1Department of Physics, Indian Institute of Engineering Science and Technology, Shibpur and Centurion University of Technology and Management, Bhubaneswar Odisha India
2Department of Physics, Indian Institute of Engineering Science and Technology, Shibpur Odisha India
Corresponding author Email: smukherjee.besu@gmail.com
Article Publishing History
Received: 04/07/2019
Accepted After Revision: 20/09/2019
Zinc, as one of the major trace elements of the human body and co-factor of more than 300 mammalian enzymes, plays an important role in maintaining crucial cellular processes including oxidative stress, DNA replication, DNA repair, cell cycle progression and apoptosis. Thus, it is evident that an alteration in zinc levels in cancer cells can cause a deleterious effect. Research has shown that low zinc concentration in cells leads to the initiation and progression of cancer and high zinc concentration shows toxic effects. Zinc-mediated protein activity disequilibrium and oxidative stress through reactive oxygen species (ROS) may be the probable mechanism of this cytotoxic effect. ZnO has a neutral hydroxyl group attached to its surface, which plays an important role in its surface charge behaviour. Our aim is to show that the effect of Zinc Oxide and Silica coated Zinc Oxide on different microbes and cancer cells. Characterization of Zn nanoparticles have been done by using different analyzing techniques i.e. UV-Vis Spectroscopy, DLS (Dynamic Light Scattering) and SEM (Scanning Electron Microscope). The effect of the Zn nanoparticles on microbes has been measured by the cup disk method where as the effect on cancer cell line (HeLa) (Human Cervical Cancer Cell Line) has been measured by Fluorescence Anisotropy, MTT assay, Reactive Oxygen Species (ROS). The effect on different enzymatic action has also been measured. Regardless of antimicrobial medicinal consideration, dismalness and mortality identified with these microorganism contaminations remain high, somewhat because of the adaptability of those life forms to create protection from almost all anti-infection agents. Our aim is to develop new drugs spot and build up the resulting age of prescription or operators to manage microorganism contaminations.
Zinc Oxide, Reactive Oxygen Species, Fluorescence Anisotropy, MTT assay.
Bhadra P, Dutta B, Bhattyacharya D, Mukherjee S. Comparative Study of the Zno and Zno Coated with Sio2 As Potential Antimicrobial and Anticancer Drugs. Biosc.Biotech.Res.Comm. 2019;12(3).
Bhadra P, Dutta B, Bhattyacharya D, Mukherjee S. Comparative Study of the Zno and Zno Coated with Sio2 As Potential Antimicrobial and Anticancer Drugs. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/2ZiYnVL
Copyright © Bhadra et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Bio nano molecules are those, whose size is comparable with nanoparticles, play an unavoidable important role in regulating various cellular cycles of the body and maintaining crucial cellular homoeostas is. With proper bio engineering, Nanoparticles can be sent in a localized condition in any system of the body and thus it can incorporate the activity of biological components, thus mimicking the biological system of the body according to the need for human benefit. Nanoparticles are highly soluble due to their small size and their solubility can be further increased by proper surface modification and the high surface area to volume ratio of those particle, make them having ample surface area to encapsulate drugs and other materials, thus providing higher therapeutic payload. Another property of these nano particles can be described as the selective targeting nature, thus Nanoparticles can specifically release a therapeutic payload onto the target, reducing the side effects on normal cells, (McNeil, et.al, 2009, Wang, et.al, 2013, Bisht and Rayamajhi, 2016; Hussain et.al, 2019). Marco et.al, 2019).
Research and development in the field of nanotechnology are growing rapidly throughout the world (Vidya et al., 2013). A major contribution of this field is the development of new materials in the nanometer scale (Sivakumar, et al., 2011; Karthikeyan et.al, 2019). These are usually particulate materials with at least one dimension of less than 100 nanometers (nm), even the particles could be zero dimension in the case of quantum dots (Vidyaet al., 2013). Metal nanoparticles have been of great interest due to their distinctive features such as catalytic, optical, magnetic and electrical properties (Garima, et al., 2011). Nanoparticles exhibit completely new or improved properties with larger particles of the bulk materials, and these novel properties are derived due to the variation in specific characteristics such as size, distribution, and morphology of the particles (Ravindra, et al., 2011, Ravindran, et.al. 2016). Particularly, nanoparticles (NP) made from metal oxides with sizes less than 100 nm exhibit antimicrobial activities owing to their special characteristics (e.g. small particle size, large surface area), which micro- or macro-sized particles do not possess. Zinc oxide, with its unique physical and chemical properties, such as high chemical stability, high electrochemical coupling coefficient, broad range of radiation absorption and high photo stability, is a multi functional material (Lou, 1991, Segets, et.al. 2009). Recent studies have shown that some NP made of metal oxides, such as ZnO NP, have selective toxicity to bacteria but exhibit minimal effect on human cells (Brayner et al. 2006; Thill et al. 2006; Reddy et al. 2007; Zhang et al. 2007, Sadhukhan et.al, 2019).
Compared with the organic materials, inorganic antibacterial reagents are more stable at high temperatures and pressures (Sawai 2003). Compare to the inorganic antibacterial materials, metal oxides such as zinc oxide (ZnO) have received increasing attention in recent years, not only because they are stable under harsh processing conditions, but also because they are generally regarded as safe materials to human beings and animals (Stoimenov et al. 2002; Fu et al. 2005; Kaushik et.al, 2019).
ZnO has a neutral hydroxyl group attached to its surface, which plays an important role in its surface charge behaviour. At high pH, ZnO exists as ZnO− due to the transfer of adsorbed protons from its surface towards aqueous solution. At low pH (acidic condition), ZnO exists as ZnOH2 + due to the transfer of protons from the aqueous environment towards its surface. The isoelectric pH of ZnO nanoparticles is 9-10 (Orel, et.al, 2015, Vinardell et.al, 2015 Roy and Jong,2019).
Thus, ZnO nanoparticles exhibit positive charge under physiological conditions such as blood or tissue fluid (which has pH 7), etc. (Degen et.al,2000, Rasmussen et.al, 2010). On the other hand, cancerous cells usually have high concentration of (negatively charged) anionic phospholipids on their outer membrane (Abercrombie et.al, 1962). The re-emergence of infectious diseases and the continuous development of antibiotic resistance among a variety of disease-causing bacteria pose a serious threat to public health worldwide (Desselberger, 2000, Vandenesch et.al, 2003). Among these pathogenic microorganisms, Enterococcus, Staphylococcus and Streptococcus are common closely related species that cause a wide variety of infections and diseases (Boyce, 1997; Lowy, 1998; Hancook & Gilmore, 2000, Laura et.al,2019).
Despite antimicrobial therapy, morbidity and mortality associated with these bacterial infections remain high, partially as a result of the ability of these organisms to develop resistance to virtually all antibiotics. New strategies are therefore needed to identify and develop the next generation of drugs or agents to control bacterial infections. CuO nanoparticles are successful in murdering a spread of microorganism. Be that as it may, ZnO nanoparticles with high focus are expected to get the disinfectant effect. The disinfectant property of such nanoparticles relies upon their size, steadiness, and focus extra to the extension medium, that gives bigger maintenance time to microorganism NP association.
Material and Methods
Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat inactivated fetal calf serum (Gibco™, Thermo Fisher Scientific), penicillin (100 U/mL) and streptomycin (100 μg/mL) (Merck, India), TMA-DPH (1-(4-Trimethylammoniumphenyl)-6-Phenyl-1,3,5-Hexatriene p-Toluenesulfonate) ( Thermo Fisher Scientific), Dry N,N,dimethylformamide (DMF; Merck, India), nickel chloride hexahydrate (NiCl2, 6H2O, Mw = 237.69 g mol−1; Merck, India), sodium hydroxide (NaOH; Merck, India), ethyl alcohol (C2H6O; Merck, India), tetraethyl orthosilicate (TEOS; Merck, India), and ammonia solution 25% (NH4OH; Merck, India) were used in this work. All the materials were used in the experiments without further purification.
Preparation of Zno@Sio2 Nanoparticle
Hydrothermal method has been used to synthesize the Zinc oxide nanoparticles (Dutta et.al., 2017). Details of that hydrothermal procedure for synthesis of metal oxide nanomaterials has been referred from (Dutta et.al., 2015). Modified Stöber (Stober et.al., 1968) method, a widely used method for synthesis of silica nanoparticles has been used to synthesize the silica coated Zinc oxide. In this typical synthesis procedure, hydrothermally synthesized Zinc oxide nanoparticles (Dutta et.al., 2017) were added to the solution of water and ethyl alcohol (in volume ration approximate 4:1). To achieve a well-dispersed mixture, the solution was sonicated for 10 min. After that, ammonia was added to the mixture (in volume ratio 1.4:50) drop by drop to catalyze the Zinc oxide nanoparticles in alcoholic media. The mixture was again sonicated for 40 min after the addition of ammonia, and finally, TEOS was added drop by drop to the mixture (in volume ratio approximately 0.4:50). The final mixture was kept under strong magnetic stirring (500 rpm) for 18 h. The well-mixed colloidal solution was centrifuged at 4000 rpm and washed by ethanol to remove the residuals from the product. The collected product was dried at 80 °C and employed for further characterization.
Anti Microbial Activity: Cup-Disc Method
The number of the zone of inhibition has been deduced from three parallel studies and those are taken as the mean value of those. These studies were compared with the known drugs available in the market. The ZnO and ZnO@SiO2 showed an average value of the zone of inhibition where the combination of these two nanoparticles showed a maximum zone of inhibition. The lower concentration of the mixed drug has an effect on the bacterial and the fungal growth which has been measured by calculating the zone of inhibition and the values are (+/-) SD of three parallel measurements.
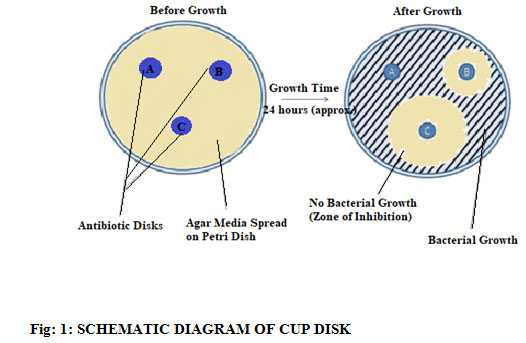 |
Figure 1: Schematic Diagram Of Cup Disk |
Cell Culture
Human cervical epithelial malignant carcinoma cell lines (HeLa) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat inactivated fetal calf serum (Gibco™, Thermo Fisher Scientific), penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere containing 5% CO2. HeLa cells at a concentration of 1.5×105 cells/mL were grown in a 25 cm2 flask of complete culture medium. At 85 % confluency HeLa cells were and trypsinized, and seeded on a 96 well tissue culture plate for overnight according to the selection of experiments.
Mtt Assay
Approximately 1 × 105 mL−1 HeLa cells in their exponential growth phase were seeded in a flat-bottomed 96-well Tissue culture plate for 24h at 37°C in a 5% CO2 incubator. Series of concentrations (5, 25, 50, 100, and 250 μg/mL) of ZnO and ZnO@SiO2 nanoparticles in the medium were added to the plate in a triplicate manner. Cytotoxicity evaluation of NiO and NiO@SiO2 nanoparticles was performed using MTT assay and MTT was added to each well and the plates incubated for 3 h in a dark chamber. 100 μl of DMSO was added to dissolve the formazan crystals and the absorbance read at 540 nm using ELISA reader (EPOCH, BIOTEK) (Zhu et al., 2001).) The % of survival was calculated using untreated cells as 100 %.
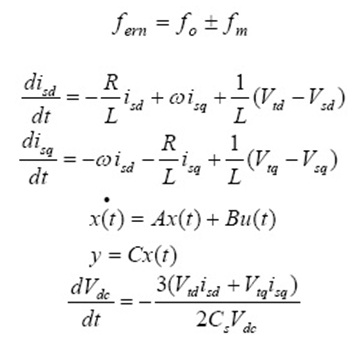
Where (A) test is the absorbance of the test sample and (B) control is the absorbance of the control sample. Non-treated cells were used as the control, and the samples were imaged using an inverted photomicroscope. The Values of MTT assay correspond to mean and standard deviations of three independent experiments.
Fluorescence Anisotropy
The fluorescence anisotropy of HeLa was assessed by the determination of TMA-DPH steady-state fluorescence polarization after the cell membrane exterior phospholipid layer permeation of the probe (Dowell, 2002; Pearson,1996; Pearson, et.al,2001, Shrivastava, et.al. 2007; Katona,2004; Lakowicz,2004; Hollan, 1996).
For the measurement of the changes in the TMA-DPH fluorescent properties following the membrane permeation, we added 2.5µM TMA-DPH to a 2 ml of cell in the measuring cuvette. The cell suspension with the fluorescent probe was incubated for 30 min at 37°C. The measurement has been done between excitation and emission state, 360 nm and 430 nm respectively.
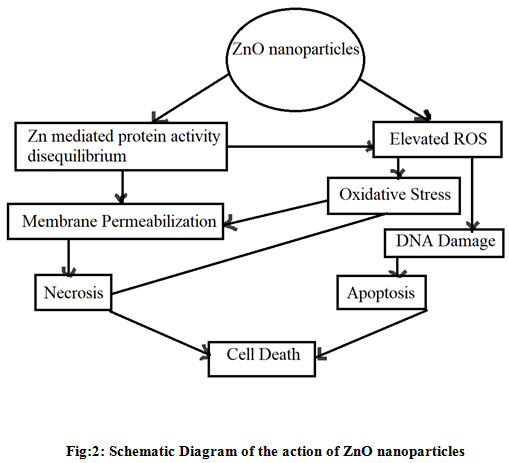 |
Figure 2: Schematic Diagram of the action of ZnO nanoparticles |
Ros Analysis
Membrane fluidity of cancer cells was shown to have a decisive role in the direct cell to cell contact and the modulation of the activity of membrane enzymes are to be affected by the increased release of reactive oxygen species (ROS) (Garden, 2001).
For the measurement of the intracellular ROS, DCF-DA was added to a 2 ml of HeLa suspensions. The cell suspension with DCF-DA was incubated for 60 min at 370 C in a dark condition. Cells without Nanoparticles were used as control. Fluorescence intensity was measured in a fluorescence spectrophotometer (model Hitachi, USA) at excitation and emission wavelengths of 504 and 529 nm, respectively.
Antioxidant Enzymes Activities
Superoxide dismutase (SOD) and Catalase (CAT) activities were measured by commercially available kits. The cells were seeded into 12- well plates at a concentration of 7×105 cells/well and all measurements were performed according to supplier’s recommendations.
Results and Discussion
The Zinc oxide Nano Particles have shown better effect than the Silica coated nano Particles. In some cases the Zinc oxide nanoparticles have shown good results in comparison to the known drugs which is a positive indication of using these nanoparticles as a potential antimicrobial drugs. We also checked their activity on the fungal growth and these particles have also shown a great effect on reducing the fungal growth. These nanoparticles can also be used to reduce the fungal growth and the contamination occurs from it. The results of the zone of inhibition in bacteria and the antibiotics have shown in table 1 and table 2 and the zone of inhibitions for fungi and available anti-fungal have shown in table 3 and table 4.
Table 1: Zone of Inhibition of Bacteria: (Values are mean +/- SD of three parallel measurements = Number of zones of inhibition.) (Concentration in µg/ml and Zone of inhibition in mm)
| Drug Name | Drug Concentration
(µg/ml) |
E. coli | S. typhi | S . aureus | S.pyrogenes |
|
ZnO |
5 | – | – | – | – |
| 25 | 13 | 12 | 14 | 12 | |
| 50 | 15 | 14 | 16 | 13 | |
| 100 | 17 | 15 | 19 | 17 | |
| 250 | 20 | 17 | 20 | 20 | |
|
ZnO@SiO2 |
5 | – | – | – | – |
| 25 | 8 | 9 | 7 | 6 | |
| 50 | 10 | 12 | 11 | 10 | |
| 100 | 13 | 13 | 14 | 12 | |
| 250 | 14 | 13 | 15 | 13 |
Table 2: Known drugs used for bacteria
| Drug Name | Drug | E.coli | S.typhi | S.aureus | S.pyrogens |
| Conc. | |||||
| (µg/m) | |||||
| Ampicillin | 5 | 13 | 14 | 11 | 10 |
| 25 | 15 | 19 | 14 | 13 | |
| 50 | 17 | 15 | 15 | 16 | |
| 100 | 18 | 17 | 19 | 18 | |
| 250 | 20 | 21 | 22 | 20 | |
| Norfloxacin | 5 | 23 | 19 | 18 | 20 |
| 25 | 25 | 20 | 20 | 22 | |
| 50 | 26 | 22 | 23 | 26 | |
| 100 | 28 | 25 | 24 | 28 | |
| 250 | 30 | 26 | 24 | 32 | |
| Amoxiline | 5 | 21 | 20 | 18 | 19 |
| 25 | 23 | 23 | 20 | 22 | |
| 50 | 25 | 24 | 23 | 23 | |
| 100 | 27 | 26 | 24 | 25 | |
| 250 | 28 | 29 | 26 | 28 | |
| Cifroflloxacin | 5 | 20 | 21 | 19 | 17 |
| 25 | 22 | 23 | 21 | 20 | |
| 50 | 26 | 24 | 23 | 20 | |
| 100 | 28 | 27 | 25 | 22 | |
| 250 | 30 | 29 | 28 | 25 |
Table 3: Anti Fungal Activity: Zone of Inhibition of Fungi: (Values are mean +/- SD of three parallel measurements = Number of zones of inhibition). (Concentration in µg/ml and Zone of inhibition in mm)
| Drug Name | Drug Concentration
(µg/ml) |
A. nigar | C. clavus | C.albicans |
|
ZnO |
5 | – | – | – |
| 25 | 14 | 17 | 15 | |
| 50 | 17 | 19 | 17 | |
| 100 | 19 | 20 | 18 | |
| 250 | 20 | 22 | 20 | |
|
ZnO@SiO2 |
5 | – | – | – |
| 25 | 8 | 7 | 5 | |
| 50 | 10 | 9 | 8 | |
| 100 | 11 | 10 | 10 | |
| 250 | 12 | 11 | 12 |
Table: 4: Known Drug Concentration
| Drug Name | Drug Concentration
(µg/ml) |
A. nigar | C. clavus | C.albicans |
|
Greseofluvin |
5 | 17 | 18 | 19 |
| 25 | 21 | 20 | 20 | |
| 50 | 22 | 23 | 21 | |
| 100 | 23 | 24 | 24 | |
| 250 | 27 | 28 | 26 | |
|
Nystitin |
5 | 19 | 17 | 18 |
| 25 | 20 | 21 | 20 | |
| 50 | 20 | 22 | 21 | |
| 100 | 23 | 24 | 23 | |
| 250 | 28 | 27 | 25 |
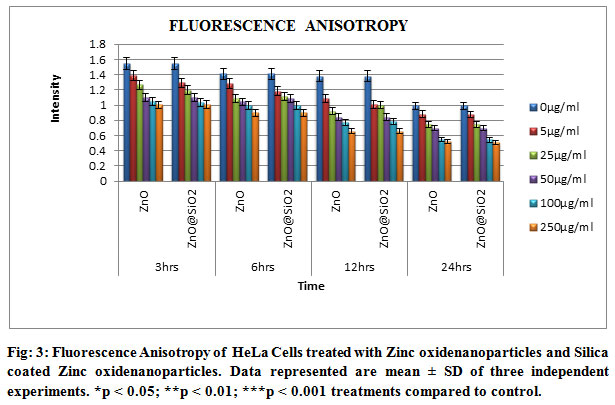
Fig 3 have shown the graph for the fluorescence anisotropy. These results have shown that the nanoparticles were interacted with the cellular membrane and dissociated the membrane proteins to enter into the cell and to interact with the cytoplasmic organelles. Thus these nanoparticles have interacted with the mitochondria and reduce the growth of the cancer cells.
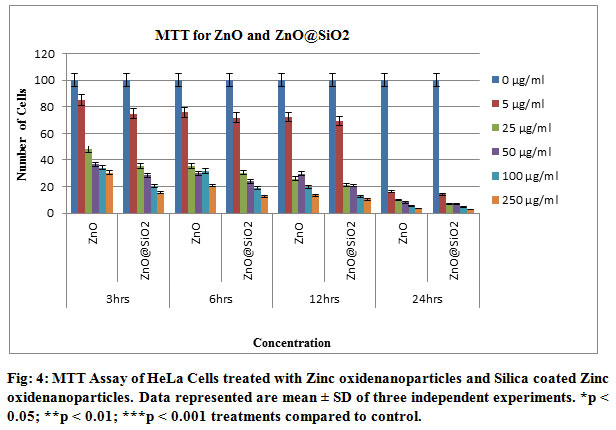
MTT assay was undertaken in order to evaluate the cell viability in cells stressed by Zinc oxide nanoparticles and Silica coated Zinc oxide nanoparticles. First we evaluated the effects of NiO Nanoparticles on HeLa cells viability. Incubation with 5µg/ml, 25µg/ml , 50µg/ml, 100µg/ml, 250µg/ml for 3, 6, 12 and 24 h resulted in a concentration dependent decrease in cell viability, the LC50 was 79.83 ± 0.856 μg/ml. Based on these results Zinc oxide nanoparticles and Silica coated Zinc Oxide nano particles at submaximal concentrations after 12 h, 50 and 100 μg/ml were selected in this study. The main findings of this assay are the LC50 of Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticles was 79.83 μg/ml and submaximal concentrations of 80 and 100 μg/ml were selected in this study. Similar results were obtained in previous findings demonstrated a dose dependent reduction of MTT-value in HeLa cells treated with Zinc oxide nano particles and Silica coated Zinc oxide nanoparticles, though cells were different ( Ahamed 2011 and Capasso, et.al. 2014).
According to the National Cancer Institute (USA), vegetables crude extracts are cytotoxic considered when their IC50 values are less than 30 μg/ml ( Da, et.al. 2013). After a large screening, Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticles (60 and 80 μg/ml) concentrations were selected due to their best actions. The present study agree with the results of Remila et al. (2015) who have demonstrated that pre-treatment of THP-1 cells with P. lentiscus extracts for 24 h strongly inhibited H2O2 damage, with maximum protection at 100 μg/ml ( Remila, et.al. 2015). The triplicate study of the cell culture has shown that the number of cells is decreasing by the increment of time and the concentration of the drugs respectively.
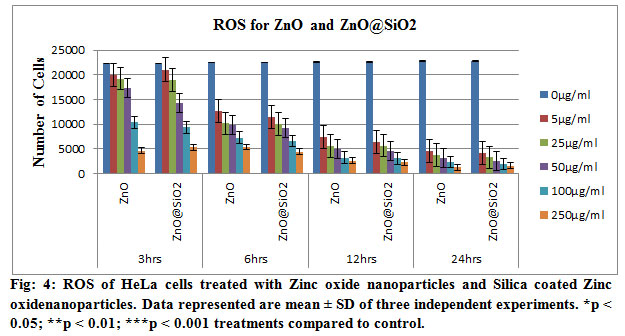
In order to investigate the effect of Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticles induced cytotoxicity mediated through ROS generation, HeLa cells were treated with the two selected concentrations of the Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticles. We detected a significant decrease of ROS level in cells treated with Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticles (Figs. 4 and 5). Oxidative stress, which is an imbalance between ROS production and the antioxidant systems favouring a ROS excess, has been identified as a common mechanism for cell damage. During oxidative stress, ROS are produced mainly from the mitochondrial electron transport chain. To minimize the damage induced by ROS, free radicals can be transformed to other less toxic molecules, for example, the superoxide anion is enzymatically converted into hydrogen peroxide by superoxide dismutase (SOD) and hydrogen peroxide may be enzymatically converted into water by catalase or glutathione peroxidase enzymes (Huerta-García et.al, 2014). Nanoparticles have been demonstrated to generate more free radicals and ROS than larger particles, likely due to their higher surface area (Sioutas, et.al, 2005). NiO Nanoparticles have been reported to reduce cell viability and to induce oxidative stress by depletion of glutathione and induction of reactive oxygen species in HEp-2 and MCF-7 cells (Siddiqui, et.al, 2013), cell death via apoptotic pathway and ROS generation in HepG2 cells in dose-dependent manner (Ahamed, et.al, 2012), Zinc oxide nano particles also increased intracellular ROS, apoptosis and necrosis in BEAS-2B and A549 cells ( Capasso, et.al, 2014).
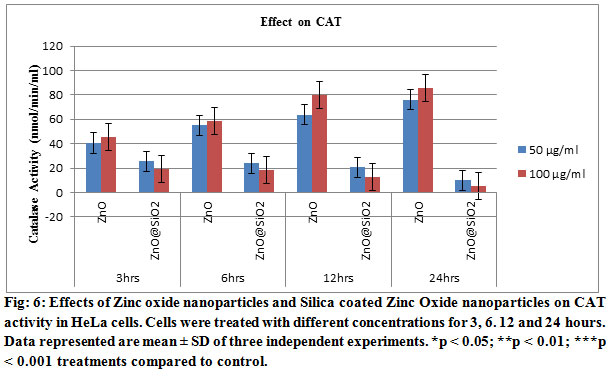
Our results confirmed that Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticlesare toxic to HeLa cells. In the Fig 6, ROS analysis has been shown in triplicate studies. These analyses showed that the requirement of the oxygen got low with the increase of time and concentration of the drug. These need of oxygen lead the cells to the apoptosis and thus the cell dies due to the treatment of the drugs. These have also coincided with the result of the MTT assay. With the increase of the concentration of the nanoparticles, the number of viable cells decreased. The nanoparticles showed the better result as the variation of the valance electron was increased as a result those reacted with the protein particles of the cells and dissociated it which leads the cells to destroy.
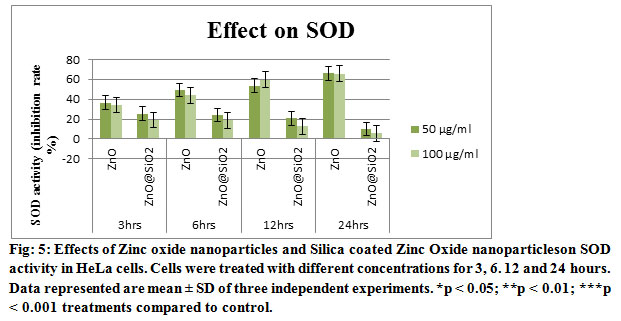
Pre-incubation of cells with both concentrations 50 and 100 μg/ml of Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticles led to enhance the antioxidant enzymes, SOD and CAT, activities shown in Figs. 5 and 6. Similarly, the Zinc oxide nanoparticles and Silica coated Zinc Oxide nanoparticles also induce a significant depletion of antioxidants. The accumulation of ROS, e.g. superoxide radicals (O2%) and hydroxyl free radicals (%OH) decrease the defensive effects of cellular antioxidant enzymes, e.g. SOD, CAT ( Li, et.al. 2012). Exposure of HT22 hippocampal cells to CuO Nanoparticles resulted decrease in the activity of SOD and the other detoxification enzymes which has been founded in this work (Niska, et.al. 2015).
Discussion
As per our research is concerned, we have found much more promising result on both anti microbial and anti cancer effect. We observed that the growth of both Gram-positive and Gram-negative bacteria was inhibited by increasing concentrations of ZnO NPs. We further explored the effect of the ZnO NPs on the cellular morphology (Hussain et.al, 2019).
The recent data of Karthikeyan et.al, (2019) have showed that the REM doped ZnO which is being costly but the procedure making of our doped particle is both cost effective and easy. Different review (Sadhukhan et.al, 2019; Kaushik et.al, 2019; Roy and Jong,2019; Laura et.al 2019; Marco et.al, 2019; Xiuting et.al,2019) of the articles lead us to do the experiments with different gram positive and gram negative bacteria and our material has shown effect in much more lower concentration both in the bacteria and cancer cells. Our Spectroscopic data analysis also confirmed the lower concentration effects on the both. This work will completely open the new era of personalized drug for each.
Conclusion
Nanoparticles in medicine are a new and emerging topic of interest for researchers. With all their promising characteristics, the in vivo application of nanoparticles is still rare and there is currently a serious lack of in vivo research into nanoparticles. Hence, a much better collaboration between clinicians, biologists and material scientists is required for the in-depth understanding of cancer biology and intelligent design of NPs for their better clinical use. This is in fact an achievable aim, considering the highly promising characteristics of ZnO Nanoparticles and their inherent nature of selectivity and toxicity towards cancer cells, making them unequivocally a key tool for next-generation cancer treatment. ZnO NP exhibited impressive antibacterial properties against different food borne pathogens as well as fungi and the inhibitory effects increased as the concentrations of ZnO nanoparticles increased. ZnO NP could distort bacterial cell membrane, leading to loss of intracellular components, and ultimately the death of cells. These results demonstrate that ZnO NP could be potentially considered as an effective antibacterial agent for protecting agricultural and food safety. Thus we have found that the ZnO can be a potential anti cancerous and antimicrobial drug for next generation of treatment.
References
Abercrombie M, Ambrose EJ.(1962) The surface properties of cancer cells: a review. Cancer Res. 22:525-48.
Afzal Hussain, Mohammad Oves, Mohamed F. Alajmi Iqbal Hussain, Samira Amir, Jahangeer Ahmed, Md Tabish Rehman, Hesham R. El-Seedi, and Imran Ali.(2019) Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv., 9, 15357
Ahamed, D. (2011) Toxic response of nickel nanoparticles in human lung epithelial A549 cells, Toxicol. In Vitro 25 930–936.
Ahamed, D. Ali, H.A. Alhadlaq, M.J. Akhtar (2013) Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2), Chemosphere 93 (2013) 2514–2522.
Boyce JM (1997) Epidemiology and prevention of nosocomial infections. The Staphylococci in Human Disease (Crossley KB Archer GL, eds), pp. 309–329. Churchill Livingstone, New York.
Brayner, R., Ferrari-Iliou, R., Brivois, N., Djediat, S., Benedetti, M.F. and Fievet, F. (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6, 866–870.
Capasso, M. Camatini, M. Gualtieri, (2014) Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells, Toxicol. Lett. 226 (2014) 28–34.
Da, MS. Gomide, F. de, O. Lemos, M.T.P. Lopes, T.M. de, A. Alves, L.F. Viccini, C.M. Coelho (2013) The effect of the essential oils from five different Lippia species on the viability of tumor cell lines, Braz. J. Pharmacogn. 23 895–902.
Degen A, Kosec M. (2000) Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. Journal of the European Ceramic Society. 20(6):667-73.
Desselberger U (2017) Emerging and re-emerging infectious diseases. J Infect 40: 3–15.
Dowell, F. E., Pearson, T. C., Maghirang, E. B., Xie, F., Wicklow, D. T. (2002). Reflectance and transmittance spectroscopy applied to detecting fumonisin in single corn kernels infected with Fusarium verticillioides. Cereal Chem. 79:222–226.
Dutta, B.; Bose, N.; Kar, E.; Das, S.; Mukherjee, S. (2016) Smart, lightweight, flexible NiO/poly (vinylidene flouride) nanocomposites film with significantly enhanced dielectric, piezoelectric and EMI shielding properties. J. Polym. Res. 24, 220.
Dutta, B.; Kar, E.; Bose, N.; Mukherjee, S. (2015) Significant enhancement of the electroactive β phase of PVDF by incorporating hydrothermally synthesized copper oxide nanoparticles. RSC Adv. 5, 105422−105434.
Fu, G., Vary, P.S. and Lin, C.T. (2005) Anatase TiO2 nanocomposites for antimicrobial coatings. J Phys Chem B 109,8889–8898.
Garden, S. R., Strachan, N. J. C. (2001). Novel colorimetric immunoassay for the detection of aflatoxin B1. Anal. Chim. Acta 444:187–191.
Garima, S., Bhavesh, R., Kasariya, K. R., Sharma, A. R and Singh, R. P., (2011), Biosynthesis of Silver nanoparticles using Ocimum sanctum (Tulasi) leaf extract and screening its antimicrobial activity. J. Nanoparticle. Res., 13(7): 2981–2988.
Gunjan Bisht and Sagar Rayamajhi (2016) ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine, 3:9 | doi: 10.5772/63437
Hancook LE Gilmore MS (2000) Pathogenicity of Enterococci. Gram-Positive Pathogens (Fischetti VA Novick RP Ferretti JJ Portnoy DA Rood JI, eds), pp. 251–258. ASM Press, Washington, DC.
Hiremath, S., Vidya, C., Antonyraj, M. A. L., Chandraprabha, M. N., Gandhi, P., Jain, A. and Anand, K., (2013), Biosynthesis of ZnO nano particles assisted by Euphorbia tirucalli (Pencil Cactus). Int. J. Curr. Eng. Technol., (1): 176-179.
Hollan, S. (1996) Membrane fluidity of blood cells. Haematologica (Budapest) 27, 109– 127.
Huerta-García, E J.A. Pérez-Arizti, S.G. Márquez-Ramírez, N.L. Delgado-Buenrostro, Y.I. Chirino, G.G. Iglesias, R. López-Marure (2014) Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells, Free Radic. Biol. Med. 73C 84–94.
Karthikeyan, A. Jafar Ahamed, Karthikeyan P, Vijaya Kumar (2019) Enhancement of antibacterial and anticancer properties of pure and REM doped ZnO nanoparticles synthesized using Gymnema sylvestre leaves extract. SN Applied Sciences. April 2019
Katona, E., Katona, G. et al. (2004) : Drug-Induced Membrane Effects in Metabolically Impaired and Nonimpaired Human T (Jurkat) Lymphoblastoid Cells. Romanian J. Biophys., 14, 29–36.
Kaushik R.Niranjan Ramar Thangam Balaraman Madhan et.al. (2019) Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Applied Surface Science Volume 479, 15 June 2019, Pages 1169-1177
Lakowicz, J. R. (2004) Principles of Fluorescence Spectroscopy, 2nd edition, Springer Science and Business Media Inc., 2004, pp. 298–299.
Laura Valenzuela Ana Iglesias Marisol Faraldos et.al. (2019) Antimicrobial surfaces with self-cleaning properties functionalized by photocatalytic ZnO electrosprayed coatings. Journal of Hazardous Materials. Volume 369, 5 Pages 665-673
Li. A, L. Han, C.C. Han (2012) Antioxidant and neuroprotective activities of essential oil, isolated from Chinese herb pairs of Angelica sinensis and Sophora flavescens, J. Appl. Pharm. Sci. 2 1–4.
Lou, X. Development of ZnO series ceramic semiconductor gas sensors. J. Sens. Trans. Technol. 1991, 3, 1‒5.
Lowy F (1998) Staphylococcus aureus infections. N Engl J Med 339: 520–532.
Marco Antonio Reyes-Torres Esmeralda Mendoza-Mendoza Ángela Merari Miranda-Hernández et.al. (2019) Synthesis of CuO and ZnO nanoparticles by a novel green route: Antimicrobial activity, cytotoxic effects and their synergism with ampicillin. Ceramics International. Available online 21 August 2019.
McNeil SE. (2009) Nanoparticle therapeutics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol.1(3):264-71.
Niska, M.J. Santos-Martinez, M.W. Radomski, I. Inkielewicz-Stepniak, (2015) CuO nanoparticles induce apoptosis by impairing the antioxidant defense and detoxification systems in the mouse hippocampal HT22 cell line: protective effect of crocetin, Toxicol. In Vitro 29 663–671.
Orel V, Shevchenko A, Romanov A, Tselepi M, Mitrelias T, Barnes CH (2015) Magnetic properties and antitumor effect of nanocomplexes of iron oxide and doxorubicin. Nanomedicine. 11(1):47-55.
Pearson, T. (1996). Machine vision system for automated detection of stained pistachio nuts. Lebensmitelw. U. Technol. 29:203–209.
Pearson, T., Wicklow, D. T., Maghirang, E. B., Xie, F., Dowell, F. E. (2001). Detecting aflatoxin in single corn kernels by transmittance and reflectance spectroscopy. Trans. ASAE 44:1247–1254.
Rasmussen JW, Martinez E, Louka P, Wingett DG. (2010) Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 7(9):1063-77
Ravindra, P. S., Shukla, V.K., Raghvendra, S. Y., Sharma, P. K., Singh, P. K. and Pandey, A.C., (2011) Biological approach of Zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett.,2(4): 313-317.
Ravindran, C. P.,Manokari, M. and Shekhawat, M. S., (2016), Biogenic production of Zinc oxide nanoparticles from aqueous extracts of Duranta erecta L. World. Sci. News., 28: 30-40.
Reddy, K.M., Feris, K., Bell, J., Wingett, D.G., Hanley, C. and Punnoose, A. (2007) Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett 90, 213902.
Remila, S. D. Atmani-Kilani, S. Delemasure, J.L. Connat, L. Azib, T. Richard, D. Atmani (2015) Antioxidant cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts, Eur. J. Integr. Med. 7 274–286.
Sadhukhan P Mousumi Kundu Shallu Rana Raj Kumar Joydeep Das Parames C. Sil.(2019) Microwave induced synthesis of ZnO nanorods and their efficacy as a drug carrier with profound anticancer and antibacterial properties. Toxicology Reports Volume 6 Pages 176-185
Sawai, J. (2003) Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods 54, 177–182.
Segets, D.; Gradl, J.; Taylor, R.K.; Vassilev, V.; Peukert, W. (2009) Analysis of optical absorbance spectra for the determination of ZnO nanoparticle size distribution, solubility, and surface energy. ACS Nano 3, 1703–1710.
Senthilkumar, S. R. and Sivakumar, T., (2014) Green Tea (Camellia sinensis) Mediated synthesis of Zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci., 6(6): 461-465.
Shrivastava, S., Chattopadhyay, A.(2007): Influence of cholesterol and ergosterol on membrane dynamics using different fluorescent reporter probes. Biochem. Biophys. Res. Commun., 356, 705–10.
Siddiqui, M. Ahamed, J. Ahmad, M.A. Majeed Khan, J. Musarrat, A.A. Al- Khedhairy, S.A. Alrokayan,(2012) Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin, Food Chem. Toxicol. 50 641–647.
Sioutas, R.J. Delfino, M. Singh, (2005) Exposure assessment for atmospheric Ultrafine Particles (UFPs) and implications in epidemiologic research, Environ. Health Perspect. 113 947–955.
Sivakumar, J., Premkumar, C., Santhanam, P. and Saraswathi, N., (2011), Biosynthesis of Silver nanoparticles using Calotropis gigantean leaf. Afr. J. Basic. Appl. Sci., 3(6): 265-270.
Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62.
Stoimenov, P.K., Klinger, R.L., Marchin, G.L. and Klabunde, K.J. (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18, 6679–6686.
Swarup Roy and Jong-Whan Rhim (2019) Carrageenan-based antimicrobial bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocolloids Volume 90, May 2019, Pages 500-507
Thill, A., Zeyons, O., Spalla, O., Chauvat, F., Rose, J., Auffan, M. and Flank, A.M. (2006) Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol 40, 6151– 6156.
Vandenesch F Naimi T Enright MC et al. (2003) Community-acquired methicillin- resistant Staphylococcus aureus carrying Panton–Valentine leukocidin genes. Emerg Infect Dis 9: 978–984.
Vidya, C., Hiremath, S., Chandraprabha, M. N., Antonyraj, L.M.A., Gopal, I.V., Jain, A. and Bansal, K., (2013) Green synthesis of ZnO nanoparticles by Calotropis gigantea. Int. J. Curr. Eng. Technol., 1: 118-120.
Vinardell M, Mitjans M.(2015) Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials. 5(2):1004.
Wang R, Billone PS, Mullett WM. (2013)Nanomedicine in Action: An Overview of Cancer Nanomedicine on the Market and in Clinical Trials. Journal of Nanomaterials. 2013:12.
Xiuting Hu Xue Jia ChaohuiZhi Zhengyu Jin Ming Miao. (2019) Improving the properties of starch-based antimicrobial composite films using ZnO-chitosan nanoparticles. Carbohydrate Polymers. Volume 210, 15 Pages 204-209
Zhang, L.L., Jiang, Y.H., Ding, Y.L., Povey, M. and York, D. (2007) Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res 9, 479–489.
Zhu Z N. Wei, H. Liu, and Z. He, (2011) Microwave-assisted hydrothermal synthesis of Ni(OH)2 architectures and their in-situ thermal convention to NiO, Advanced Powder Technology, vol. 22, no. 3, pp. 422–426.