Department of Biotechnology, University Institute of Engineering and Technology, Kurukshetra University, Kurukshetra, Haryana, India
Corresponding author Email: sunkhatak@rediffmail.com
Article Publishing History
Received: 10/03/2018
Accepted After Revision: 11/06/2018
Prosopsis cineraria commonly known as Khejri and Tecomella undulata known as Rohida are Golden Trees of Thar Desert in India belonging to the family Fabaceae and Begnoniaceae respectively. Both the plants are multipurpose tree whose almost all parts are used in pharmaceutical industry for preparing medicines. The medicinal uses of this plant has necessitated large scale production and as raw material to medicinal industry, leading to its over-exploitation. Both the trees are important component of desert Ecosystem of India as biomass producer and enrich desert soil, fix atmospheric nitrogen and provide a green coverage. Both contribute to ecological stability of the region and providing extensive support to human beings, livestock and the nutrient deficient soils. The plant tissue culture techniques can play an important role in propagation and qualitative improvement of plants of medicinal aspects. Axilary nodes, and shoot tips were aseptically cultured on MS basal medium fortified with different concentrations of cytokinins (BAP, Kinetin) and auxins (NAA, IAA,IBA) along with sucrose as energy source. The plant growth regulators act in synergistic way to proliferate shoots of almost 1-5cm lengths which were rooted on rooting media to regenerate the whole plant. In vitro developed complete seedlings were acclimatized and propagated in vitro for mass cultivation.
Bud Break; Bap; Micro Propagation; Tecomella, Prosopsis
Khatak S, Sharma A, Saini R. Comparative Analysis of Plant Growth Regulators on Bud Break in Prosopsis and Tecomella for Sustainable Agriculture in Arid and Semi-Arid Regions of India. Biosc.Biotech.Res.Comm. 2018;11(2).
Khatak S, Sharma A, Saini R. Comparative Analysis of Plant Growth Regulators on Bud Break in Prosopsis and Tecomella for Sustainable Agriculture in Arid and Semi-Arid Regions of India. Biosc.Biotech.Res.Comm. 2018;11(2). Available from: https://bit.ly/2ZjkniB
Introduction
Woody trees are vital to arid environments because of their ecofriendly and multipurpose nature, and the fact that they are well able to tolerate drought situations. Prosopis and Tecomella are the principal genera in these regions, and both have great biological diversity and ecological plasticity. These are used worldwide in arid regions to improve the local economy. These tree species are biologically diverse and are well adapted to stress as a result of multiple interbreeding species. Leguminous tree products are economically important sources of food, fodder, firewood, and timber. Improvement in quality attributes through selection, modification and mass production of germplasm is desirable. Propagation through seeds is the most common practice for raising quality trait seedlings for new plantations in arid areas. such as cuttings, suckers, air layering and tissue cultures are available but more efforts towards their refinement are still required, particularly with regards nursery and laboratory techniques, before commercial cultivation (Baksha et al., 2007; Biswas et al., 2009; Roy, 2008).Plant cell tissue culture has offered a very novel technique to mass multiply, true to type and providing disease resistant plants in controlled conditions.
Prosopis cineraria commonly known as khejri belongs to pea family, Fabaceae and is a multipurpose tree of desert in Western Rajasthan. It is also called kalptaru, ‘wonder tree’ and the ‘king of desert. (Singh et al. 2013, Tarachand et. al. 2012). It is native to arid portions of Western and the Indian subcontinent, including Afghanistan, Iran, India, Oman, Pakistan, Saudi Arabia, the United Arab Emirates, and Yemen. In India it is found in the various parts of Rajasthan, Gujarat, Haryana, Uttar Pradesh and Tamil Nadu (Rathore et al., 1991).It is regarded as a backbone of rural economy being a good biomass producer and fixes atmospheric nitrogen and provides a green coverage and in turn helps in the enrichment of desert soil. It contributes to ecological stability of the region and providing extensive support to human beings, livestock and the nutrient deficient soils (Chaudhry 2011, Panwar et al., 2014 and Hua et al. (2015).
The tree is well adapted to arid and semi-arid conditions of the Indian desert, due to their well-developed and expansive tap root system which reach up to a length of 20 m, often reaching out the ground water resources (Gehlot et al., 2008). Pods of this plant locally called “Sangri” are considered as dry fruits of desert. Pods contain various phytoconstituents like tannins (gallic acid), steroids (stigma sterol, campestral, sitosterol, etc.), Flavones derivatives (prosogerin A, B, C, D, and E), alkaloids (spicigerine, prosophylline), etc. have been isolated from the sangri pods (Gehlot et al., 2008).The ashes of bark are rubbed over the skin to remove hair. Fresh Leaves juice mixed with lemon juice is used for dyspepsia; extract of crushed pods is used for earache, toothache, pain relief from fractured bones (Garg and Mittal 2013).
The whole plant is used in the Indigenous System of Medicine as a folk remedy for various ailments like leprosy, dysentery, bronchitis, asthma, leucoderma, piles, muscular tremor and wandering of the mind. It is also known to possess anthelmintic, antibacterial, antifungal, antiviral and anticancer activities. Tecomella undulata belongs to family Begnoniaceae is an important medicinal plant. Wide range of therapeutic activities has been attributed to this plant. The plant is excellent blood purifier hence rewarding in hepatitis. Bark forms a major constituents of various herbal formulations like Livo-plus, Liv-52, Livosan,Herboliv, Amylcure for curing inflammatory hepatic disease. The leaves have oleanolic acid, ursolic acid and betulinic acid which are strong prohibitors of HIV (Nandwani et al., 1995; 1996). It has been reported to be used in phyto remediation of soil contaminated with crude petroleum oil its common agro forestry tree in arid and semi arid regions used for research, including bio fertilizer aspect as well as afforestation programmes (Rathore et al., 1991 and Shekhawat et al., 1993).
The plant is propagated mainly using seeds and the tree is slow growing species. Till date the woody plants lack suitable methods for vegetative propagation on mass scale. Seeds are on prime importance for extensive plantation. The seeds are available from month of April to June. Freshly harvested seeds have more potential for germination as compared to unripe fruits or ripe fruits collected in June. So present investigation was undertaken with an objective to devise one suitable protocol for bud initiation and plant propagation using nodal tissues in both Tecomella and Prosopsis (Bhansali, 1993).
Material and Methods
The seedlings of Prosopsis and Tecomella were used in the present study for bud break and shoot proliferation, which were procured from Tau Devi Lal Herbal Park, Near Khizrabaad Highway, Yamunanagar District, Haryana, India. Axillary nodes were selected as explants to avoid genetic alterations and somaclonal variations observed using indirect regeneration. The disease free axillary buds were collected from 4 weeks old healthy plant. The explants were excised and the contaminants were washed under running tap water for 4-5 minutes, followed by washing in the liquid detergent Tween -20 (few drops / 100 ml solution) and then rinsed with running tap water. The cleaned explants were surface sterilized with variable concentration of mercuric chloride (0.1%-0.5%) under laminar air flow. The explants were subjected to different time intervals to optimize the sterilization procedure and then rinsed 4-5 times with sterilized distilled water. After trimming the cut ends, equal sized, surface sterilized explants were cultured on the culture medium defined by Murashige and Skoog, (1962). Among all experiments, a treatment of (0.1% and 0.3%) HgCl2 for 3 minutes proved to be the best for sterilization in Prososopsis and Tecomella, a large percentage (85%) of explants. The surface sterilization was optimized and this helped in preventing blackening of tissue on exposure to mercuric chloride and establishment of clean cultures.
Axillary nodes were excised from the young seedlings grown in vivo conditions and were cultured on different shoot proliferation media (Table 1,2) consisting of different concentration and combination of cytokinin and auxin as plant growth regulator. Axillary node explants were implanted vertically. Test tubes and flask containing the explants were sealed with sterilized cotton plugs and incubated for 4 weeks at 25±2°C under a 16 hour photoperiod. Radiation source was supplied by soft white fluorescence tubes. For each treatment, a minimum of 3 replicates were carried out. Percent shoot proliferation and multiple shoot formation was recorded after four weeks of culture.
Results and Discussion
The bud break, shoot proliferation and exploring the potential for multiple shoots regeneration in Prosopsis and Tecomella is an utmost requirement for propagation of this plant.Sterilization of explants is a crucial step in plant tissue culture and to achieve 100 percent sterilization explants were subjected to various concentration of mercuric chloride (0.1%-0.5%) along with antifungal supplement and a prior ethanol treatment. Maximum sterilization (80%) was observed using a concentration of 0.3% of mercuric chloride for 3 min. A higher concentration of 0.5% of mercuric chloride burn the tissue and cause blackening at the edges resulting in 23 % sterilization while a lower concentration of 0.1% of mercuric chloride resulted in only 40% sterilization. It was found that mercuric chloride alone at a concentration of 0.3% for 3 minutes is sufficient to sterilize the explants without a prior treatment of ethanol and antifungal supplements.
The axillary nodes were cut in equal size and surface sterilized using standard procedure. A concentration of 0.1% HgCl2 for 3 minutes resulted in 85% of sterilization of explants while as compared a lower concentration of HgCl2 was less effective in sterilization process. Even a concentration of 0.05 for 5 minutes resulted in 51% decontamination of explant. However a pretreatment of ethanol (rinse) and Bavistin (45min) resulted in higher percentage of healthy sterilized tissue.
The axillary nodes excised from in vivo grown young seedlings were sterilized using standard mercuric chloride solution at 0.1% concentration for 3 min which was sufficient to obtain 80% sterilization. The proliferation of shoots from cultured explant was remarkably influenced by the type of concentration of the growth regulator used. Axillary node cultured on MS medium supplemented with different concentration of BAP alone showed the best results.
Tecomella Undulata
Complete plant development through tissue culture strongly relies on synergistic effects of growth hormone or say auxin and cytokinin which play a significant role in cell differentiation and whole plant growth in Tecomella. Different concentrations of cytokinin and auxins were used alone or in combination for initiation of shoot proliferation. BAP was taken alone without any auxin in combination at different concentrations (0.1,0.5,1.0 mg/L) resulted in bud break and shoot proliferation of up to 2cm from sterilized axillary nodes cultured . Further increase in BAP concentration from 1.5 to 2.0 mg/L resulted in shoot proliferation 1-2 cm in length with a regeneration frequency of 50% in approximately 10 days. Similar results were obtained using axillary nodes in Prosopsis by Pareek et al., 2012 and Kumar and Singh (2009) who supported the effectiveness of BAP and KIN in regeneration of multiple shoots in Prosopsis. Similar concentration of BAP alone at 0.2 mg/l found to be effective in axillary bud break in Baccopa monnieri, (Sharma et al., 2010).
While on further increase in BAP concentration from 2 to 3 and 3.5mg/l found to be inhibitory. No bud break or initiation in shoot proliferation was noticed on higher concentration which clearly demonstrates that there is decrease in plant cell differentiation with much higher concentration of hormones. With an increase in concentration of growth regulators the days required to bud break also increased which is further supported by our present studies. The results totally contradict the finding of Kumar and Singh (2009) who observed one to multiple shoots on medium containing higher concentration of BAP (5.0mg/l) along with IAA at (1.0mg/l). Indole acetic acid was used as auxin in combination with BAP as cytokinin. The concentration of BAP was kept constant (1.0mg/l) and IAA as auxin was used in combination at four different concentrations (0.01,0.02,0.05,0.1mg/l) resulted in bud break and shoot proliferation of 1-2cm in length with an increase in regeneration frequency from 50%to 65% and bud break initiated in lesser time period of 8 days. This combination reveals the synergistic action of cytokinin to act and promote cell differentiation along with auxin. After getting positive response of BAP along with IAA, the concentration of BAP was raised to 1.5mg/l and was kept constant while the concentration of IAA was altered (0.01,0.02,0.05,0.1mg/l).The shoot length increased in size to 3 cm as compared to earlier with bud break and shoot proliferation in 7 days.
The regeneration frequency also get increased to 95%.Our results well corroborate with the findings of Lal and Singh(2010) in Celastrus paniculatus using different cytokinin BAP and Kinetin(0.5,1.0,2.0mg/L) along with auxins (IAA, NAA, and 2,4-D) using nodal explants from mature tree of this species and observed less number of shoots per explants and 100% bud break on MS medium supplemented with BAP.(1.0mg/L). Yadav et al in 2011 reported that season of collection of explants showed direct influence on bud break in Celastrus paniculatus using shoot tip explants obtaining highest percentage (90%) bud break and multiple shoot formation(4/explant)on MS medium containing 1.0 mg/l BAP.
On further increase in BAP concentration from 1.5 to 2.0mg/l with IAA in combination at four different concentrations (0.01, 0.02, 0.05, 0.1mg/l) no significant increase was observed. Although the bud break and shoot proliferation was observed which was almost same resulting in 1-2 shoots / explants of 1-2 cm in length with a regeneration frequency of 60% only .The results clearly depicts the synergy between auxin and cytokinin to act in combination for bud break and shoot proliferation but a higher concentration of BAP was found to be inhibitory whether used alone or in combination with IAA. Present results strongly contradict to that observed by Warrier et al in 2010 in Aegle marmelos where higher concentration of BAP (2.5mg/l) was beneficial for inducing multiple shoots. (Table-1 and figure 1)
| Table 1: Composition of MS medium supplemented with different growth regulators and result obtained after incubation of Tecomella undulata nodal culture. | |||||||||
|
S.No. |
Media Code | Growth Hormones (mg/L) | No. of shoots | Shoot length | % of regeneration | Days of initiation | |||
| BAP | IAA | ABA | NAA | ||||||
| 1 | MS – 1 | — | — | — | — | Control | |||
| 2 | MS – 2 | 0.1 | — | — | — | Bud break | — | 16 | 9 |
| 3 | MS – 3 | 0.5 | — | — | — | Bud break | — | 30 | 10 |
| 4 | MS – 4 | 1.0 | — | — | — | Bud break | — | 25 | 9 |
| 5 | MS – 5 | 1.5 | — | — | — | 1 | 1.5 | 50 | 13 |
| 6 | MS – 6 | 2.0 | — | — | — | 1 | 1.0 | 45 | 12 |
| 7 | MS – 7 | 2.5 | — | — | — | Bud break | — | 35 | 11 |
| 8 | MS – 8 | 3.5 | — | — | — | — | — | — | 11 |
| 9 | MS – 9 | 1.0 | 0.01 | — | — | — | — | — | 8 |
| 10 | MS – 10 | 1.0 | 0.02 | — | — | 1 | 1.5 | 60 | 7 |
| 11 | MS – 11 | 1.0 | 0.05 | — | — | 1 | 1.0 | 55 | 8 |
| 12 | MS – 12 | 1.0 | 0.1 | — | — | 2 | 1.0 | 65 | 7 |
| 13 | MS – 13 | 1.5 | 0.01 | — | — | 2 | 1.5 | 80 | 7 |
| 14 | MS – 14 | 1.5 | 0.02 | — | — | 3 | 2.5 | 95 | 7 |
| 15 | MS – 15 | 1.5 | 0.05 | — | — | 1 | 1.5 | 80 | 9 |
| 16 | MS – 16 | 1.5 | 0.1 | — | — | Bud break | — | 50 | 11 |
| 17 | MS – 17 | 2.0 | 0.01 | — | — | Bud break | — | 54 | 7 |
| 18 | MS – 18 | 2.0 | 0.02 | — | — | Bud break | — | 70 | 8 |
| 19 | MS – 19 | 2.0 | 0.05 | — | — | Bud break | — | 65 | 7 |
| 20 | MS – 20 | 2.0 | 0.1 | — | — | 1 | 1.0 | 60 | 9 |
| 21 | MS – 21 | 2.5 | 0.01 | — | — | 1 | 1.5 | 65 | 10 |
| 22 | MS -22 | 2.5 | 0.02 | — | — | 2 | 1.0 | 55 | 9 |
| 23 | MS – 23 | 2.5 | 0.05 | — | — | 2 | 1.0 | 60 | 11 |
| 24 | MS -24 | 2.5 | 0.1 | — | — | 2 | 1.0 | 55 | 10 |
| 25 | MS -27 | 1.5 | — | — | 0.01 | Bud break | — | 70 | 8 |
| 26 | MS -28 | 1.5 | — | — | 0.1 | Bud break | — | 65 | 9 |
| 27 | MS -29 | 2.0 | — | — | 0.01 | 1 | 1.5 | 55 | 7 |
| 28 | MS – 30 | 2.0 | — | — | 0.1 | — | — | — | 8 |
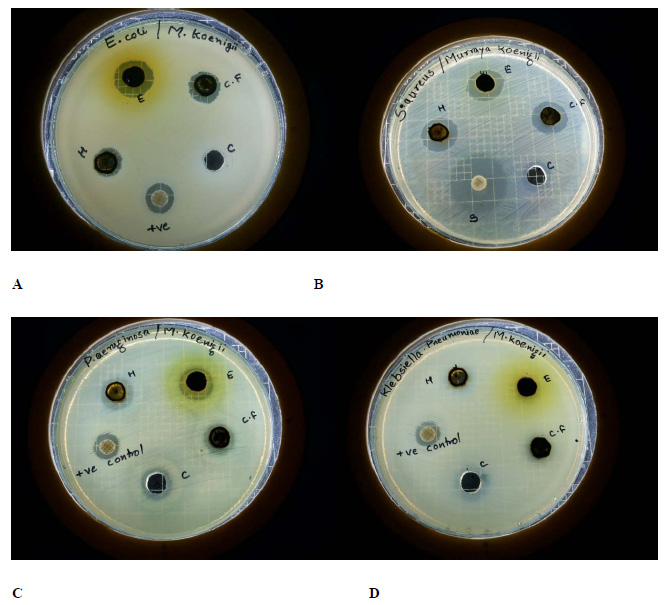 |
Figure 1: Bud break on MS medium supplemented with different growth regulators in Tecomella undulate. A) BAP (0.1mg/L),B) (0.5mg/L)andC) (1.0mg/l) |
Prosopsis Cineraria
Different cytokinins and auxins were employed alone or in combination for bud break and shoot proliferation in Prosopsis. Eighteen different media combinations were used for Prosopsis micropropagation which was recalcitrant to grow taking different concentrations of growth hormones. Six different medium were used having BAP (0.5.1.0,1.5, 2.0mg/l) along with NAA as auxin at two different concentrations of (0.5 and 1.0 mg/l). Four medium consisted of BAP along with IBA where IBA was kept constant at a concentration of (1.0mg/l) while the BAP concentration was raised from (1.0 mg /l to 4.0 mg/l). Further six medium consisted of kinetin in combination with two different auxins ie. NAA and IBA. NAA was kept constant while kinetin was used at two different concentrations of (2.0 and 3.0 mg/l),while with IBA the concentration was kept constant at 1.0 mg/l and kinetin was used at very high concentration of(5.0,7.0,9.0,10.0mg/l)to search out the possibility of multiple shoots at much higher concentration.
Six more combinations of auxin and cytokinin includes Zeatin in combination with IBA,where IBA concentration was kept constant at 1.0 mg/l but Zeatin concentration was altered (1.0,1.5,2.0,2.5,3.0,4.0). Although a higher concentration of Zeatin was also used similar to kinetin (5.0,7.0,9.0,10.0mg/l) but the higher concentration as observed earlier in case of Tecomella were found to inhibitory and no bud break or shoot proliferation was observed in Prosopsis.
| Table 2: Composition of MS medium supplemented with different growth regulators and result obtained after incubation of Prosopis cineraria nodal culture. | |||||||||
| S. No | Media code | Growth hormones (mg/L) | No. of shoots | Shoot length (cm) | % of Regeneration | Day of initiation | |||
| Zeatin | IBA | BAP | NAA | ||||||
| 1 | MS – 1 | 1.0 | 1.0 | — | — | — | — | 30 | 30 |
| 2 | MS – 2 | 1.5 | 1.0 | — | — | 2 | 1.5 | 30 | 30 |
| 3 | MS – 3 | 2.0 | 1.0 | — | — | 1 | 1.5 | 50 | 30 |
| 4 | MS – 4 | 2.5 | 1.0 | — | — | 1 | 5 | 50 | 30 |
| 5 | MS – 5 | 3.0 | 1.0 | — | — | 1 | 5 | 60 | 40 |
| 6 | MS – 6 | 4.0 | 1.0 | — | — | 1 | 4.5 | 60 | 40 |
| 7 | MS – 7 | — | 1.0 | 1.0 | — | 2 | 2.5 | 30 | 40 |
| 8 | MS – 8 | — | 1.0 | 2.0 | — | 1 | 2.5 | 40 | 40 |
| 9 | MS – 9 | — | 1.0 | 3.0 | — | 1 | 3.0 | 25 30
35 30 |
|
| 10 | MS – 10 | — | 1.0 | 4.0 | — | 1 | 2.5 | ||
| 11 | MS – 11 | — | — | 0.5 | 0.5 | Bud break | — | 15 | 15 |
| 12 | MS – 12 | — | — | 1.0 | 0.5 | Bud break | — | 20 | 20 |
| 13 | MS – 13 | — | — | 1.5 | 0.5 | 2 | 1.5 | 20 | 20 |
| 14 | MS – 14 | — | — | 2.0 | 0.5 | 1 | 1.0 | 20 | 20 |
| 15 | MS – 15 | — | — | 0.5 | 1.0 | Bud break | — | 20 | 10 |
| 16 | MS – 16 | — | — | 1.0 | 1.0 | Bud break | — | 10 | 15 |
| 17 | MS – 17 | — | — | — | 1.5 | 1 | 2 | 10 | 30 |
| 18 | MS – 18 | — | — | — | 1.5 | 1 | 4 | 15 | 40 |
On increasing the concentration of BAP step by step from 0.5to2.0mg/l and keeping the NAA concentration constant at 0.5mg/l. At lower concentrations bud break was observed in 15 to 20 days at 0.5 and 1.0mg/l of BAP while on further increase in concentration (1.5and 2.0mg/L)1-2 shoots per explants were observed of 1-1.5cm in length with higher regeneration frequency as compared to lower concentrations used within same time frame (Figure 2). When NAA concentration was raised from 0.5 to 1.0mg/l in combination with BAP at two different concentrations of 0.5and 1.0mg/l no significant change was observed, moreover the medium resulted in bud break only.
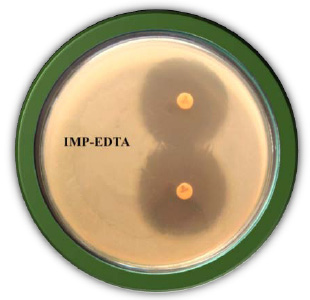 |
Figure 2: Shoot propagation on MS medium supplemented with growth regulators (BAP 1.5mg/l + NAA 0.5mg/l) |
Similar findings were reported by Singh et al. (2014) in Shorea robusta a woody valuable tree species using nodal explants. They reported that BAP at 1.0mg/l along with NAA at a concentratons of 0.5mg/l found to be the best medium for shoot initiation and proliferation. Similar findings observed by Tyagi et al (2010) in Capparis deciduas using axillary shoots observed shoots of 2cm in length when cultured on MS medium supplemented with 2mg/l BAP along with 0.5mg/l NAA.in 2010.
Girijashanker (2011) in Acacia auriculiformis a multipurpose tree of medicinal forestry observed higest percentage of shoots induction on BAP (2mg/l )along with NAA at (0.1mg/L). In combination of Zeatin with IBA, IBA was kept constant at 1.0mg/l while the zeatin concentration at different concentration resulted in 1-2 shoots per explants of 1-5cm in length in 45 days with a regeneration frequency of 55%. Similar combinations have been reported by Hua et al. (2015) to be effective in Pitaya for shoot proliferation, they used 3.0uM Zeatin in combination with IBA at 0.5mg/l resulting more vigorous multiplication of shoots. With an increase in Zeatin concentration has positive effects on bud break and shoot length elongation as a maximum of 5cm length shoots were observed on 4.0mg/l concentration, while an further increase in concentration of Zeatin has inhibitory effects on cell differentiation same as kinetin.
The results depicts that higher concentrations of hormone should not be taken as alternative to mass propagate plants in shorter period of time. BAP was used at different concentrations (1.0,2. 0,3.0,4.0), while IBA concentration was kept constant at 1.0mg/l resulted in 1-2 shoots per explants of 2-3cm in length in 35-40 days approximately. NAA was kept constant at a concentration of (1.5mg/l) and using kinetin at two different concentration (2.0 and 3.0mg/l) the shoot length increased in size from 2 to 4cm almost double while the initiation process of shoot proliferation also shorten by 10 days from 40 to 30 days. The results well corroborate with that of Dhabhai et al. (2010) in Acacia nilotica a nitrogen fixing tree through direct regeneration using nodal segments cultured on MS medium supplemented with Kinetin (1.0mg/l along with NAA at 0.6mg/l they observed highest no of shoots of 2-3 cm in 15-20 days of culture. Multiple shoots were not observed on any of the media used only one or two shoots were observed otherwise the shoots just proliferated to 2-4 cm in 15 days. Similar findings were observed in Vitex negundo, where MS medium supplemented with BAP individually enhanced the induction of multiple shoots within an average time of 8 to 12 days, while BAP (2.0mg/L) in combination with NAA (0.5 mg/L) resulted in a higher percentage (93%) of multiple shoot formation. The results were further supported in Gloriosa superba, Rauvolfia serpentine and Boerrhavia diffusa (Baksha et al., 2007; Hassan and Roy, 2005 Biswas et al., 2009). MS media without growth regulators failed to induce bud break from nodal segments of Tecomella which is probably due to insufficient level of endogenous growth regulators in explants to induce bud break and it required an exogenous
supply.
Our present investigation focused on initial bud break. Supplementation of cytokinins like BAP at different concentration induces bud break as well as initiation of shoot. Cytokinin supplemented media nullifies the effect of apical dominance and promote axillary bud prolifiration into shoots. This is due to exogenous applications of cytokinins disturbs the internal polarity and change the genetically physiology of explants, resulting in organogenesis. Similar effectiveness of cytokinins in promoting in vitro auxillary bud break have been reported in many forest plants. Auxins and cytokinins have synergistic action in plant cells proliferation and differentiation. The ratio of auxin to cytokinin has major impact on growth and bud break. The release or absorption of one growth regulator is regulated by the presence of other.
Conclusion
Multiplication of germplasm through plant tissue culture methods is one of the applications of biotechnology via which elite trees can be mass produced rapidly. MS medium without growth regulators failed to induce bud break from nodal segments of Tecomella and Prosopsis probably due to insufficient level of endogenous growth regulators in explants to induce bud break and it requires an exogenous supply of growth hormones. Our present investigation was merely focused on initial bud break and shoot proliferation with axillary buds in Tecomella and Prosopsis. Both the plants have importance as medicinal value as reported in ancient literature. Both can withstand the adverse conditions prevalent in semi-arid and arid regions. With temperature 500ºF in winters and 110-114ºF in summers. The tree provides grazing material to livestock and plays an important role in livelihood and ecosystem preservance. Both are excellent soil binders and important constituents of arid vegetation system. The National Research Centre Institute for Arid Horticulture, Bikaner (CIAH), Central Arid Zone Research Institute (CAZRI), Jodhpur and Arid Zone Forestry Research Institute (AFRI), Jodhpur are working to collect germplasm, and to improve and propagate fruit and forest trees found in hot arid zones. Still there is a need to cope up with overgrazing and exploitation of both trees as important structure for livelihood of local people residing in deserts areas. New protocols are indeed a basic requirement to propagate such trees on large scale multiplication through tissue culture technology .Conclusively this study has established the bud break and shoot initiation from axillary node of both woody taxa. Further research in order to develop plantlets and their acclimatization to field conditions will be next stepping stone .Meanwhile this protocol offers an efficient method to initiate shoot proliferation and also paved the path to improve vegetation coverage in arid and semi-arid regions of India.
References
Baksha R., Jahan M. A. A., Khatun R. and Munshi J.L (2007) In vitro rapid clonal propagation of Rauvolfia serpentine (Linn.) Benth, Bangladesh J Sci Ind Res Vol 42 No 1: Pages 37-44
Bhansali R.R. (1993) Bud culture for shoot multiplication and plantlets formation of Tecomella undulata (Rohida) – a woody tree of the arid zone Trop. Sci. Vol.33: Pages 1-8
Biswas A., Bari M. A., Roy M. and Bhadra S.K (2009) Clonal propagation through nodal explants culture of Boerrhaaviaa diffusa L.– a rare medicinal plant Plant Tissue Cult Biotechnol Pages 53-59
Chaudhary P. (2011) Prosopis cineraria (L) Druce: A life line tree species of the Thar Desert in Danger Journal of Biodiversity and Ecological Sciences Vol 1No 4: Pages 289-293
Dhabhai K., Sharma M. M. and Batra A. (2010) In vitro clonal propagation of Acacia nilotica (L) – A nitrogen fixing tree Researcher Vol 2 No 3: Pages 26-32
Garg A. and Mittal S. (2013) Review on Prosopis cineraria: A potential herb of Thar dessert drug invention today Vol 5: Pages 60-65
Gehlot P., Bohra N. K. and Purohit D. K. (2008) Endophytic mycoflora of Inner bark of Prosopis cineraria – a Key Stone Tree Species of Indian Dessert
Girijashankar V. (2011) Micropropagation of multipurpose medicinal tree Acacia auriculiformis Journal of Medicinal plants Research. Vol 5 No3: Pages 462-466
Hassan S. A. K. M. and Roy S. K. (2005) Micropropagation of Gloriosa superba L. through high frequency shoot proliferation Plant tissue cult. Vol 15: Pages 67-74
Hua Q., Chen P., Liu W., Ma Y., Liang R., Wang L., Wang Z., Hu G. and Qin Y. (2015) A protocol for rapid In vitro propagation of genetically diverse Pitaya Plant Cell Tiss Organ Cult. Vol 120: Pages 741-745
Kumar S. and Singh S. (2009) Micropropagation of Prosopis Cineraria(L.) Druce a multipurpose desert tree. Research. Vol 1: Pages 28-32
Lal D. and Singh N. (2010). Mass multiplication of Celastrus paniculatus Willd- an important medicinal plant under In vitro conditions using nodal segments J of American Sci. Vol 6: Pages 55-61
Murashige T. and Skoog F. (1962) A revised medium for rapid growth and bioassays with Tobacco Tissue cultures Plant Physiol. Vol 15: Pages 473-497
Nandwani D., Mathur N. and Ramawat K.G. (1995) In vitro shoot multiplication from cotyledonary node explants of Tecomella undulate Gartenbauwissenschaft Vol 60: Pages 65-68
Nandwani D., Sharma R. and Ramawat K.G. (1996) High frequency regeneration in callus cultures of a tree – Tecomella undulate Gartenbauwissenschaft Vol 61 No.3: Pages147-150
Panwar D., Pareek K. and Bharti C.S. (2014) Unripe pods of Prosopis cineraria used as a vegetable (Sangri) In Shekhawati region. Int. J. Of Science and Engi. Research Vol 5 No 2
Pareek A., Sharma D. and Pareek L. (2012) In vitro micropropagation through cotyledonary nodal segments in Prosopis cineraria Research Journal of Pharmaceutical, Biological and Chemical Sciences Vol 3 No 3: Pages 309-313
Rathore T.S., Singh R.P. and Shekhawat N.S. (1991) Clonal propagation of desert Teak (Tacomella undulate) through tissue culture Plant.Sci. Vol 79 No2: Pages 217-222
Roy P.K. (2008) Rapid multiplication of Boerhaavia diffusa L.through in vitro culture of shoot tip nodal explants Plant Tissue Cult Biotechnol. Vol 18 No 10: Pages 49-56
Sharma S., Kamal B., Rathi N., Chauhan S., Jadon V., Vats N., Gehlot A. and Arya S. (2010) In vitro rapid and mass multiplication of highly valuable medicinal plant Bacopa monnieri (L.) Wettst. Afri J Biotechnol. Vol 9 No 49: Pages 8318-8322
Shekhawat N. S., Rathore T.S., Singh R.P., Deora N.S. and Rao S.R. (1993) Factors affecting In vitro clonal propagation of Prosopis cineraria Plant Grow Regul. Vol 12: Pages 273-280
Singh M., Sonkusale S., Niratker, C.H. and Shukla P. (2014). Micropropagation of Shorea Robusta: An economically important woody plant Journal of Forest Science Vol60 No 2: Pages 70-74
Singh S., Naresh V. and Sharma S. (2013) Pharmacognostic studies on the leaves of Prosopis cineraria (L) Druce Growing in south Haryana, India Vol 2 No 1
Tarachand, Bhandari A., Bhupendra, Kumaawat K, Sharma A. amd Nagar N. (2012) Physicochemical and preliminary phytochemicals screening of pods of Prospis cineraria (L) Druce Der Pharacia Sinica, Vol 3 No. 3: Pages 377- 381
Tyagi P., Khanduja S. and Kothari S.L., (2010) In vitro culture of Capparis deciduas and assessment of clonal fidelity of the regenerated plants Biol Plants. Vol 54: Pages 126-130
Warrier R., Viji J. and Priyadharshini P.(2010) In vitro propagation of Aegle marmelos L.(Corr.) From Mature Trees Through enhanced Axilliary Brancing Asian J. Exp. Biol.Sci. Vol 1 No 3 : Pages 669-676
Yadav K., Lal D. and Singh N., (2011) Influence of explanting season on In vitro multiplication of Celastrus paniculatus wild. – An endangered medicinal herb Agric. Tech Vol 7 No 5: Pages 1355-1361


