1Department of Biotechnology, D.D.U Gorakhpur University, Gorakhpur, Uttar Pradesh- 273 009, INDIA
2Environmental Biotechnology and Genomics Division, CSIR-National Environmental Engineering Research
Institute (NEERI), Nehru Marg, Nagpur-440 020, INDIA
3College of Agriculture, Department of Biotechnology, S.V.P university of Agriculture and Technology, Meerut 250 110, INDIA
Corresponding author email: dinesh_yad@rediffmail.com
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 14/09/2020
Development of effective regeneration protocol is a prerequisite for genetic transformation of pigeonpea owing to its recalcitrance behavior in tissue culture conditions. Screening of cultivars is considered to be one important factor for investigating the regeneration ability under in vitro conditions. Selected eleven Indian cultivars of pigeonpea were studied for multiple shoot bud induction and regeneration using apical meristem explants. The response of these cultivars under the influence of variable concentration of three different hormones namely 6-benzyl amino purine (BAP), kinetin (KIN) and thiadiazuron (TDZ) was investigated. BAP was found to be better compared to kinetin and TDZ for in vitro regeneration of these cultivars. It was observed that higher concentration of BAP was effective for multiple shoot bud induction and IPA-242 was promising revealing a maximum of 7 buds per explants at 3.0 mgL-1 of BAP. Similarly IPA-204 showed best response under the influence of different concentration of TDZ and a maximum of 10 buds per explants was observed at 0.30 mgL-1 of TDZ.
The overall response of these cultivars under different concentration of kinetin was poor though IPA-2013 was found to be best with 4 buds per explants at 3.0 mgL-1 of kinetin. The rooting of the shoots derived from the apical meristem explants was found to be better when treated with 1-NaphthaleneAcetic Acid (NAA) as compared to Indole-3 Acetic Acid (IAA) and Indole-3 Butyric Acid (IBA). Further it was observed that 0.2mgL-1 of NAA worked best for most of the cultivars for rooting as evident from number of primary roots. The screening of these cultivars of pigeonepea for in vitro regeneration ability exclusively from apical meristem explants has widened the scope of developing efficient regeneration and genetic transformation protocols.
Apical Meristem, Multiple Shoot Bud Induction, Pigeonpea, Hormones, Regeneration
Kashyap V, Sarangi B. K, Yadav M. K, Yadav D. Comparative Assessment of Selected Indian Cultivars of Pigeonpea (Cajanus cajan L. Millsp) for in vitro Regeneration Using Apical Meristem Explants. Biosc.Biotech.Res.Comm. 2020;13(3).
Kashyap V, Sarangi B. K, Yadav M. K, Yadav D. Comparative Assessment of Selected Indian Cultivars of Pigeonpea (Cajanus cajan L. Millsp) for in vitro Regeneration Using Apical Meristem Explants. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2FlOb5v
Copyright © Kashyap et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Pigeonpea[Cajanuscajan (L.) Millspaugh]is an important protein rich grain legume predominately grown in Indian subcontinent, South East-Asia and East Africa, the genome of which has been sequenced (Singh et al. 2012 and Varshney et al. 2012). The crop productivity is hindered due to several constraints like limited genetic resources, low level of genetic diversity, plethora of biotic and abiotic stresses (Bohra et al. 2010). Conventional plant breeding, molecular breeding and genomic assisted breeding approaches are being used for used legume crop improvement (Pratap et al. 2018; Bohra et al. 2020).The identification of genes associated with desirable agronomic traits in pigeonpea is comparatively easier due to the availability of genome sequence and could be used for transgenic production. Still the availability of efficient and reproducible in-vitro regeneration protocol is lacking in pigeon pea and other legumes in general as these are considered to be recalcitrant to in-vitro regeneration under tissue culture conditions (Chandra and Pantel 2003; Pratap et al. 2018).
Substantial efforts have been made to develop efficient Agrobacterium-mediated genetic transformation and transgenic pigeonpea production (Geetha et al. 1999, Lawrence and Koundal 2001, Satyavathi et al. 2003, Prasad et al. 2004, Surekha et al. 2005; Sharma et al.2006; Surekha et al. 2014; Ghosh et al. 2017; Karmakar et al. 2019).In pigeonpea in-vitro regeneration via organogenesis using different explants like leaf, cotyledons, cotyledonary nodes, embryonal axes, leaf petiole, embryo, embryonal axis attached cotyledons, auxillary buds and apical meristem among different cultivars has been extensively reviewed (Krishna et al. 2010 and Pawar et al. 2014). Leaf tissues were predominately used as explants source for in vitro regeneration of pigeonpea (Eapen and George 1993, Singh et al. 2002, Dayal et al. 2003, Kashyap et al. 2011, Asande et al. 2016, Abhijeeta and Rajesh, 2018).
Other explants source like cotyledons and cotyledonary nodes (Banala et al. 2016 and Jasani et al. 2017), embryonal axes (Raut et al. 2015), leaf petiole (Nalluri and Karri 2017), embryonal axis attached cotyledons (Karmakar et al. 2019) and auxiliary bud (Vijay Kumar et al. 2016; Kumar et al. 2016) have also been recently reported for in vitro regeneration of pigeonepea with different cultivars. There are only few reports of apical meristem as explants source for direct organogenesis (Kumar et al. 1984; Cheema and Bawa 1991; Franklin et al. 1998 and Parekh et al.2014) attempted with cultivars AL 15, ICP 6917, ICP 6974, ICP 7119, ICP 7263, Vamban, one wild and GT 102 (Karmakar et al. 2019).
Genotype dependent varying regeneration responses have been reported in pigeonpea using variable explants sources, though apical meristem has not been extensively studied. The screening of more cultivars for direct organogenesis exclusively for apical meristem explants needs to be attempted for evaluating the variability in the in vitro regeneration efficiency. Based on the literature survey an attempt has been made to evaluate eleven selected Indian cultivars of pigeonpea for multiple shoot bud induction and regeneration. The effects of variable concentration of growth regulators BAP, Kinetin and TDZ for multiple shoot bud formation among these cultivars were also assessed to reveal genotype dependent variability (Karmakar et al. 2019).
MATERIAL AND METHODS
The eleven cultivars of pigeonpea procured from ICAR- Indian Institute of Pulses Research, Kanpur were IPA-2013, IPA-3088, Pusa-9, IPA-34, IPA-204, IPA-242, T-7, IPA-61, IPA-337, IPA-341 and IPA-98-3 and were used the present study. The seeds prior to germination were surface sterilized using 1% cetrimide solution, 70% ethanol and 0.2% HgCl2 as reported earlier (Kashyap et al. 2011; Kashyap et al. 2014) The apical meristem explants of approximately 1.0 cm size were excised aseptically from 10 day germinated seedlings. The standard MS culture medium (Murashige and Skoog 1962) with variable concentration of growth hormones BAP, Kinetin and TDZ was used for multiple shoot bud induction and regeneration studies .
The explants with or without shoot initials were sub cultured repeatedly after 15 days. Numbers of shoot buds were counted after 30 days of inoculation. For each experimental set up 10 explants were used with each concentration and experiment was repeated twice. After each successive subculture within 15 days, the well-developed shoots were rooted on MS media with different concentration of NAA, IAA and IBA. The explants with or without shoot initials were sub cultured repeatedly after 15 days. Numbers of shoot buds were counted after 30 days of inoculation. For each experimental set up 10 explants were used with each concentration and experiment was repeated twice. After each successive subculture within 15 days, the well-developed shoots were rooted on MS media with different concentration of NAA, IAA and IBA. The culture conditions of cool white fluorescent light at 25±20C with 16 hours light and 8 hour dark interval was maintained in plant tissue culture lab.
RESULTS AND DISCUSSION
Genetic transformation has immense potential for legume crop improvement but due to the lack of efficient regeneration methods, limited success has been achieved (Pratap et al. 2018). Plant regeneration through organogenesis has been preferred in pigeonpea genetic transformation and several efforts have been made to investigate the factors influencing in-vitro regeneration using different cultivars. In-vitro regeneration by organogenesis of pigeonpea has been attempted using diverse explants like leaf, cotyledons, cotyledonary nodes, embryonal axes, leaf petiole, embryo, epicotyls, embryonal axis attached cotyledons, auxiliary buds and apical meristem with more than fifty diverse cultivars (Krishna et al. 2010, Pawar et al. 2014 and Pratap et al. 2018). Several factors like genotype selection, explants tissues, media composition, and plant growth regulators substantially influence the plantlet regeneration via organogenesis in legumes that is amenable to efficient genetic transformation (Krishna et al. 2010, Pawar et al. 2014 and Pratap et al. 2018).
Screening of diverse genotypes or cultivars is considered to be the major factor for deciphering the inherent regeneration potential via organogenesis (Chandra Venkata et al. 2019; Bohra et al. 2020). More than fifty pigeonpea genotypes have been studied for in vitro regeneration both via organogenesis and somatic embryogenesis to reveal the inherent regeneration ability (Krishna et al. 2010). In the present study selected eleven Indian cultivars of pigeonopea were assessed for regeneration via organogenesis using apical meristem explants under influence of variable concentration of growth regulators namely BAP, Kinetin and TDZ as reported with leaf and plumule junction explants (Kashyap et al. 2011 and Kashyap et al. 2014).
These selected Indian cultivars of pigeonpea when subjected to variable concentration of BAP hormone ranging from 0.5-4.0 mgL-1 revealed variability in regeneration ability as evident from number of buds per explants as shown in Table-1. The cultivar IPA-242 showed best response with a maximum of 7 buds per explants in the presence of MS media supplemented with 3.0 mgL-1 BAP. The response of cultivars IPA-2013, IPA-2014 and IPA-61 was also comparatively better at higher concentration of BAP (Kashyap et al. 2014).
Table 1. Effect of BAP on multiple shoot bud induction using apical meristem explants (number of shoots / explant) for eleven cultivars of pigeon pea after 4 weeks of culture with an average of 10 replicates and means with different letters differ significantly at p=0.05.
| BAP (mgL-1) → |
0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| Cultivars↓ | Number of shoots (Mean±S.D.) | |||||||
| IPA-2013 | 1.7±0.4a | 1.7±0.4a | 3.3±0.6b | 2.4±0.4a | 3.1±1.2b | 4.4±0.6ab | 4.4±1.3b | 3.9±1.5b |
| IPA-3088 | 3.5±0.5b | 3.7±1.0b | 4.4±0.4b | 5.9±3.0b | 5.3±0.6b | 3.9±0.9b | 4.3±1.1b | 4.7±2.7b |
| Pusa-9 | 1.9±0.7a | 2.6±1.2a | 1.0±0.0a | 3.3±0.7a | 1.0±0.0a | 1.3±0.4a | 3.5±1.5b | 4.7±0.4ab |
| IPA-34 | 2.7±0.7b | 1.0±0.0a | 2.8±0.9b | 1.0±0.0a | 2.2±0.4a | 3.0±0.0b | 3.8±0.6ab | 3.8±1.8b |
| IPA-204 | 1.0±0.0a | 3.0±0.0b | 4.3±0.4b | 3.5±0.5b | 1.5±0.5a | 4.6±1.9ab | 3.7±0.45b | 1.7±0.8b |
| IPA-242 | 1.0±0.0a | 1.2±0.4a | 1.0±0.0a | 1.9±0.3a | 1.9±1.1a | 6.2±0.6a | 1.4±0.7a | 3.7±0.4a |
| T-7 | 1.0±0.0a | 1.4±0.9a | 1.4±0.8a | 1.6±1.2a | 2.0±0.8a | 2.3±1.1a | 2.6±1.2b | 4.7±1.0ab |
| IPA-61 | 1.0±0.0a | 1.0±0.0a | 5.7±0.9ab | 1.0±0.0a | 3.2±2.0b | 4.6±0.9b | 4.9±1.4b | 3.8±0.6a |
| IPA-337 | 1.0±0.0a | 1.0±0.0a | 3.4±0.6ab | 1.0±0.0a | 3.1±0.3b | 1.0±0.0a | 1.1±0.3a | 1.0±0.0a |
| IPA-341 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
| IPA-98-3 | 1.0±0.0a | 3.5±0.5b | 1.0±0.0a | 1.0±0.0a | 3.3±0.4b | 1.0±0.0a | 3.2±0.4b | 4.1±0.6ab |
Overall higher concentration of BAP was found to be better for direct organogenesis as reported earlier irrespective of explants used (Krishna et al. 2010). The shoot bud induction for all the eleven cultivars with their best responsive concentration of BAP is shown in Figure-1(a-k). Mulitple shoot bud induction and regeneration exclusively in the presence of BAP has earlier been reported for cultivars ICP 6917, ICP6974, ICP 7119, ICP 7263 Vamban and one wild species (Kumar et al. 1984 and Franklin et al. 1998). A total of 12 numbers of maximum shoots has been reported from apical meristem explants in the presence of BAP (Franklin et al. 1998).
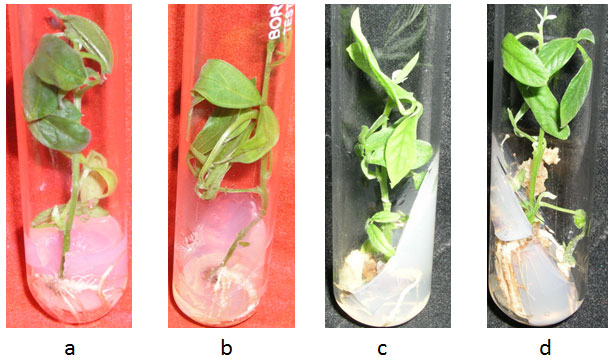
Figure 1: Multiple shoot bud induction from apical meristem explants of different cultivars of pigeonpea showing their best response in MS media supplemented with variable concentration of BAP (in mgL-1). (a)IPA-2013 (3.0), (b)IPA-3088 (2.0), (c)Pusa-9 (4.0), (d)IPA-34 ( 3.5), (e)IPA-204(3.0), (f)IPA-242(3.0), (g)T-7 (4.0), (h)IPA-61(1.5), (i)IPA-337 (1.5), (j)IPA-341 (1.0), (k)IPA-98-3 (4.0).
The response of these cultivars was also evaluated in the presence of different concentration of TDZ ranging from 0.05-0.4 mgL-1 (Table-2). The response of cultivar IPA-204 was found to be best with 0.30 mgL-1 of TDZ resulting in a maximum of 10 buds per explants. To the best of our knowledge there are no reports of in vitro multiple shoot bud induction and regeneration from apical meristem of pigeonpea in the presence of TDZ (Krishna et al. 2010).The concentration of TDZ in the range of 0.25-0.30 mgL-1 was found to be effective for shoot bud induction for these cultivars of pigeonpea. In case of cultivars IPA-242, IPA-337, IPA-341 and IPA-98-3 only single bud was observed irrespective of different concentration of TDZ used.
Table 2. Effect of TDZ on multiple shoot bud induction using apical meristem explants (number of shoots / explant) for eleven cultivars of pigeon pea after 4 weeks of culture with an average of 10 replicates and means with different letters differ significantly at p=0.05.
| TDZ
(mgL-1)→ |
0.05 | 0.1 | 0.15 | 0.20 | 0.25 | 0.30 | 0.35 | 0.40 |
| Cultivars↓ | Number of shoots (Mean±S.D.) | |||||||
| IPA-2013 | 2.9±0.3a | 1.0±0.0a | 3.0±0.0a | 3.0±0.0a | 3.0±0.0a | 2.0±0.0b | 2.0±0.0b | 4.0±0.0ab |
| IPA-3088 | 3.0±0.0a | 1.0±0.0a | 3.1±0.3a | 3.2±0.4a | 6.1±0.5a | 4.7±0.4a | 4.6±0.4a | 4.5±0.5a |
| Pusa-9 | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 2.8±0.4a | 4.5±0.9a | 1.0±0.0a |
| IPA-34 | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 3.6±0.4a |
| IPA-204 | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 7.4±1.1a | 1.0±0.0a | 1.0±0.0a |
| IPA-242 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
| T-7 | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 3.7±0.4a |
| IPA-61 | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 1.0±0.0a | 3.1±0.3a | 1.0±0.0a |
| IPA-337 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
| IPA-341 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
| IPA-98-3 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
Figure 2: Multiple shoot bud induction from apical meristem explants of different cultivars of pigeonpea showing their best response in MS media supplemented with variable concentration of TDZ (in mgL-1). (a)IPA-2013 (0.4), (b)IPA-3088 (0.25), (c)Pusa-9 (0.35), (d)IPA-34 (0.40), (e)IPA-204(0.30), (f)IPA-242(0.15), (g)T-7 (0.40), (h)IPA-61(0.35), (i)IPA-337 (0.25), (j)IPA-341 (0.05), (k)IPA-98-3 (0.15).
Similarly when these cultivars were subjected to different concentration of kinetin ranging from 0.5-4.0 mgL-1, they showed variability in terms of multiple shoot bud induction and cultivar IPA-2013 showed best response with a maximum of 5 buds per explants with 3.0 mgL-1 kinetin. It was also observed that many of the cultivars like IPA-204, IPA-242, T7, IPA-61, IPA-337, IPA-341 and IPA-98-3 showed no response for multiple shoot bud induction under different concentration of kinetin. In general, higher concentration of kinetin was found to be effective for shoot bud induction for most of the cultivars.
Similar studies has been perfomed with cultivar AL-15subjected to different concentration of kinetin ranging from 0.1- 9.0 mgL-1. The lower concentration in the range of 0.5-3.0 mgL-1 was found to be better resulting in healthy shoots while higher concentration resulted in the formation of clustersalong with BAP (Cheema and Bawa 1991). Among these three hormones tested, BAP was found to be comparatively better as compared to kinetin and TDZ for in vitro multiple shoot bud induction and regeneration as reported earlier (Kumar et al. 1984, Cheema and Bawa 1991 and Franklin et al. 1998).
Comparative assessment of BAP, Kinetin and TDZ either singly or in combination for multiple shoot bud induction attempted for a genotype GT-102 also revealed BAP to be better hormone (Parekh et al. 2014). Multiple shoot buds obtained from apical meristem explants were subjected to rooting on full strength MS basal medium supplemented with three different hormones viz. NAA, IAA and IBA at three different concentrations namely 0.1, 0.2 and 0.3 mgL-1. The response for rooting was found to be better with 0.2mgL-1 of NAA for most of the cultivars resulting in a maximum number of primary roots (Franklin et al. 1998). The overall response to rooting of all the eleven cultivars at three different concentrations of NAA is shown in Table-3.
Table 3. Rooting responses of in- vitro regenerated shoots from apical meristem explants under different concentrations of NAA. Date recorded after 4 weeks of culture with 10 replicates for each treatment and experiment was repeated twice.
| Cultivars | NAA 0.1 mg/l | NAA 0.2 mg/l | NAA 0.3 mg/l | |||
| % of
rooting |
Number of primary roots Mean±S.D.
|
% of rooting | Number of primary roots Mean±S.D | % of rooting | Number of primary roots Mean±S.D | |
| IPA-2013 | 100 | 5.0±0.7 | 100 | 4.7±0.5 | 70 | 1.4±0.9 |
| IPA-3088 | 80 | 5.7±2.9 | 70 | 2.9±1.9 | 80 | 1.6±0.8 |
| Pusa-9 | 0 | NR | 90 | 4.2±1.5 | 80 | 1.9±1.0 |
| IPA-34 | 100 | 4.6±1.4 | 50 | 1.6±1.9 | 0 | NR |
| IPA-204 | 100 | 6.1±0.5 | 50 | 1.8±2.0 | 100 | 3.8±0.5 |
| IPA-242 | 80 | 3.2±1.2 | 70 | 1.4±0.9 | 0 | NR |
| T-7 | 100 | 2.0±0.0 | 100 | 6.2±0.4 | 0 | NR |
| IPA-61 | 100 | 5.0±0.0 | 80 | 3.1±1.5 | 100 | 2.0±0.0 |
| IPA-337 | 0 | NR | 80 | 6.4±3.2 | 0 | NR |
| IPA-341 | 0 | NR | 0 | NR | 0 | NR |
| IPA-98-3 | 0 | NR | 0 | NR | 0 | NR |
The percentage of rooting varied from 50 to 100% among these cultivars and IPA-337was found to be best among others for rooting with NAA (Figure-3).
Figure 3: Rooting response of apical meristem derived shoots of few cultivars of pigeonpea on MS media supplemented with different concentration of NAA (in mgL-1) (a) IPA-3088 (0.1), (b) IPA-204 (0.1), (c) T-7 (0.2) and (d) IPA-337 (0.2).
The response in the presence of three different concentration of IAA was also evaluated and it was found to be variable for cultivars though IPA-337 gave the best response at 0.2 mgL-1of IAA. The response of rooting was poor with different concentration of IBA for most of the cultivars in contrast to what has been reported for the cultivar Vamban-1 (Franklin et al. 1998).The percentage acclimatization of multiple shoot buds with proper rooting in soil ranged from 25 to 75% with cultivar IPA-337, IPA-61 and IPA-204 showed 75, 70 and 65% acclimatization. The assessment of these eleven pigeonpea cultivars for direct organogenesis attempted with apical meristem explants has clearly revealed that variability in regeneration potential is genotype dependent. Further cultivar IPA-242 seems promising for direct organogenesis with apical meristem as explants source though substantial standardization for enhancing the regeneration efficiency is still needed to develop efficient regeneration protocol suitable for genetic transformation (Franklin et al. 1998).
CONCLUSION
Several cultivars of pigeonpea like AL 15, ICP 6917, ICP 6974, ICP 7119, ICP 7263, Vamban and GT 102 have been reported for direct organogenesis using apical meristem explants earlier. To the best of our information these selected cultivars of pigeonpea were not studied for in vitro regeneration earlier and hence an attempt has been made to decipher the potential of these cultivars for direct organogenesis exclusively for apical meristem as explants. Among the three growth hormones BAP, TDZ and kinetin studied for in vitro regeneration among these cultivars, multiple shoot bud induction and regeneration was found to be better with higher concentration of BAP as reported earlier.
Genotype-dependent response for organogenesis under the influence of variable concentration of growth regulators was observed for these cultivars. The best responsive cultivars for multiple shoot bud induction and in vitro regeneration under variable concentration of BAP, Kinetin and TDZ treatments were IPA-242, IPA-2013 and IPA-204 respectively. A maximum of 7 buds observed with IPA-242 at higher concentration of BAP has immense potential for developing efficient regeneration protocol using apical meristem explants which could be further tested for its amenability for genetic transformation.
ACKNOWLEDGMENTS
Authors highly acknowledge Director, ICAR-IIPR Kanpur for providing seeds of pigeonpea. Authors are also grateful to Department of Biotechnology, DDU Gorakhpur University for their support to carry out this research work.
REFERENCES
Abhijeeta KN and Rajesh BM (2018) In vitro plant regeneration in pigeonpea (Cajanus cajan L Millsp) using various explants. Legume Research-An International Journal 41(2):226-229.
Asande LK, Indieka AS, Adero MO et al. (2016). In vitro regeneration of pigeonpea using leaf explants. African Journal of Crop Science 24 (2): 191-201.
Banala M, Marka R, Pamulaparthi A and Nanna RS (2016). In vitro direct regeneration through cotyledon culture in pigeonpea [Cajanus cajan (L.)Millsp.] and evaluation of genetic fidelity using RAPD markers. Vegetos 29:4.
Bohra A, Mallikarjuna N, Saxena K et al. (2010). Harnessing the potential of crop wild relatives through genomic tools for pigeonpea improvement. J Plant Biol 37(1): 85-100.
Bohra A, Saxena KB, Varshney RK and Saxena RK (2020). Genomics-assisted breeding for pigeonpea improvement. Theoretical and Applied Genetics 133: 1721-1737.
Chandra A and Pental D (2003). Regeneration and genetic transformation of grain legume: An overview. Current Science 84(3): 381-387.
Chandra Venkata SK, Nadigatla Veera Prabha Ram GR, Saxena RK et al. (2019). Pigeonpea impreovment: An amalgam of breeding and genomics research. Plant Breeding 138:445-454.
Cheema HK and Bawa J (1991). Clonal multiplication via multiple shoots in some legumes (Vigna unguiculata and Cajanus cajan). Acta Hortic 289: 93–94.
Dayal S, Lavanya M, Devi P and Sharma KK (2003). An efficient protocol for shoot regeneration and genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) by using leaf explants. Plant Cell Rep 21: 1072–1079.
Eapen S and George L (1993). Plant regeneration from leaf discs of peanut and pigeon pea: Influence of benzyladenine, indole acetic acid and indoleacetic acid-amino acid conjugates. Plant Cell Tiss Org Cult 5: 223–227.
Franklin G, Jeyachandran R, Melchias G and Ignacimuthu S (1998). Multiple shoot induction and regeneration of pigeon pea (Cajanus cajan (L.) Millsp.) cv. Vamban: from apical and axillary meristem. Curr Sci 74: 936–937.
Geetha N, Venkatachalam P and Lakshmi Sita G (1999). Agrobacterium mediated genetic transformation of pigeonpea (Cajanus cajan L) and development of transgenic plant via direct organogenesis. Plant Biotechnology 16: 213-218.
Ghosh G, Ganguly S, Purohit A. et al. (2017). Transgenic pigeonpea events expressing Cry1Ac and Cry2Aa exhibit resistance to Helicoverpa armigera. Plant Cell Report 36(7): 1037-1051.
Jasani HV, Umretiya NG, Kapuria MN et al. (2017). In vitro regeneration of pigeonpea [Cajanus cajan (L.) Millsp.] genotype GT 101 using cotyledonary node. Indian Journal of Science and Technology, (45), 9.
Karmakar, S., Molla, K, A., Gayen, D. et al. (2019). Development of a rapid and highly efficient Agrobacterium-mediated transformation system for pigeon pea [Cajanu scajan(L.) Millsp]. GM Crops & Food, 10(2), 115–138,
Kashyap V, Sarangi BK, Yadav MK and Yadav D (2011). Assessment of in vitro multiple shoot bud induction from leaf explants among eleven Indian cultivars of pigeonpea (Cajanus cajan (L) Millsp.). Biotechnology 10(6): 534-539.
Kashyap V, Sarangi BK, Yadav MK and Yadav D (2014). In vitro multiple shoot bud induction and regeneration from plumule junction explants of pigeonpea (Cajanus cajan (L) Millsp.) cultivars. African Journal of Biotechnology 13(41): 4061-4069.
Krishna G, Reddy PS, Ramteke PW and Bhattacharya PS (2010). Progress in tissue culture and genetic transformation research in pigeonpea [Cajanus cajan (L.) Millsp.]. Plant Cell Rep 29: 1079-1095.
Kumar AS, Reddy TP and Reddy GM (1984). Multiple shoots from cultured explants of pigeon pea and Atylasia species. SABRAO J 16:101–105.
Kumar V, Lokesha R, Janagoudar BS and Munniswamy S (2016). An efficient protocol for whole plant regeneration via auxillary bud explants and molecular confirmation of pigeonpea [Cajanus cajan (L.) Millsp.] regenerated plant. Legume Genomics and Genetics 7(4):1-9.
Lawrence PK and Koundal KR (2001). Agrobacterium tumefaciens mediated transformation of pigeon pea (Cajanus cajan L. Millsp.) and molecular analysis of regenerated plants. Current Science, 80, 1428–1432.
Murashige T and Skoog F (1962).A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497.
Nalluri N and Karri VR (2017). Efficient shoot induction and plant regeneration of pigeonpea [Cajanus cajan (L.) Millsp.] ICCPL87 variety using leaf petiole explants. International Journal of Applied Biology and Pharmaceutical Technology 8(4): 30-35.
Nalluri N and Karri VR (2019). In vitro regeneration of ICP 8863 pigeonpea (Cajanus cajan (L) Millsp) variety using leaf petiole and cotyledonary node explants and assessment of their genetic stability by RAPD analysis. Indian Journal of Science and Technology 12(9).
Parekh MJ, Mahatma MK and Kapadia CV (2014). In vitro regeneration of pigeonpea (Cajaus cajan(L.) Millisp) genotype GT-102 using apical meristem. Journal of Cell and Tissue Research 14(1): 4099-4103.
Pawar BD, Jadhav AS, Pawar SV. et al. (2014).An efficient regeneration system for pigeonpea (Cajanus cajan L.). Journal of Crop Improvement 28(6): 825-833.
Prasad V, Satyavathi VV, SanjayaValli KM. et al. (2004). Expression of biologically active hemagglutinin-neuraminidase protein 4 of Peste des petits ruminants virus in transgenic pigeon pea [Cajanus cajan (L.) Millsp.]. Plant Sci 166: 199–205.
Pratap A, Prajapati U, Singh CM. et al. (2018). Potential, constraints and applications of in vitro methods in improving grain legumes. Plant Breeding 137: 235-249.
Raut RV, Dhande GA, Rajput JC and Ingale AG (2015). Rapid and highly competent shoot regeneration of pigeonpea (Cajanus cajan) using variable explants by in vitro culture system. Journal of Pharmacognosy and Phytochemistry 4 (4).
Satyavathi VV, Prasad V, Khandelwal A. et al. (2003). Expression of hemagglutinin protein of Rinder pest virus in transgenic pigeon pea (Cajanus cajan (L.) Millsp.) plants. Plant Cell Rep 21: 651–658.
Sharma KK, Lavanya M and Anjaiah V (2006). Agrobacterium-mediated production of transgenic pigeon pea (Cajanus cajan L. Millsp.) expressing the synthetic Bt Cry1AB gene. In Vitro Cell and Dev Biol-Plant 42: 165–173.
Singh ND, Saho L, Sonia and Jaiwal PK (2002).In vitro shoot organogenesis and plant regeneration from cotyledonary node and leaf explants of pigeon pea (Cajanus cajan L. Millsp.). Physiol Mol Biol Plant 8:133–140.
Singh NK, Gupta DK, Jayaswal PK. et al. (2012). The first draft of pigeonpea genome sequence. J Plant Biochem Biotechnol 21(1): 98-112.
Surekha CH, Beena MR, Arundhati A. et al. (2005). Agrobacterium-mediated genetic transformation of pigeon pea (Cajanuscajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci 169: 1074–1080.
Surekha CH, Kumari KN, Aruna LV. Et al. (2014). Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant Cell Tissue and Organ Culture 116(1):27-36.
Varshney RK, Chen W, Li Y. et al. (2012). Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource poor farmers. Nature Biotechnology, 30(1): 83-89.
Vijay Kumar S, Lokesha R, Janagoudar BS and Muniswamy S (2016). An efficient protocol for whole plant regeneration via auxiliary bud explants and molecular confirmation of pigeonpea [Cajanus cajan (L.) Millsp.] regenerated plants. Legume Genomics and Genetics 7(4): 1-9.