Kuban State Technological University, Krasnodar, Russia.
Article Publishing History
Received: 19/09/2021
Accepted After Revision:
The work is devoted to a comparative study of the features of the structural and mechanical properties of soybean lecithins, produced in Russia, in their relationship with the composition and other characteristics. Considering that the cultivation of transgenic agricultural crops is prohibited in Russia, Russian plant raw materials and products of its processing, including lecithin, are of interest and are in demand in a number of European countries. Despite the fact that the main raspberry raw material in Russia is sunflower, the volume of processing of soybeans grown without the use of genetic modification methods, the main plantations of which are located in the Far East, ranks second after sunflower.
Lecithin production technologies in Russia are mostly focused on the production of so-called “raw” liquid lecithins and do not provide for the operation of their subsequent conditioning in order to ensure special characteristics of the composition and consumer properties. Despite this, raw soy lecithins produced by Russian enterprises mostly meet the requirements of GOST 32052-2013 and the European Union E 322 requirements and can be positioned as standard liquid lecithins. At the same time, during the release of such lecithins, problems associated with an increase in their viscosity during storage are periodically observed. This article presents the results of comparative studies of the composition and properties of liquid soybean lecithins of various consistencies and presents conclusions on the factors that determine the features of their structural and mechanical properties.
Chemical Composition, Lecithin, Phospholipids, Rheological Properties and Viscosity.
Butina E, Gerasimenko E, Kalmanovich S, Bugaets I, Dubrovskaya I, Iljinova S, Kopytova N, Sonin S. Comparative Rheological Properties of Soy Lecithins Produced in Russia. Biosc.Biotech.Res.Comm. 2021;14(4).
Butina E, Gerasimenko E, Kalmanovich S, Bugaets I, Dubrovskaya I, Iljinova S, Kopytova N, Sonin S. Comparative Rheological Properties of Soy Lecithins Produced in Russia. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/31vI3mF”>https://bit.ly/31vI3mFeg</a>
Copyright © Butina et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Lecithins are one of the most demanded food additives obtained as a secondary product in the processing of oilseeds (Shahidi 2005; Considine and Kulik 2006; Hernandez and Quezada 2008; Nieuwenhuyzen and Tomás 2008; Hoogevest et al. 2014; Ahmad and Xu 2015). Lecithins available on the modern market differ in the type of feedstock and in the specific composition and technological and functional properties formed as a result of special technological methods for processing raw lecithin obtained in the process of degumming vegetable oils (Joshi et al. 2006; Nieuwenhuyzen and Tomás 2008; Ahmad and Xu 2015).
Another difference in lecithins, the importance of which has increased recently, is the specificity of the origin of the feedstock: with the use of GMOs (genetically modified organisms) or without GMOs (Heiser 2016). The European market prefers lecithin obtained from non-GMO raw materials (Cabezas et al. 2012; Ahmad and Xu 2015; Schneider 2019). This lecithin is the basis for the production of organic lecithin, the market for which has been actively growing over the past ten years (Cabezas et al. 2012; Ahmad and Xu 2015).
Considering that the cultivation of transgenic agricultural crops is prohibited in Russia (State Duma of the Federal Assembly of the Russian Federation 1997), Russian plant raw materials and products of processing, including lecithin, are of interest and are in demand in certain European countries (Fedorova 2019; Sinegovsky 2019).
Lecithin production technologies in Russia are mostly focused on the production of so-called “raw” liquid lecithins and do not provide for the operation of their subsequent conditioning in order to ensure special characteristics of the composition and consumer properties. Despite this, raw soybean lecithins produced by Russian enterprises typically comply with the requirements of GOST (State standard of Russian Federation) 32052 and the European Union E 322 requirements and, therefore, can be positioned as standard liquid lecithins.
At the same time, problems associated with an increase in their viscosity during storage are periodically observed. According to the results of some studies, the lecithin viscosity is a complex function of the content of the acetone insoluble matter, moisture, minerals, and acid value (Shahidi 2005; Nieuwenhuyzen and Tomás 2008; Ahmad and Xu 2015; Heiser 2016; Sinegovsky 2019).
As a general rule, the higher the content of acetone insoluble matter and moisture, the higher the level of the viscosity of liquid lecithins, and, on the contrary, a high acid value leads to a decrease in the viscosity (Ahmad and Xu 2015). However, in scientific publications, the results of comparative studies of the composition and properties of raw lecithins of different viscosity are not sufficiently presented, which does not allow for the prediction of the quality of finished products of raw lecithins by monitoring the incoming raw materials. This work is devoted to the study of these aspects.
MATERIAL AND METHODS
Samples of raw liquid soybean lecithins produced at Russian enterprises from Russian raw materials were used as the objects of research. In appearance, samples 1 and 2 were a homogeneous viscous liquid, and samples 3 and 4 were in the form of a plastic mass. The rest of the organoleptic characteristics of the lecithin samples corresponded to the established requirements: color—from yellow to brown; smell and taste—typical for soy lecithin.
The determination of the organoleptic parameters, as well as the physicochemical properties, including the content of the toluene insoluble matter and acetone insoluble matter, content of moisture and volatile substances, acid value, peroxide value, color of 10% solution in toluene, and viscosity at 25 ° C, was performed in accordance with the control methods regulated by GOST 32052 and corresponding international standards (Federal Agency for Technical Regulation and Metrology 2013).
The determination of the rheological characteristics of lecithins was conducted using a Brookfield rotary viscometer, model LVDV-II + Pro, spindle LV4 (code 64). The composition of fatty acid acyls of the phospholipid complex was determined according to ISO 12966 (European Committee for Standardization, 2015) using a gas chromatograph “Kristall-5000” (ZAO SKB Khromatek Russia), column SOLGEL-WAX 30 m × 0.32 mm ID SOLGEL- WAX × 0.5 µm). The group composition of phospholipids was investigated by high performance liquid chromatography (HPLC) using an “Agilent 1260 Infinity” high performance liquid chromatograph (Agilent Technology, USA), a LiChrospher 100 250×4 mm column, Diol (5 µm).
This analysis was in accordance with the methods given in the previous studies, as well as by thin layer chromatography (TLC) with subsequent processing of chromatograms by scanning densitometry (Paronyan and Scriabin 2007; Deutsche Forschungsgemeinschaft (DFG) 2007; The American Oil Chemists’ Society 2017). When implementing the TLC method, we used high-performance “Sorbfil” plates with a silica gel particle size distribution of 8–12 µm.
The solvent system chloroform/methanol/water (65:25:4) was used as the mobile phase. For the development of chromatograms, a solution of phosphoric-molybdic acid (PMA) in ethyl alcohol with a concentration of 5% was used. The spots obtained were identified using taps and specific tests for individual phospholipid groups. The content of unsaponifiable substances was determined according to the method of (Paronyan and Scriabin 2007).
The composition of unsaponifiable substances was determined by gas chromatography–mass spectrometry using a “Kristall 5000 MS” gas chromatograph and the NIST (National Institute of Standards and Technology, USA) library. The content and composition of tocopherols was determined in accordance with EN 12822 (Federal Agency for Technical Regulation and Metrology, 2015). The content and composition of sterols were determined according to ISO 12228-1 using a “Kristall 5000” gas chromatograph.
The content of polyvalent metal ions was determined using an atomic absorption spectrometer “GTA 120 240FS AA” (Agilent Technologies) with flame and electrothermal atomization, USA. The studies were performed on the equipment of the Research Center of Food and Chemical Technologies of the Federal State Budgetary Educational Institution of Higher Education “Kuban State Technological University” (European Committee for Standardization, 2014).
RESULTS AND DISCUSSION
The results of the study of the main physical and chemical characteristics of lecithin samples, typically normalized for lecithins by domestic and foreign standards, are presented in Table 1.
Table 1. Physicochemical indicators of lecithins
| Indicator | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Requirements of GOST (State standard of Russian Federation) 32052 | Requirements
European Union E 322 |
| acetone insoluble matter,% | 60.1 | 62.25 | 61.47 | 61.13 | No less than
60.0 |
No less than
60.0 |
| toluene insoluble matter,% | 0.30 | 0.27 | 0.19 | 0.16 | No more than
0.30 |
No more than
0.30 |
| moisture and volatile substances,% | 0.19 | 0.19 | 0.11 | 0.31 | No more than 1.0 | No more than 2.0 |
| Acid value,
mg KOH/g |
25.1 | 26.4 | 18,7 | 16.9 | No more than 36.0 | No more than 35.0 |
| Peroxide value
meq/kg |
less than 0.1 | 1,2 | 1.0 | 1.5 | No more than 10.0 | No more than 10.0 |
| Color of a 10% solution in toluene: | ||||||
| mg I2 | 79 | 58 | 58 | 53 | No more than 80.0 | – |
| units Gardner scale | 11.9 | 11.0 | 11.0 | 10.9 | Not standardized | – |
| Viscosity at 25 оС, Pa * s | 6.9 | 9.3 | Over 2000 | No more than 12.0 | – | |
The analysis of the physicochemical indicators presented in Table 1 shows that, with the exception of the viscosity at 25 °C, all lecithin samples met the requirements, regulated by GOST 32052 and the European Union for the quality of liquid lecithins. The quality of lecithin, characterized by the above indicators, is formed, as a rule, under the influence of technological factors. The acetone insoluble matter characterizes the content in lecithin of the main groups of polar lipids, such as phospholipids and glycolipids, as well as carbohydrates (Van Nieuwenhuyzen and Tomás, 2008).
Phospholipids typically make up about 45%, glycolipids—11%, and carbohydrates—4% (Nieuwenhuyzen and Tomás 2008; Ahmad and Xu 2015). Thus, differences between the studied lecithin samples in the values of physicochemical indicators (Table 1) do not allow us to conclude the reasons for the differences in the consistency and viscosity of liquid samples 1 and 2 and plastic samples 3 and 4.
Along with the considered indicators, an important indicator of the quality of lecithins is the acid value, which characterizes both the content of free fatty acids that have passed into the phospholipid emulsion during degumming in the composition of neutral lipids and the actual content of acidic forms of phospholipids. Research has demonstrated that the acid value of lecithins correlates with the level of their viscosity (Nieuwenhuyzen and Tomás 2008; Van Nieuwenhuyzen 2014).
All other things being equal, lecithins with a higher acid value have a lower viscosity than lecithins with a lower acid value. For example, according to early scientific publications, the acid value of liquid commercial lecithins was 24.7–34.4 mg KOH/g, in contrast to plastic samples characterized by an acid value of 21.9–22.9 mg KOH/g (Pardun 1964; Ahmad and Xu 2015; Sinegovsky 2019).
The results of determining the acid value in the analyzed lecithin samples confirm the above position, namely, the acid value of the samples of soybean lecithins No. 3 and 4, having a plastic consistency, ranged from 16.9 to 18.7 mg KOH/g, which is significantly lower (in 1.4 times on average) than the acid value of liquid samples of soybean lecithins No. 1 and 2, which ranged from 24.4 to 26.4 mg KOH/g. To reduce the viscosity of lecithins, foreign companies often use the addition of distilled fatty acids to lecithins, if the value of the acetone insoluble matter allows.
This operation is combined with the dilution of lecithins with refined deodorized vegetable oil (Yaron and Letan 1975; Nieuwenhuyzen and Tomás 2008; Nieuwenhuyzen 2014). To determine the content of free fatty acids in lecithin samples, the acid value of their acetone miscella was analyzed in accordance with the recommendations (Shahidi 2005; Nieuwenhuyzen and Tomás 2008; Sinegovsky 2019). The results are shown in Table 2.
Table 2. Acid value of lecithins
| Lecithin sample | Acid value, mg KOH/g | ||
| general | due to the presence of free fatty acids | due to the presence of acidic forms of phospholipids | |
| Sample 1 | 24.4 | 5.2 | 19.2 |
| Sample 2 | 26.4 | 7.1 | 19.3 |
| Sample 3 | 18,7 | 2.4 | 16.3 |
| Sample 4 | 18,6 | 2.1 | 16.6 |
As can be seen from the presented data, the difference between the acid value of the samples of soy lecithins No. 1 and 2 and No. 3 and 4 is, on average, 2.8 mg KOH/g, which corresponds to the content of free fatty acids in terms of oleic acid, which is 1.4%. According to the literature, the addition of 2% oleic acid to lecithins results in a decrease in viscosity by half on average. Considering that the difference in the viscosity of liquid (No. 1, 2) and plastic (No. 3, 4) samples of soy lecithins is, on average, more than 250 times, it is not possible to explain this solely by the influence of a larger amount of free fatty acids (Pardun 1964; Yaron and Letan 1975; Sinegovsky 2019).
Lecithins are structured non-Newtonian systems, the structural and rheological properties of which are determined by the interactions between their constituent components, which are phospholipids, neutral lipids, and minor accompanying substances, including carbohydrates and unsaponifiable lipids. The peculiarities of interactions between phospholipids depend on their group and fatty acid composition, as well as on their chemical composition, since phospholipids in unrefined oils can be represented both by individual molecules (in the form of associates) and complex compounds with unsaponifiable lipids and polyvalent metal ions (Arutyunyan and Kornena 1986; Sinegovsky 2019).
Considering this, at the next stage of research, we studied the group composition of phospholipids, the composition of fatty acids, the composition of the accompanying minor components and polyvalent metal ions present in the lecithin samples. The results of the study of the group composition of phospholipids are presented in Table 3.
Table 3. Group composition of phospholipids
The name of the phospholipid groupThe content of the phospholipid group, % of the totalSample 1Sample 2Sample 3Sample 4Phosphatidylinositols (PI)21.619.913.214.4Phosphatidylcholines (PC)24.721.024.323.1Phosphatidylethanolamines (PEA)26.527.231.529.5Phosphatidic and polyphosphatidic acids (PA and PPA)15.315.413.513.2Di- and phosphatidylglycerols (DPG and PG)12.016.417.519.8
From the presented data, the samples of liquid lecithins No. 1 and 2 differ from the samples of plastic lecithins No. 3 and 4 by a higher content of phosphatidylinositols and a lower content of phosphatidylethanolamines. At the same time, phosphatidylethanolamines are the predominant group of phospholipids in the samples of plastic soy lecithins. Considering that phosphatidylinositols have pronounced acidic properties, the data obtained are consistent with the revealed differences in the acid value values of liquid and plastic samples of soy lecithins. The results of studying the composition of fatty acid acyls of lecithin samples, as well as the acetone-insoluble polar lipids included in their composition, are presented in Tables 4 and 5 (Arutyunyan and Kornena 1986; Fedorova 2019).
Table 4. Composition of fatty acids in the studied lecithin samples
| Fatty acid name | Fatty acid content, % of the total | |||
| Sample 1 | Sample 2 | Sample 3 | Sample 5 | |
| C16: 0 Palmitic | 18.58 | 17.83 | 20.94 | 20.76 |
| C18: 0 Stearic | 4.50 | 4.04 | 4.40 | 4.12 |
| C18: 1 Oleic | 14.47 | 14.80 | 9.49 | 10.08 |
| C18: 2 Linoleic | 54.84 | 51.39 | 48.70 | 54.93 |
| C18: 3 Linolenic | 5.67 | 5.52 | 7.38 | 8.11 |
| C22: 0 Behenic | 2.04 | 6.42 | 9.09 | 2.01 |
| sum of saturated | 25.02 | 28.28 | 34.43 | 26.89 |
| sum of polyunsaturated | 60.51 | 56.91 | 56.08 | 63.04 |
Table 5. Composition of fatty acids of acetone-insoluble polar lipids in the studied lecithin samples
| Fatty acid name | Fatty acid content, % of the total | |||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| C16: 0 Palmitic | 22.01 | 22.51 | 20.46 | 21.19 |
| C18: 0 Stearic | 4.97 | 4.32 | 3.93 | 3.03 |
| C18: 1 Oleic | 10.56 | 10.17 | 9.69 | 7.86 |
| C18: 2 Linoleic | 54.11 | 52.72 | 57.68 | 55.94 |
| C18: 3 Linolenic | 6.18 | 5.49 | 7.60 | 9.59 |
| C22: 0 Behenic | 2.17 | 4.79 | 0.64 | 2.38 |
| sum of saturated | 29.15 | 31.62 | 25.03 | 26,60 |
| sum of polyunsaturated | 60.29 | 58.21 | 65.28 | 65.53 |
Our analysis of the data presented in Table 5 showed that the acetone-insoluble polar lipids that are part of the studied lecithin samples contained fatty acid acyls characteristic of the corresponding types of vegetable oils. At the same time, no regularities in the differences in the compositions of fatty acid acyls for liquid and plastic soy lecithins were revealed.
Among the fatty acid acyls of the acetone-insoluble polar lipids included in the samples of plastic soy lecithins No. 3 and 4, a slightly higher content of polyunsaturated fatty acids was observed; however, this difference is not decisive. Nevertheless, this difference is consistent with the data on the group composition of phospholipids (Table 3), namely, with the predominance of phosphatidylethanolamines in the composition of plastic samples of soy lecithins, which, along with phosphatidylcholines, are characterized by the highest degree of unsaturation of their constituent acyls of fatty acids. At the same time saturated fatty acids predominate in the composition of phosphatidylinositols (Arutyunyan and Kornena 1986; Fedorova 2019).
The results of the study of the minor accompanying substances of lecithins are presented in Table 6. From the presented data, significant differences between the studied lecithins were seen in the content of tocopherols and polyvalent metal ions. The content of tocopherols correlated with the content of polyunsaturated fatty acids in the lecithin samples (Table 4) and generally corresponded to the composition and content of tocopherols in the feedstock.
Table 6. The composition of the minor accompanying substances of lecithins
| Indicator | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
| Mass fraction of unsaponifiable lipids,% | 1.79 | 0.57 | 1.12 | 0.88 |
| including: | ||||
| Aliphatic alcohols | 0.09 | 0.02 | 0.48 | 0.09 |
| Squalene | 0.010 | 0.005 | 0.010 | 0.011 |
| Sterols | 0.287 | 0.325 | 0.342 | 0.377 |
| including: | ||||
| campesterol | 0.067 | 0.099 | 0.108 | 0.118 |
| stigmasterol | 0.068 | 0.082 | 0.094 | 0.103 |
| β-sitosterol | 0.152 | 0.144 | 0.140 | 0.156 |
| Content of tocopherols, mg/100 g, including: |
60.04
|
75.45
|
93.32
|
94.77
|
| α-tocopherol | 20.22 | 7.44 | 11.25 | 9.85 |
| β + γ -tocopherol | 60.04 | 68.01 | 82.07 | 84.92 |
| δ-tocopherol | Lack of | Lack of | Lack of | Lack of |
| Carbohydrate content,% | 8.4 | 5.9 | 7,7 | 7.4 |
| Content of ions of polyvalent metals, mg/kg: | ||||
| Cu | 0.57 | 0.07 | 0.22 | 0.08 |
| Fe | 27.40 | 9.87 | Less than 0.1 | Less than 0.1 |
| Pb | Less than 0.01 | Less than 0.01 | 0.09 | Less than 0.01 |
| Zn | 11.09 | Less than 0.1 | Less than 0.1 | 1.79 |
In the samples of liquid lecithins No. 1 and 2, there was a significantly greater number of polyvalent metals, compared to the samples of plastic soy lecithins No. 3 and 4. There was an almost complete absence of Fe (less than the detection limit of the method) in the samples of plastic lecithins No. 3 and 4; however, in the samples of liquid lecithins, this metal was predominant. These data are in part consistent with the data on the phospholipid group composition of the studied lecithin samples (Tables 3 and 4), according to which, in liquid lecithin samples 1 and 2, the content of the acidic forms of phospholipids, including phosphatidylinositols and phosphatidic and polyphosphatidic acids, formed stable complex compounds with polyvalent metals, significantly more than in the plastic samples of lecithins No. 3 and 4. Differences in the phospholipid group composition cannot explain the almost complete absence of Fe in the samples of plastic soy lecithins No. 3 and 4, although acidic groups of phospholipids present in a smaller amount in the composition of these samples of lecithins, can form stable complexes with Fe (Arutyunyan and Kornena 1986; Bogdanor 1988; Smitss et al. 1988; Ahmad and Xu 2015).
Salts of polyvalent, predominantly divalent metals are used in foreign technologies to reduce the viscosity of standard liquid lecithins (Van Nieuwenhuyzen and Tomás, 2008; Van Nieuwenhuyzen, 2014; Ahmad and Xu, 2015). This technological method is based on the ability of the polar groups of phospholipids to interact with ions of polyvalent metals and to form complex compounds, which provides screening of the polar groups of phospholipid molecules and leads to a decrease in the interaction energy and the ability to form micelles.
The reason for the low content of polyvalent metal ions in the composition of lecithins can be both the characteristics of the raw materials used and the use of special technological methods, consisting in the preliminary treatment of unrefined oil with complex-forming agents that form stable complexes with the polyvalent metals (Bogdanor 1988; Smitss et al. 1988; Ahmad and Xu 2015; Fedorova 2019).
The purpose of such treatment is typically to increase the degree of degumming of phospholipids and to increase the yield of lecithin, although other goals may be pursued, to achieve the desired functional properties of the lecithins (Bogdanor 1988; Smitss et al. 1988; Ahmad and Xu 2015).
Thus, the low content of polyvalent metal ions in the composition of soybean plastic lecithin samples No. 3 and 4 may be one of the reasons for their high viscosity. Analysis of the composition of other related substances in the lecithin samples (Table 7) showed the following. In the composition of lecithins of plant origin, carbohydrates are found in both free and bound forms (Arutyunyan and Kornena 1986). Bound carbohydrates are present both in the composition of glycolipids and in the form of structural elements of phospholipid molecules.
The presence of free carbohydrates is mainly explained by the formation of mixed associates as a result of the appearance of intermolecular hydrogen bonds between the P = O, P-OH, and NH2 groups of phospholipid molecules and the C = O, C-OH groups of carbohydrate molecules. According to Arutyunyan and Kornena (1986), phospholipids isolated from unrefined vegetable oils may contain free carbohydrates in an amount from 4% to 12% by weight of the extracted phospholipid complex (Arutyunyan and Kornena 1986; Ahmad and Xu 2015; Fedorova 2019).
Table 7. Effective viscosity of the studied lecithin samples
| Shear rate, s-1 | Effective viscosity value, Pa‧s | |||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| 0.5 | 8.4 | 10.2 | ||
| 1.0 | 7.2 | 9.6 | Over 2000 | Over
2000 |
| 2.0 | 6.9 | 9.3 | ||
| 2.5 | 7.0 | 9.3 | ||
| 4.0 | 6.9 | 9.3 | ||
| 5.0 | 6.8 | 9.2 | ||
| 10.0 | 6.8 | 9.1 | ||
| 20.0 | 6,7 | 9.1 | ||
| 50.0 | 6,7 | 9.1 | ||
The content of carbohydrates in the studied samples of soy lecithins generally corresponded to the literature data, while no correlation dependences with their consistency were revealed. A similar conclusion can be drawn with regard to the quantitative and qualitative composition of the unsaponifiable lipids present in the lecithin samples under study (Arutyunyan and Kornena 1986; Ahmad and Xu 2015).
At the next stage of research, the structural and rheological properties of lecithin samples were studied using the method of rotational viscometry. Lecithins with a content of acetone insoluble matter of about 60% are pseudoplastic non-Newtonian systems. Along with the effective viscosity, the structural and rheological properties of non-Newtonian systems are determined by a number of other rheological characteristics, the most complete representation of which is provided by the rheological flow curves with their subsequent mathematical approximation (Evdokimov and Eliseev 2005; Kuznetsov et al. 2005; Sinegovsky 2019).
The rheological flow curves were performed on the basis of experimental data obtained by investigating the samples on a Brookfield LVDV-II + Pro rotary viscometer. Samples of the studied lecithins were placed in a thermostated cell and, after exposure at a predetermined temperature for 30 minutes, a measurement was performed. The range of variation of the shear rate was from 0.5 to 50 s-1. The results of measurements carried out at 25 ° C are presented in Table 7. As can be seen from the presented data, the viscosity of the samples of plastic lecithins No. 3 and 4 exceeded the upper limit of the measuring range of the device, which is 2000 Pa‧s, at all shear rates.
The rheological curves of the flow of samples of liquid lecithins No. 1 and 2 are shown in Figures 1 and 2. The analysis of Figures 1 and 2 showed that the rheological flow curves of lecithin samples No. 1 and 2 are typical for structured non-Newtonian systems and, therefore, are typical for standard liquid lecithins, which are dispersed systems by their nature with a characteristic macro- and microstructure. The rheological flow curves are described by the Ostwald-de-Ville power equation, which is typical for the flow of pseudoplastic non-Newtonian food systems (Kuznetsov et al. 2005; Sinegovsky 2019).
Figure 1: Rheological flow curves of the lecithin sample No 1 at 25 оC
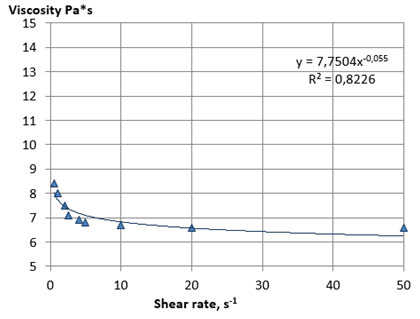
Figure 2: Rheological flow curves of the sample lecithin No 2 at 25 оС
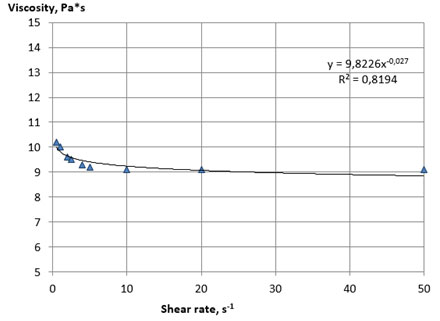
A fairly rapid decrease in viscosity occurred with an increase in mechanical impacts, due to the processes of destruction of the initial structure of the system and the orientation of the particles of the dispersed phase in the direction of flow, which provides a decrease in the resistance of the system to the displacement of layers relative to each other. The viscosity practically does not change after reaching the limiting shear rates, and the rheological flow curves take the form characteristic for Newtonian systems. The main rheological characteristics of the lecithin samples No. 1 and 2, calculated from the flow curves, are presented in Table 8.
Table 8. Rheological properties of lecithins (Samples 1 and 2)
| Indicator | Indicator value | |
| Sample 1 | Sample 2 | |
| Effective viscosity at minimum shear rate (system with intact structure), Pa‧s |
8.4 |
10.2 |
| Effective (dynamic) viscosity of a system with an extremely destroyed structure, Pa ∙ s |
6,7 |
9.1 |
| Structural destruction coefficient | 1,2 | 1.1 |
Samples of lecithins No. 1 and 2 were characterized by close values of the coefficient of destruction of the structure, which indicates a similar nature of their structural organization and plastic strength of the structure. The viscosity of lecithins, like other similar non-Newtonian systems, decreases with increasing temperature. Considering this, we investigated the effect of temperature on the structural and mechanical properties of the plastic samples of lecithins No. 3 and 4. The experiment was carried out by thermostating the samples in the measuring cell of a viscometer with a sequential increase in temperature with steps of 1 ° C until the viscosity value reached below the upper limit of the measurement range of the device used (less 2000 Pa*s).
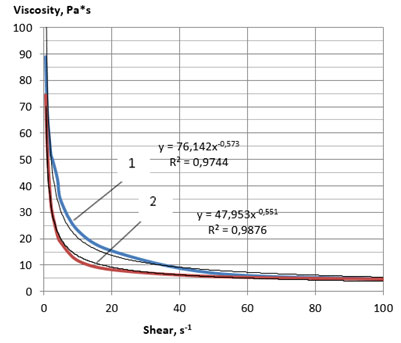
We found that the temperature that ensured the attainment of a viscosity value of less than 2000 Pa*s was 59 oC for sample 3 and 52 oC for sample 4. To obtain comparable data, rheological flow curves were built at a temperature of 60 ° C, which made it possible to carry out measurements for both samples of plastic lecithins (Figures 3, 4). At a temperature of 60 °C, the values of the effective viscosity of the samples of liquid lecithins 1 and 2 were no longer a function of the shear rate and took similar values to 1.5 Pa s. The main rheological characteristics of the plastic lecithin samples, calculated from the flow curves, are presented in Table 9.
Table 9. Rheological properties of lecithins (Samples 3 and 4)
| Indicator | Indicator value | |
| Sample 3 | Sample 4 | |
| Effective viscosity at the minimum shear rate (system with intact structure, direct measurement), Pa‧s |
371 |
88.8 |
| Effective (dynamic) viscosity of a system with an extremely destroyed structure, Pa ∙ s |
2.9 |
4.6 |
| Structural destruction coefficient | 128 | 19 |
| Effective viscosity at the minimum shear rate (reverse measurement), Pa‧s |
76.8 |
74.4 |
Comparative analysis of the obtained flow curves (Figures 1, 2 and 3, 4) showed that the samples of soy plastic lecithins No. 3 and 4 had a stronger structure even at a temperature of 60 °C compared with the samples 1 and 2 at a temperature of 25 °C. Samples 3 and 4 also demonstrated thixotropic properties, since, with a decrease in the intensity of mechanical shear action, they demonstrated the ability to partially restore the broken bonds between structural components.
Figure 3: Rheological flow curves of the lecithin sample No. 3 at 60 °C:
1—direct measurement, 2—reverse measurement
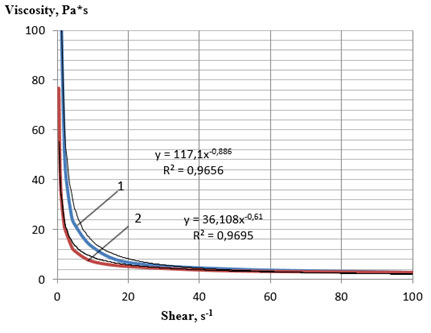
Figure 4: Rheological flow curves of the lecithin sample No. 4 at 60 °C:
1—direct measurement, 2—reverse measurement
The high strength of the coagulation structure and indication of thixotropic properties for the plastic samples of soy lecithins No. 3 and 4 can be explained on the basis of the following concepts.
Being surfactants, phospholipid molecules in a non-polar substance (oil) interact with each other through polar groups. Such interactions are based on the electrostatic nature, in contrast to the entropic nature of the interaction of hydrocarbon radicals in aqueous substances (Mittela, 1980). According to the theory of stepwise surfactant aggregation in a non-polar substance, the energy gain from replacing the polar group-carbon interaction with the interaction between polar groups is so significant that, in a non-polar substance, even at low surfactant concentrations (10-7–10-6 mol/dm3), the latter form stable aggregates on a small scale, called “premicellar” (Sinegovsky 2019).
Thus, phospholipids, being surfactants, in a non-polar substance are predominantly present not in the form of individual molecules, but in the form of associates and micelles of various scales, which undergo a number of structural transformations during degumming and subsequent drying. As a result of such transformations, the resulting liquid lecithin becomes a dispersed system with a coagulation structure and consists of micellar aggregates formed by phospholipids and other polar lipids. Such aggregates interact with each other through interlayers of a dispersion medium consisting of neutral lipids (oil) (Sinegovsky 2019).
The more screened the polar groups, the lower the relative polarity of the surfactant and, accordingly, the lower the scale of the formed micelles and the lower the energy of intermicellar interactions. The described mechanism underlies the technological method of reducing the viscosity of lecithins by adding bivalent metal ions to the phospholipid emulsion before drying, for example, in the form of calcium chloride (Nieuwenhuyzen and Tomás 2008; Ahmad and Xu 2015).
A decrease in the energy of intermicellar interaction can also be provided by an increase in the thickness of the interlayer of the dispersion medium, or an increase in its relative polarity. In practice, such effects are achieved by adding refined and deodorized oil or distilled fatty acids to lecithin (Yaron and Letan 1975; Nieuwenhuyzen and Tomás 2008; Ahmad and Xu 2015). Thus, an increase in the viscosity of lecithins during storage at a normal level of the mass fraction of moisture and volatile substances (no more than 1%) and, in the absence of intensive oxidative processes, may be due to the following reasons:
– a high content of insoluble acetone substances, and, accordingly, a low content of neutral lipids, which does not ensure the creation of layers of a dispersion medium of sufficient thickness and leads to an enlargement of micelles and an increase in the degree of their interaction during storage;
– a high relative polarity of phospholipid molecules, leading to the formation of a developed coagulation structure with a high energy of interaction of micellar aggregates, which, during storage, leads to their enlargement and, as a consequence, may be accompanied by a partial separation of the dispersion medium.
Thus, summarizing the results obtained, we can conclude that the main reason for the increase in viscosity and the formation of a plastic consistency of the samples of soy lecithins No. 3 and 4 is the formation of a developed coagulation structure consisting of micellar aggregates interconnected by strong electrostatic and hydrogen bonds, as a result of the high amount of phospholipids with active polar groups in lecithins at a low content of free fatty acids, which can reduce the activity of such interactions through a “wedging” action.
Soybean liquid lecithins, characterized by similar values of the indicators acetone insoluble matter, moisture and volatile substances, toluene insoluble matter, peroxide value, and color differed significantly in consistency from liquid fluid to viscous plastic. Samples of soy lecithins with a plastic consistency were characterized by a lower acid value of no more than 19 mg KOH/g. Comparative analysis of the group composition of phospholipids of the studied samples of soybean lecithins showed that the samples of lecithins with low viscosity differed from the samples of plastic lecithins by a higher content of phosphatidylinositols and a lower content of phosphatidylethanolamines.
At the same time, phosphatidylethanolamines were the predominant group of phospholipids in the samples of plastic soy lecithins. Acetone-insoluble polar lipids, which were part of the studied lecithin samples, contained fatty acid acyls, characteristic of the corresponding types of vegetable oils. At the same time, no regularities in the differences in the compositions of fatty acid acyls for liquid and plastic soy lecithins were revealed.
Samples of low viscosity liquid lecithins contained significantly higher amounts of polyvalent metals compared to the samples of plastic soy lecithins. There was almost a complete absence of Fe (less than the detection limit of the method) in the samples of plastic lecithins, while, in the samples of liquid lecithins, this metal was predominant. The content of carbohydrates and unsaponifiable lipids in the studied samples of soy lecithins generally corresponded to the literature data, and no correlations with their consistency were revealed.
The samples of soy lecithins with a plastic consistency had a stronger structure at a temperature of 60 °C than the samples of lecithins with a low viscosity at a temperature of 25 °C. At the same time, the samples of plastic lecithins had thixotropic properties, as, with a decrease in the intensity of mechanical shear action, they demonstrated the ability to partially restore broken bonds between structural components (Schneider 2019).
CONCLUSION
The findings of the present study suggest that the main reason for the increase in viscosity and the formation of a plastic consistency of the samples of soybean lecithins No. 3 and 4 was the formation of a developed coagulation structure, consisting of micellar aggregates interconnected by strong electrostatic and hydrogen bonds, as a result of the predominance of phospholipids with active polar groups in the lecithins at low contents of free fatty acids, which can reduce the activity of such interactions through the “wedging” action.
ACKNOWLEDGEMENTS
This study was financially supported by the Russian Science Foundation, project no. 21-16-0053. Moreover, the research was carried out using the equipment of the Research Center of Food and Chemical Technologies of KubSTU (CKP_3331).
REFERENCES
Ahmad, MU and Xu, X (2015). Polar lipids. Biology, chemistry, and technology AOCS Press.
Arutyunyan, NS and Kornena, EP (1986). Phospholipids of vegetable oils Moscow: Agropromizdat, Page 256.
Bogdanor, JM (1988). The effect of acid pretreatment of phospholipid removal. Journal of the American Chemical Society Vol 65 No 4 Page 512.
Cabezas, DM, Madoery, R, Diehl, BWK, et al. (2012). Emulsifying properties of different modified sunflower lecithins. Journal of The American Oil Chemists Society Vol 89 Pages 355-361. http://dx.doi.org/10.1007/s11746-011-1915-8
Considine, GD and Kulik, PH (2006). Van Nostrand’s scientific encyclopedia John Wiley and Sons.
Deutsche Forschungsgemeinschaft (DFG) [German research foundation] (2007) Einheitsmethode F-1 6a (07).
European Committee for Standardization (2014). ISO 12228-1: 2014 Determination of individual and total sterols by gas chromatography – Part 1: animal and vegetable fats and oils. ISO Switzerland.
European Committee for Standardization (2015). ISO 12966 Animal and vegetable fats and oils — Gas chromatography of fatty acid methyl esters. ISO Switzerland.
Evdokimov, IN and Eliseev, NYu (2005). Molecular mechanisms of liquid and gas viscosity. Basic concepts. RGU Russia.
Federal Agency for Technical Regulation and Metrology (2013). GOST 32052-2013 Food additives. Lecithins E322. General technical conditions Standartinform Russia.
Federal Agency for Technical Regulation and Metrology (2015). EN 12822-2014 Foodstuffs – determination of vitamin e by high performance liquid chromatography – measurement of alpha-, beta-, gamma- and delta- tocopherols. Standartinform Russia.
Fedorova, EB (2019). Russian lecithin market: 20 years of changes. In V International business forum WORLD SOYA, International Scientific and Practical Conference Functional phospholipids in the food and pharmaceutical industry St. Petersburg Russia (16).
Heiser, J (2016). Lecithin the natural and powerful substance. In 15th International Conference Phospholipids: New Opportunities in Technology, Analytical Chemistry and Applications Krasnodar Russia.
Hernandez, E, and Quezada, N (2008). In Phospholipids technology and applications, Uses of phospholipids as functional Ingredients (Edited by F.D. Gunstone) The Oily Press Scotland Pages 83–94.
Hoogevest, P, Van Prusseit, B and Wajda, R (2014). Phospholipids: natural functional ingredients and actives for cosmetic products. Inform Vol 25 No 3 Pages 182-188.
Joshi, A, Paratkarb, SG and Thorat, BN (2006). Modification of lecithin by physical, chemical and enzymatic methods. European Journal of Lipid Science and Technology Vol 108 Pages 363-373. http://dx.doi.org/10.1002/ejlt.200600016
Kuznetsov, OA, Voloshin, EV and Sagitov, RF (2005). Rheology of food masses GOU OSU Russia.
Mittela, K (1980). Micelle formation, solubilization and microemulsions KL Mitell Mir Russia.
Nieuwenhuyzen, WV (2014). The changing world of lecithins. INFORM Vol 25 No 4 Pages 254-259.
Nieuwenhuyzen, WV and Tomás, MC (2008). Update on vegetable lecithin and phospholipid technologies. European Journal of Lipid Science and Technology Vol 110 Pages 472-486. http://dx.doi.org/10.1002/ejlt.200800041
Pardun, H (1964). Analytical methods of qualitative evaluation of soybean lecithin. Fette, Seifen, Anstrichmittel Vol 66 Page 467.
Paronyan, VKh and Scriabin, NM (2007). Analytical control and assessment of the quality of fat and oil products DeLi print Russia.
Schneider, M (2019). Lecithin and phospholipids yesterday – today – tomorrow. In V International Business Forum World Soy St. Petersburg Russia May 22-23 2019.
Shahidi, А (2005). Bailey’s Industrial Oil and Fat Products, 6(6) Set John Wiley and Sons.
Sinegovsky, MO (2019). Problems and prospects of soybean production in the Far Eastern Federal District. In V International Business Forum WORLD SOYA St. Petersburg Russia May 22-23 2019.
Smitss, A, Kakuda, Y and MacDonald, BE (1988). Effect of degamming reagents on the recovery and nature of lecithins from crude canola, soybean and sunflower oils. Journal of the American Chemical Society Vol 65 No 7 Pages 1151-1156. https://doi.org/10.1007/bf02660572
State Duma of the Federal Assembly of the Russian Federation (1997). Federal Law of the Russian Federation of 17.12.1997 No. 149-FZ On seed production as amended on 03.07.2016. Available at: http://www.kremlin.ru/acts/bank/11763
The American Oil Chemists’ Society (2017). In Official Methods and Recommended Practices of the AOCS, Determination of Lecithin Phospholipids by HPLC – AOCS Official Method Ja 7b-91 AOCS USA.
Yaron, A and Letan, A (1975). Consistency of commercial soybean lecithins. Journal of Texture Studies Vol 6 No 4 Pages 541-548. https://doi.org/10.1111/j.1745-4603.1975.tb01427.x


