where coated with collagen type IV, followed with PBS washes, the scaffolds where incubated in culture media overnight. Cells were seeded at the rate of 1, 00,000 cells per scaffold/ plain silk sheet. In this experiment, cells were cultured under three distinct conditions on both scaffolds as well as plain silk sheets (control) as explained in the introduction section.
Biochemical or Molecular Assay
Once obtaining the distinct cell culture samples, we perform molecular assays where each sample was processed or examine for DNA quantification and RT-PCR. A snippet of the DNA quantification and quantitative real-time polymerase reaction is given in the sub-sequent sections.
DNA Quantification
In this study, the proliferation of the cells was assessed under control and treated conditions, which was evaluated at day 1, 3 and 5 by measuring the DNA content using the Pico-green dsDNA quantification kit (Invitrogen). To perform DNA quantification, we used a recent protocol proposed in Kumar (2016). Summarily, the cells were lysed using lysis buffer (0.02% SDS with Proteinase K 0.2 mg/ml). The lysate was mixed with the Picogreen dye to determine DNA content by measuring the fluorescence intensity in a well-plate reader with 485 nm excitation and 528 nm emissions.
Quantitative real time polymerase reaction
To assess quantitative real-time polymerase reaction, cells were cultured on silk scaffolds and control plain silk for two different time points 7 days and 14 days. For molecular gene expression studies RNA was isolated using the RNase Mini Kit (Quigen) as per the manufacturer’s instruction. In this study, we used a total of 2 µg of RNA for cDNA synthesis using a high-capacity cDNA reverse transcription kit (Applied Bio-systems) as per the manufacturer’s instruction. Quantitative real-time PCR (qRT-PCR) was carried out using a Power up SYBR Green master mix (Thermo Scientific) with 10ng of the cDNA as the template. Further, we normalized the gene expression to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and the fold change was calculated by means of 2-ΔΔct. To study gene expressions, we used EMT markers Epithelial-cadherin (E-cad), Neural-cadherin (N-cad), Vimentin, Snail, Slug, Twist, Paxillin, along with Matrix metalloproteinase-2 (MMP2), Sox2 and HF1 primers.
Results and Discussion
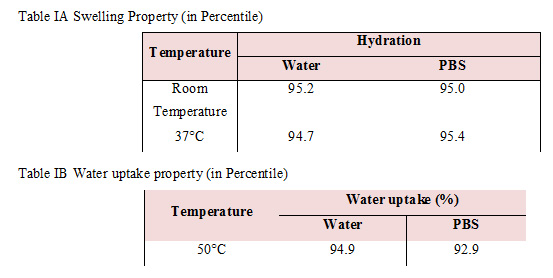
We have characterized the mechanical properties of the developed silk scaffold and characterized the biochemical properties of the cells grown in them through molecular assays such as DNA quantification and Quantitative real time polymerase chain reaction. We have used plain sheet of silk as control for our biological assays onHFIP + silk scaffolds. We further discuss the results obtained and our inferences. Since porosity is a biologically-relevant mechanical property, we have characterized it by measuring the swelling (Table IA) and water-uptake (Table IB) of our scaffold. Swelling was measured at room temperature and at 37°C for water and PBS. The swelling at room temperature was higher when hydrated with water in comparison to hydration with PBS. Interestingly, at 37°C, swelling was higher when hydrated with PBS We estimated water uptake at 50°C using equation (4) (Table IB). We observed that the water uptake under water is higher than with PBS
These results show that these porous scaffolds are suitable for 3D tissue culture. Such scaffolds can be used to understand various properties of the cell such as adhesion, polarity, and morphology.The stiffness of silk/HFIP sponges is higher (5.8 kPa) in comparison to 4% lyophilized scaffold (2.8 kPa). Moreover, the stiffness of the silk scaffold is closer to the stiffness of the breast tissue in vivo when compared to the stiffness of standard tissue culture dishes, which is in the order of giga pascals Paszek (2005). This is relevant because mechanical forces and stiffness of the cell microenvironment is known to alter gene expression patterns and cell signaling. In addition, the rigidity of the matrix can also affect stem cell separation, the migration of cells through cell membrane receptors, and activation of actin cytoskeleton Engler (2006), Mendoza (2010), Menezes (2008), Buxboim (2010), Erler (2009), Kim (2009). The porous nature of the scaffold enables circulation of oxygen and nutrients that could eventually help in elimination of cell detachment and its debris. On contrast, such features are not visible in the 2D monolayer culture that typically exhibits cellular uniformity (in terms of shape and size) along with contact inhibition at confluence. Hence, we have chosen HFIP-silk scaffolds for further experiments.
Cells cultured on the 3D scaffold typically have more space to proliferate and hence take more time to reach confluence. Cells form clusters in between the single layers of cells towards the scaffold’s surface which eventually gives rise to the multilayer clumps. Lack of detoxification results in growth arrest which then later develops into necrosis in the central regions. This mimics breast tumor in vivo and is therefore a more realistic breast cancer model. Figures 1 and 2 show the SEM images of the scaffold and cells cultured in them. Cells were cultured for a period of 7 and 14 days (Fig. 2).
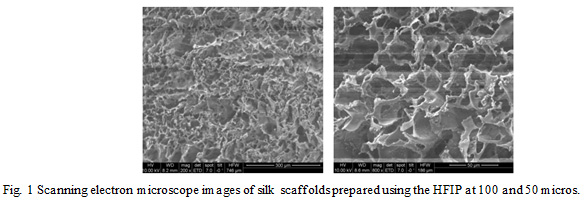 |
Figure 1: Scanning electron microscope images of silk scaffolds prepared using the HFIP at 100 and 50 micros. |
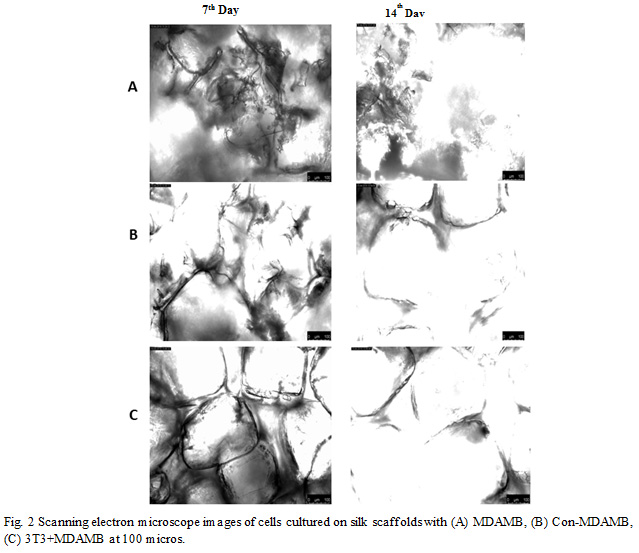 |
Figure 2: Scanning electron microscope images of cells cultured on silk scaffolds with (A) MDAMB, (B) Con-MDAMB, (C) 3T3+MDAMB at 100 micros. |
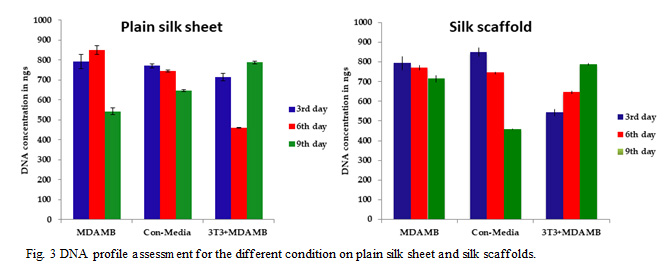 |
Figure 3: DNA profile assessment for the different condition on plain silk sheet and silk scaffolds. |
We have measured the DNA concentration of MDAMB cells cultured on control and silk scaffolds and under three conditions, normal, conditioned media, and de cellularized ECM matrix for a period of 3, 6 and 9 days. (Fig. 3) In the control at normal conditions, the proliferation increases from day3 to day 6 and then it decreases. In the case of MDAMB cells grown under conditioned media, the cell proliferation is consistently decreasing from day 3 to day 9 for both control and silk scaffolds. This could be due to the secreted factors present in the conditioning media. In contrast, the cells cultured on control and silk scaffolds containing on decellularized ECM matrices are proliferating. This shows the importance of cell-ECM interaction in tumor formation.
The following results exhibit the quantitative real-time polymerase reaction assessment of the silk scaffolds. Fig. 4 a,b,c,d exhibit the molecular change in cell cultured on plain and scaffolds on 7th and 14th day. We have characterized the fold-changes of different EMT markers; Epithelial-cadherin (E-cad), Neural-cadherin (N-cad), Vimentin, Snail, Slug, Twist, Paxillin, along with Matrix metalloproteinase-2 (MMP2), Sox2 and HF1 primers. Results (Fig. 4) reveal that cells cultured on plain scaffold showed both epithelial and mesenchyme characters, on 7th day increase in E-cad and Paxillin levels contributing to the epithelial character but on the 14th day change in levels of slug, snail, twist, MMP2 along with N-cad and Vimentin contributing to mesenchyme character, with increase in stemness and hyoxia condition contributes to processes involved in metastasis like chromosomal instability, invadopodia, angiogenesis etc.
Cells on scaffolds with time showed mesenchyme characteristic. Gene expression levels of Slug, Snail and twist contributes to increase in cell stemness (SOX2) and metastasis.
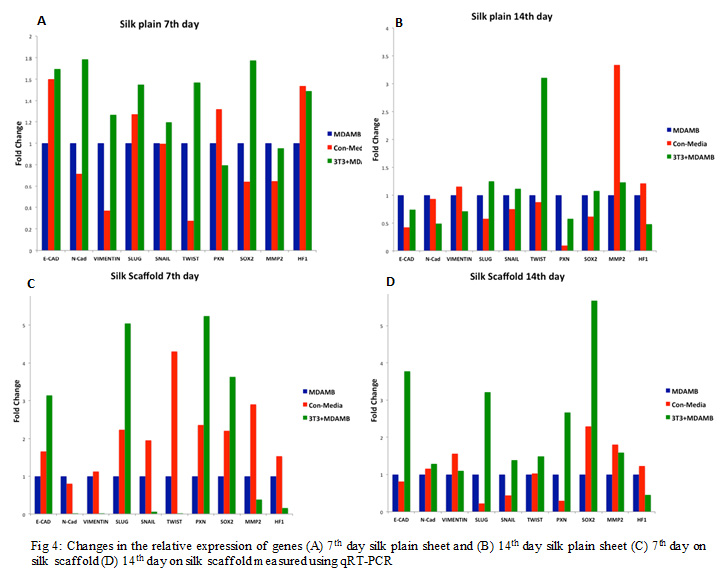 |
Figure 4: Changes in the relative expression of genes (A) 7th day silk plain sheet and (B) 14th day silk plain sheet (C) 7th day on silk scaffold (D) 14th day on silk scaffold measured using qRT-PCR |
The key limitations of the major at hand approaches or cell models are its inability to examine the efficacy of anti-cancer agents and varied cell-interactions occurring in the TME. Such limitations revitalize researchers to develop more efficient and advanced in vitro tumor models. In our work a novel breast cancer model was developed a 3D in vitro culture model by mimicking the architecture and the stiffness of the breast tumor. . This approach enabled spatial cell-development on a porous, scaffolding structure that mimics the in vivo tissue-like microenvironment for the cells. The concurrent use of different culturing conditions for cells characterizes robustness of the proposed model to form a “tumor organ”. Such culture mechanism enabled the in-depth assessment of the direct as well as reciprocal interactions amongst the cancer cells. The bio-material used in this cancer cell model was natural silk fibroin, which is cost-efficient, accessible, non-toxic that after removal of sericin layer exhibits no activating adaptive immune response. Initially, we emphasized on selecting the best scaffolding technique for breast cancer tumor cell’s culture. In our study, we exhibited that silk fibroin scaffolds with the pore size ranging 250- 500 μm can be significant towards optimal cell proliferation and associated infiltration. Here, two commercially available cell lines were used for 3D culture.
Considering the significance of the TME in cancer biology, different in vitro culture models have been proposed by Belli (2018), Kolenda (2018). However; not much significant efforts are made towards assessing direct as well as reciprocal cell interactions analysis that often takes place in between stromal and tumor cells. There are some cancer models which employ indirect co-culture, by applying cell-growth within distinct compartments or by using conditioned media (CM) from the specific kind of cells. Such approaches enable merely understanding the impacts of paracrine signaling but avoid exhibiting direct cell-to-cell interactions. In this study we developed a system that enables assessing direct cell-to-cell interactions. The scaffolds allowed cell attached, proliferating and ECM secretion, affirming the fact that silk fibroin can be a potential and better biomaterial to support cell culture. This study revealed perceptible differences in morphology (in cells cultured in 3D culture). It is found that in the culture cell model, it becomes intricate to distinguish singular cells by SEM images due to cell-embedding into the thick layer of extracellular matrix generated by cells. In addition, this study revealed different growth kinetic and morphological changes in 3D culture model.
The gene expression patterns of the cells were examined. Cells cultured on 2D silk sheets, were characterized to be more epithelial phenotype when compared to cells cultured on 3D scaffold. Factually, such phenotypic inferences depend predominantly on the morphology of cell. In the considered breast cancer cells, breaking of β -catenin often cause significantly rise in cell mobility along with mesenchymal, vimentin expression that refers an EMT Yamori (1997). The cell-transition into a 3D environment signifies their higher mesenchyme phenotype nature. Noticeably such features were observed more in those genes which are conscientious towards extracellular matrix production and remodeling. Thus, this study revealed that the 3D culture enables cells the environmental cues required to maintain their physiology.
CONCLUSION
We have developed and characterized a silk-protein-based scaffold for mimicking the tissue microenvironment and stiffness in vitro. Breast cancer cells (MDAMB-231) were cultured with ECM secreted by fibroblasts (3T3) on these scaffolds. Two different scaffolds, namely, HFIP-based sponges and lyophilized scaffolds were developed. The geometry of the scaffolds was characterized using SEM; physical properties such as swelling, and water-uptake were measured. We characterized the cells cultured on these scaffolds by measuring their morphology, proliferation and gene expression levels of a panel of cancer-associated genes. Our preliminary results show that cells in the scaffold show enhanced expression of genes associated with invasion, migration, ECM-remodeling and metabolism. It is pertinent to note that even though MDAMB-231 cells are metastatic, they gradually lose their mesenchymal phenotype when grown in 2D culture dishes. In contrast, when grown on our scaffolds, they exhibit their true metastatic potential. Moreover, our scaffolds are flexible, cost effective, biocompatible and easy-to-handle. Hence, our system could be an attractive in vitro model for studying breast cancer metastasis in vitro, testing the efficacy and molecular mechanisms of the anti-cancer drugs.
ACKNOWLEDGMENT
This work was supported by the Indian Council of Medical Research, New Delhi, India.
CONFLICT of INTEREST
Authors declare no conflicts of interest in the publication.
REFERENCES
Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. (2003) Silk-based biomaterials. Biomaterials. 24:401–16.
Amann A, Zwierzina M, Koeck S, Gamerith G, Pechriggl E, Huber JM, Lorenz E, Kelm JM, Hilbe W, Zwierzina H, Kern J. (2017) Development of a 3D angiogenesis model to study tumour – endothelial cell interactions and the effects of anti-angiogenic drugs. Sci Rep. 7:2963.
Angelucci C, Maulucci G, Lama G, Proietti G, Colabianchi A, Papi M, Maiorana A, De Spirito M, Micera A, Balzamino OB, Di Leone A, Masetti R, Sica G. (2012) Epithelial-stromal interactions in human breast cancer: effects on adhesion, plasma membrane fluidity and migration speed and directness. PLoS One. 7:e50804.
Bai Geetha R, Muthoosamy K, Manickam S, Hilal-Alnagbi A. (2019) Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int J of Nanomedicine. 14:5753-5783.
Belli Carmen, Trapani Dario, Viale Giulia, D’Amico Paolo, Duso Bruno Achutti, Vigna Paolo Della, Orsi Franco, Curigliano Giuseppe. (2018) Targeting the microenvironment in solid tumors. Cancer treatment Reviews 65:22-32.
Buxboim A. and D. E. Discher. (2010) Stem cells feel the difference. Nature Methods. vol. 7, no. 9, pp. 695–697.
Chang TT, Hughes-Fulford M. (2008) Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng Part A. 15:559–67.
Chiew GG, Wei N, Sultania S, Lim S, Luo KQ. (2017) Bioengineered three-dimensional co-culture of cancer cells and endothelial cells: a model system for dual analysis of tumor growth and angiogenesis. Biotechnol Bioeng 21-2 34 -42.
Edmondson R, Broglie JJ, Adcock AF, Yang L. (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Tech. 12:207–18.
Egeblad M, Nakasone ES, Werb Z. (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 18:884–901.
Engler A.J, Sen S, Sweeney H. L, and Discher D. E. (2006) Matrix elasticity directs stem cell lineage specification. Cell, vol. 126, no. 4, pp. 677–689.
Erler J. T. and Weaver V. M. (2009) Three-dimensional context regulation of metastasis. Clinical and Experimental Metastasis. vol. 26, no. 1, pp. 35–49.
Erler JT, Weaver VM. (2009) Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 26:35–49.
Esendagli D, Esendagli G, Yilmaz G, Guc D. (2014) Spheroid formation and invasion capacity are differentially influenced by co-culture of fibroblast and macrophage cells in breast cancer. Mol Biol Rep. 41:2885–92.
Hanahan D, Coussens LM. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 21:309–22.
Jastrzebska K, Kucharczyk K, Florczak A, Dondajewska E, Mackiewicz A, Dams-Kozlowska H. (2015) Silk as an innovative biomaterial for cancer therapy. Rep Pract Oncol Radiother. 20:87–98.
Kearns V, MacIntosh AC, Crawford A, Hatton PV. (2008). Silk-based biomaterials for tissue engineering. Top Tissue Eng, 2–19.
Kim SA, Lee EK, Kuh HJ. (2015) Co-culture of 3d tumor spheroids with fibroblasts as a model for epithelial-mesenchymal transition in vitro. Exp Cell Res. 335:187–96.
Kim Y.J, Bae H.I, Kwon O. K, and Choi M.S. (2009) Three dimensional gastric cancer cell culture using nanofiber scaffold for chemosensitivity test. International Journal of Biological Macromolecules, vol. 45, no. 1, pp. 65–71.
Kolenda Tomasz, Przybyla Weronika, Kapalczynska Marta, Teresiak Anna, Zajaczkowska Maria, Blizniak Renata, Lamperska Katarzyna M. (2018). Tumor microenvironment – unknown niche with powerful therapeutic potential. Reports of practical oncology and radiotherapy. 23:143-153.
Kumar S., Raj S., Sarkar, K. and Chatterjee K. (2016) Engineering a multi-biofunctional composite using poly (ethylenimine) decorated graphene oxide for bone tissue regeneration Nano, vol. 8, pp. 6820-6836.
Kundu B, Saha P, Datta K, Kundu SC. (2013) A silk fibroin based hepatocarcinoma model and the assessment of the drug response in hyaluronan-binding protein 1 overexpressed HepG2 cells. Biomaterials. 34:9462–74.
Lee GY, Kenny PA, Lee EH, Bissell MJ. (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 4:359–65.
Mendoza M. Miron, Seemann J, and Grinnell F. (2010) The differential regulation of cell motile activity through matrix stiffness and porosity in three-dimensional collagen matrices. Biomaterials. vol. 31, no. 25, pp. 6425–6435.
Menezes G. C, Mendoza M Miron, Ho C.H, Jiang.H, and Grinnell F. (2008) Oncogenic Ras-transformed human fibroblasts exhibit differential changes in contraction and migration in 3D collagen matrices. Experimental Cell Research. vol. 314, no. 16, pp. 3081–3091.
Onion D, Argent RH, Reece-Smith AM, Craze ML, Pineda RG, Clarke PA, Ratan HL, Parsons SL, Lobo DN, Duffy JP, Atherton JC, McKenzie AJ, Kumari R, et al.(2016) 3-dimensional patient-derived lung cancer assays reveal resistance to standards-of-care promoted by stromal cells but sensitivity to histone deacetylase inhibitors. Mol Cancer Ther. 15:753–63.
Paszek M.J, Zahir N, Johnson K.R, Lakins J.N, Rozenberg G.I, Hammer D, Weaver V.M. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell. 8, 241-254.
Polonio-Alcala Emma, Rabionet Marc, Guerra Antonio J, Yeste Marc, Ciurana Joaquim, Puig Teresa. (2018) Screening of additive manufactured scaffolds designs for triple negative breast cancer 3D cell culture and stem-like expansion. Int J of Molecular Sciences. 19.
Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD. (2015) 3D cell culture systems: advantages and applications. J Cell Physiol. 230:16–26.
Rockwood Danielle N, Preda Rucsanda C , Yucel Tuma, Wang Xiaoqin, Lovett Machael L and Kaplan David L. (2011) Material fabrication for Bombyx mori silk fibroin. Protocol. 6(10): 1612-1631.
Sethi P, Jyoti A, Swindell EP, Chan R, Langner UW, Feddock JM, Nagarajan R, O’Halloran TV, Upreti M. (2015) 3D tumor tissue analogs and their orthotopic implants for understanding tumor-targeting of microenvironment-responsive nanosized chemotherapy and radiation. Nanomedicine. 11:2013–23.
Sundelacruz S, Kaplan DL. (2009) Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Sem Cell Dev Biol. 20:646–55.
Sung KE, Su X, Berthier E, Pehlke C, Friedl A, Beebe DJ. (2013) Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One. 8:e76373.
Talukdar S, Mandal M, Hutmacher DW, Russell PJ, Soekmadji C, Kundu SC. (2011) Engineered silk fibroin protein 3D matrices for in vitro tumor model. Biomaterials. 32:2149–59.
Tan PH, Aung KZ, Toh SL, Goh JC, Nathan SS. (2011) Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology. Biomaterials. 32:6131–7.
Valdez J, Cook CD, Ahrens CC, Wang AJ, Brown A, Kumar M, Stockdale L, Rothenberg D, Renggli K, Gordon E, Lauffenburger D, White F, Griffith L. (2017) On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials. 130:90–103.
Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D, Eccles SA. (2012) Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 10:29.
Wang Ying, Mirza Sameer, Wu Shaohua, Zeng Jiping, Shi Wen, Band Hamid, Band Vimla, Duan Bin Duan. (2018) 3D hydrogel breast cancer models for studying the effects of hypoxia on epithelial to mesenchymal transition. Oncotarget. 9:63 32191-32203.
Yamori T, Sato S, Chikazawa H, and Kadota T. (1997) Anti-tumor efficacy of paclitaxel against human lung cancer xenografts. Japanese Journal of Cancer Research. vol. 88, no. 12, pp. 1205– 1210.
Yodmuang S, McNamara SL, Nover AB, Mandal BB, Agarwal M, Kelly TA, Chao PH, Hung C, Kaplan DL, Vunjak-Novakovic G. (2015) Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 11:27–36.


