Department of Biology, Turabah University College, Taif
University, P.O.Box 11099,Taif 21944, Saudi Arabia.
Corresponding author email: o.saeeed@tu.edu.sa
Article Publishing History
Received: 13/10/2021
Accepted After Revision: 20/12/2021
The most common endocrinopathy in women is polycystic ovarian syndrome (PCOS). Obesity is linked to PCOS and tumor necrosis factor-a (TNF-α), which is followed by hyperandrogenism and enhanced insulin resistance. Previous case-control and meta-analysis studies on the TNF-a gene and PCOS women relied heavily on the -C850T polymorphism. The aim of this study was to investigate the Elisa levels and -C850T (rs1799724) polymorphism in TNF-α gene with PCOS women in the Saudi women. In this case-control study, 50 PCOS patients and 50 healthy controls were recruited, and plasma levels of TNF-α were evaluated using an enzyme linked immunosorbent assay (Elisa), and extracted DNA was utilized to explore the -C850T polymorphism using Polymerase Chain Reaction (PCR).
The limited and digested PCR products were run on an Agarose gel to test for the -C850T polymorphism. Elevated Elisa levels were found in CT genotype and gene polymorphism studies showed 12% of CT genotypes was documented in PCOS women and 14% in control women. None of the genotypes or allele frequencies were associated with a positive relationship between PCOS women and controls. The CT genotype had higher TNF-α levels than the CC genotype, and the C850T polymorphism was not related with PCOS in women, according to the findings of this study.
PCOS, C850T, TNF-α, Elisa and PCR.
Nassir O. C850T Polymorphism in Tumor Necrosis Factor Alpha gene in Women with Polycystic Ovarian Syndrome: Physiological Genetic Validation. Biosc.Biotech.Res.Comm. 2021;14(4).
Nassir O. C850T Polymorphism in Tumor Necrosis Factor Alpha gene in Women with Polycystic Ovarian Syndrome: Physiological Genetic Validation. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3I0vm3P“>https://bit.ly/3I0vm3P</a>
Copyright © Nassir This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
PCOS (Polycystic Ovarian Syndrome) is one of the most common metabolic spectrum diseases in the human population, as well as one of the most commonly identified endocrine abnormalities (Ma et al., 2021). According to Rotterdam criteria, PCOS women were impacted, with a prevalence of 15-20% among reproductive-age women (Aboeldalyl et al., 2021). Ovarian hyperandrogenism and micropolycystic morphology in PCOS patients are clinical and biochemical manifestations (Aversa et al., 2020). Currently, the etiology of PCOS is unknown, and there is no cure; therefore, management is based on symptoms, treatment, and risk factor reduction (Tatkare, 2021).
The genetics contribution of PCOS is well established to be a complex condition in which heritable genetics is suggested due to familial clustering symptoms (Combs et al., 2021). PCOS is associated with numerous of clinical symptoms, Obesity, metabolic syndrome, decreased glucose tolerance, and type 2 diabetes mellitus (T2DM), cardiovascular disease, and dyslipidemia, and prior research has shown a correlation between gestational diabetes mellitus (GDM) and PCOS ( Pan et al 2015, Lentscher et al., 2021).
Signaling proteins produced by various types of immune cells have a significant effect on other cells and organisms. Leukocytes, oocytes, and follicular cells produce it in the ovary. Many genes have been proposed as contributing factors to PCOS, however none have yet been accepted as a main cause of this clinical disorder. TNF-α (tumor necrosis factor alpha) levels in muscle and adipose tissue have been associated to an increased risk of insulin resistance in humans. To comprehend the pathophysiology of PCOS, it is necessary to comprehend the involvement of TNF-α. TNF-α is a proinflammatory cytokine that has been proven to be elevated in at least a portion of PCOS women ( Thathapudi et al., 2014, Guo et al., 2015, Gnanadass et al., 2021).
Several TNF-α promoter polymorphisms have been found and connected to TNF-α transcription regulation. The polymorphisms (SNPs) in the promoter region of the TNF-α gene have been thoroughly investigated (Guzmán-Flores et al., 2013). Kato group discovered the -C850T polymorphism in the Promoter region of TNF-α gene, which is proven to be multifunctional and cytokine is produced mostly by macrophages and lymphocytes. TNF-α gene is located on the 6p21.3 chromosome and spans around 3kb. It has four exons (Khan et al., 2015).
Elisa and C-850T polymorphism studies were carried out in PCOS women from the Saudi population in this study. There have been no previous reports with Elisa, although one Saudi study (Alkhuriji et al.) revealed in PCOS women with cytokine markers, including TNF-α gene polymorphism. The purpose of this study is to look into TNF-α levels in PCOS women using Elisa tests and C-850T (rs1799724) polymorphism analyses in the TNF-α gene.
Table 1. Demographic and biochemical data obtained with PCOS cases and control women
| PCOS (n=50) | Controls (n=50) | P Value | |
| Age | 32.1±5.74 | 34.64±4.74 | 0.39 |
| Weight, kg | 72.5±11.89 | 80.71±14.94 | 0.02* |
| Height, cm | 158.44±5.40 | 159.71±9.29 | 0.47 |
| Body Mass Index (BMI). | 28.69±5.01 | 31.94±7.54 | 0.04* |
| Fasting Blood Glucose (FBG), mmol/L | 4.98±0.79 | 4.91±0.85 | 0.75 |
| Follicle Stimulating Hormone (FSH), IU/L. | 6.81±2.63 | 6.48±2.87 | 0.65 |
| Luteinizing Hormone (LH), IU/L. | 7.79±4.72 | 5.61±2.41 | 0.06 |
| Tumor Necrosis Factor alpha (TNF-α), pg/mL | 11.57±0.57 | NA | NA |
Arithmetic means ± standard error, * indicates significant difference between PCOS and control (P<0.05).NA, not applicable.
MATERIAL AND METHODS
Selection of PCOS and control women: In this study, we have recruited 50 patients diagnosed with polycystic ovarian syndrome from Saudi Arabia. Ethical grant was approved from Institutional Review Board and all the women participated in this study has signed the informed consent form. The women who denied in signing the consent form was excluded from this study. Based on Rotterdam criteria, PCOS women was diagnosed and included in this study.
Table 2. Genotype and allele frequencies between PCOS women and control subjects
| C-850T | PCOS (n=50) | Controls (n=50) | Statistical analysis |
| CC | 44 (88%) | 43 (86%) | – |
| CT | 06 (12%) | 07 (14%) | OR-0.84 [95%CI:0.26-2.69] p=0.72 |
| TT | 00 (0%) | 00 (0%) | OR-0.97 [95%CI:0.01-50.36] p=0.99 |
| C | 94 (0.94) | 93 (0.93) | – |
| T | 06 (0.06) | 07 (0.07) | OR-0.84 [95%CI:0.27-2.61] p=0.77 |
| CC vs TT+CT | 06 (12%) | 07 (14%) | OR-0.83 [95%CI:0.26-2.69] p=0.76 |
The women who doesn’t fit into the Rotterdam criteria were excluded from this study. The women without any history of infertility is considered as normal controls were involved in this study. The women with any history of infertility or PCOS will be excluded in this study as normal controls. Based on inclusion and exclusion criteria, 60 control subjects were recruited in this study. Based on Mohamadin et al studies, the cases and controls were selected (Mohamadin et al., 2010).
Sample collection:One milliliter of ethylenediaminetetraacetic acid blood was collected from each patient and used for DNA isolation. Another one milliliter of blood was used for measuring Elisa levels with the extraction of plasma from blood.
Table 3. Anova analysis involved with C850T genotypes in PCOS women
| CC (n=44) | CT (n=06) | TT (n=00) | P values | |
| Tumor Necrosis Factor alpha levels (TNF-α), pg/mL | 11.4±0.48 | 12.5±0.28 | 0.0±0.0 | 0.001** |
| Body Mass Index (BMI). | 28.5±5.27 | 30.4±1.78 | 0.0±0.0 | 0.01* |
| Fasting Blood Glucose (FBG), mmol/L | 4.94±0.80 | 5.29±0.69 | 0.0±0.0 | 0.68 |
| Follicle Stimulating Hormone (FSH), IU/L. | 6.85±2.79 | 6.55±0.85 | 0.0±0.0 | 0.79 |
| luteinizing Hormone (LH), IU/L. | 7.53±4.67 | 9.71±5.10 | 0.0±0.0 | 0.29 |
Arithmetic means ± standard error, * indicates significant difference between PCOS and control (P<0.05).
**indicates highly significant difference between PCOS and control (P<0.001).
Elisa studies:Plasma samples were separated from 50 PCOS women and using TNF-α kit (Cat# BMS223HS, Invitrogen, USA) as per the prescribed protocol from Invitrogen company. The enzyme reaction was ended by adding 100ul of stop solution and read the absorbance on a spectrophotometer using 450nm and 620nm as the primary and reference wave lengths. All the samples were performed in a duplicate. Finally, the concentration of TNF-α of each sample was calculated from the obtained standard curve. The reagents were stored between 2-8°C and lyophilized controls were stored at -20°C based on the recommended protocol.
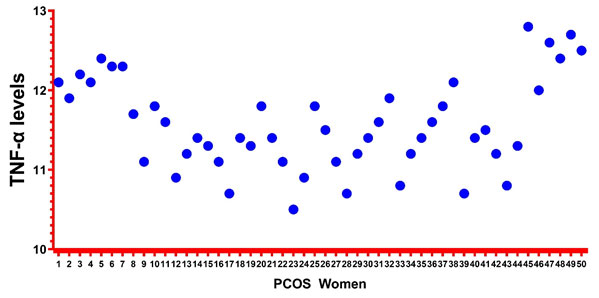
Figure 1: Representation of Elisa levels using GraphPad
DNA analysis: Genomic DNA was extracted from collected 1ml of the blood using Qiagen kit and the technique was implemented as per the recommended protocol. The separated DNA was measured with NanoDrop spectrophotometer and stored in the freezer at -80°C. Genotyping was performed with polymerase chain reaction and followed with RFLP analysis. PCR reaction was carried out with a total volume of 50µl for rs1799724 polymorphism, which consists of 10pmols of primers (+/-), 20ng of genomic DNA, 30µl of Qiagen master mix and 15µl of purified water. The cycling conditions was carried out in thermal cycles based on initial denaturation at 94°C for 10 mins, 94°C for 30 seconds of denaturation, 66C for 30s as annealing temperature, 72°C for 1 min as extension and 72°C for 10 mins as final extension which was followed for 40 cycles and holds at 4°C (Al-Otaiby et al., 2021, Alshammary and Khan, 2021).
The amplified obtained PCR product was 131bp and after digesting with HindII restriction enzyme at 18 hours for room temperature the digested PCR product produces 105 and 26bp at C allele and 131bp at T allele. All PCR products were separated using a 3% agarose gel electrophoresis and stained with ethidium bromide. The complete RFLP analysis was performed based on the Khan et al studies (Khan et al., 2015).
Statistical analysis:The distribution for normality of continuous variables was examined with Kolmogorov-Smirnov test between cases and controls. The mean ± SD, was used to present the normal distribution and HWE equilibrium was performed with -850C/T controls was assessed using a software defined (Khan et al., 2015). Genotype and allele frequencies were obtained from PCOS cases and controls using OR, 95%CI and specific p values. The ANOVA was performed with C-850T polymorphism with three genotypes in PCOS women (Khan et al., 2019). Elisa studies on TNF-α levels showed different levels in PCOS cases, which was represented using Graphpad software. The Elisa levels were measured in duplicates for 90% of PCOS cases.
RESULTS AND DISCUSSION
Demographic and Biochemical Details: Both the demographic and biochemical values of both PCOS and controls were shown in Table-1. The mean age of the PCOS cases and controls were found to be 32.1±5.74 and 34.64±4.74. The weight was found to be high among controls rather than cases. The mean height was found to be 158.4 in PCOS and 159.7 in controls. The PCOS cases were found to be overweight and controls were confirmed as obesity. The mean FBG was found to be 4.9 in both Cases and controls. The FSH levels were found to be high in PCOS cases than controls and elevated LH levels were found in PCOS cases than controls.
Elisa details:The Elisa levels were measured for 50 PCOS cases using Elisa Invitrogen kit. The mean levels were found to be 11.57. The minimum and maximum values for TNF-a levels were found to be 10.5 and 12.8. The values were recorded in pg/ml. The details were shown in Table-1. Simultaneously, figure-1 represents the Elisa levels in PCOS cases.
Genotyping details: The genotyping for -850C-T polymorphism was performed between PCOS cases and controls was shown in Table-2. CC and CT genotypes for PCOS cases was documented to be 88% and 12% and 86% and 14% was found in controls. None of the TT genotypes was documented in none of the PCOS cases and women. Genotype (CT vs CC-OR-0.84 [95%CI:0.26-2.69] p=0.72 and TT vs CC- OR-0.97 [95%CI:0.01-50.36] p=0.99) and dominant model (CC vs TT+CT- OR-0.83 [95%CI:0.26-2.69] p=0.76) don’t show any statistical association when compared between PCOS cases versus control subjects. The minor allele frequency of T allele showed 6% in PCOS and 7% in controls, whereas in C allele, PCOS women was confirmed to be 94% and 93% in controls (T vs C- OR-0.84 [95%CI:0.27-2.61] p=0.77).
In this case-control study, Anova analysis was performed between CC and CT genotypes in PCOS cases and compared with TNF-α levels, BMI, FBG, FSH and LH levels which is tabulated in Table-3. All the parameters involved in this study such as TNF-α levels, BMI, FBG, FSH and LH levels showed higher values in CT genotype when compared with CC genotypes. Both TNF-α levels (p=0.001) and BMI values (p=0.01) showed significant association when compared with CC, CT and TT genotypes. As usual, there was no values obtained in TT genotypes.
In the present study, the genotype and Elisa levels were measured for TNF-α gene. Genotyping was performed with PCOS cases and controls and the study results showed negative association either with genotypes (CT vs CC-OR-0.84 [95%CI:0.26-2.69] p=0.72 and TT vs CC- OR-0.97 [95%CI:0.01-50.36] p=0.99) and dominant model (CC vs TT+CT- OR-0.83 [95%CI:0.26-2.69] p=0.76) and allele frequencies (T vs C- OR-0.84 [95%CI:0.27-2.61] p=0.77). However, Elisa levels were measured only PCOS cases and duplicates was measure in above 90% of plasma samples. The minimum level was obtained is 0.5µl and maximum value was 2.8µl.
However, in CC genotypes, the lower and upper levels of TNF-α values were found to be 0.5-2.4pg/ml, whereas in CT genotypes, 2-2.8pg/ml levels were measured. The current study results confirmed as there was negative association with -850C/T polymorphism in PCOS cases versus controls. Additionally, there was no strong association when compared with genotyping and biochemicals levels such as FBG, FSH and LH (p>0.05) and positive association was documented with TNF-α levels and BMI (p<0.05), when performed Anova analysis.
PCOS is defined as the presence of multiple ovaries and prolonged oligo-anovulation in a proinflammatory state, which causes ovarian dysfunction and metabolic disorders. C-reactive protein levels were commonly elevated in PCOS women, and this low-grade inflammatory marker may lead to a serious of cardiac problems, as adipose tissue is associated with inflammation. TNF-α is one of the documented inflammatory markers in which their biological role of function varies with complexed mechanism. TNF-α plays enormous therapeutic roles in the body with immunostimulation. TNF-α and PCOS were associated in an in vitro study on rats, which found that TNF-α can alter the reproductive axis. It can also induce theca cell growth, stereogenesis, and has an apoptotic impact (Deligeoroglou et al., 2012, Ebejer and Calleja-Agius, 2013).
This study has performed based on limited sample size. From each group 50 cases and 50 controls were selected. The study results confirmed the negative association and this study is in agreement with the previous studies confirmed in PCOS women in global studies (Deepika et al., 2013, Escobar-Morreale et al., 2001, Guo et al., 2015, Korhonen et al., 2002, Milner et al., 1999, Vural et al., 2010, Yun et al., 2011) and only single study from India has confirmed the positive association (Thathapudi et al., 2014). Additionally, a previous meta-analysis carried out in PCOS and cytokine inflammatory markers which include -C850T polymorphism showed the negative association (Wu et al., 2015).
Global studies have performed the Elisa studies with TNF-α gene in PCOS women (Escobar-Morreale et al., 2001, Ihsan et al., 2018, Korhonen et al., 2002, Milner et al., 1999, Thathapudi et al., 2014) and our levels were found to be low when compared with other study results and global studies have also compared their serum/plasma levels with in controls. In this study, only PCOS cases were studies and controls were excluded. This study has certain limitations as only 50 PCOS cases and 50 controls were studies and Elisa levels were measured only in PCOS women and excluded the controls. The other limitation of this study was only C-850T polymorphism was studies and documents as negative association. The strength of this study was carried out with 50 PCOS women and 50 controls selected from Saudi women. RFLP analysis was performed in this study to detect the polymorphism frequencies.
CONCLUSION
In conclusion, this study confirms the negative association with -850C/T polymorphism in TNF-α gene and elevated Elisa levels were observed in CT genotypes. The -850C/T polymorphism which was previously documented from Saudi population, global and meta-analysis studies.
REFERENCES
Aboeldalyl, S., James, C., Seyam, E., Ibrahim, E. M., Shawki, H. E.-D. & Amer, S. (2021). The Role Of Chronic Inflammation In Polycystic Ovarian Syndrome—A Systematic Review And Meta-Analysis. International Journal Of Molecular Sciences, 22, 2734.
Al-Otaiby, M., Althnayan, R., Binmethem, A., Alenezy, R. B., Alhadlg, M. A., Alaqeel, A., Alqahtani, S. H., Ghufran, N., Alotaibi, A. A. & Alayed, N. (2021). The Prevalence Of Factor V Leiden (Arg506gln) Mutation In King Khalid University Hospital Patients, 2017–2019. Nagoya Journal Of Medical Science, 83, 407.
Alkhuriji, A., Al Omar, S., Babay, Z., El-Khadragy, M., Allharbi, W. & Alnafjan (2018) A. Lack Of Association Between Il10 And Tnfα Gene Polymorphisms And Polycystic Ovary Syndrome In Saudi Women.
Alshammary, A. F. & Khan, I. A. (2021). Screening Of Obese Offspring Of First-Cousin Consanguineous Subjects For The Angiotensin-Converting Enzyme Gene With A 287-Bp Alu Sequence. Journal Of Obesity & Metabolic Syndrome, 30, 63.
Aversa, A., La Vignera, S., Rago, R., Gambineri, A., Nappi, R. E., Calogero, A. E. & Ferlin, A. (2020). Fundamental Concepts And Novel Aspects Of Polycystic Ovarian Syndrome: Expert Consensus Resolutions. Frontiers In Endocrinology, 11.
Combs, J. C., Hill, M. J. & Decherney, A. H. (2021). Polycystic Ovarian Syndrome Genetics And Epigenetics. Clinical Obstetrics And Gynecology, 64, 20-25.
Deepika, M., Reddy, K. R., Yashwanth, A., Rani, V. U., Latha, K. P. & Jahan, P. (2013). Tnf-Α Haplotype Association With Polycystic Ovary Syndrome–A South Indian Study. Journal Of Assisted Reproduction And Genetics, 30, 1493-1503.
Deligeoroglou, E., Vrachnis, N., Athanasopoulos, N., Iliodromiti, Z., Sifakis, S., Iliodromiti, S., Siristatidis, C. & Creatsas, G. (2012). Mediators Of Chronic Inflammation In Polycystic Ovarian Syndrome. Gynecological Endocrinology, 28, 974-978.
Ebejer, K. & Calleja-Agius, J. (2013). The Role Of Cytokines In Polycystic Ovarian Syndrome. Gynecological Endocrinology, 29, 536-540.
Escobar-Morreale, H. C. F., Calvo, R. M., Sancho, J. & San Millán, J. L. (2001). Tnf-Α And Hyperandrogenism: A Clinical, Biochemical, And Molecular Genetic Study. The Journal Of Clinical Endocrinology & Metabolism, 86, 3761-3767.
Gnanadass, S. A., Prabhu, Y. D. & Gopalakrishnan, A. V. (2021). Association Of Metabolic And Inflammatory Markers With Polycystic Ovarian Syndrome (Pcos): An Update. Archives Of Gynecology And Obstetrics, 1-13.
Guo, R., Zheng, Y., Yang, J. & Zheng, N. (2015). Association Of Tnf-Alpha, Il-6 And Il-1beta Gene Polymorphisms With Polycystic Ovary Syndrome: A Meta-Analysis. Bmc Genetics, 16, 1-13.
Guzmán-Flores, J. M., Escalante, M., Sánchez-Corona, J., García-Zapién, A. G., Cruz-Quevedo, E. G., Munoz-Valle, J. F., Moran-Moguel, M. C., Saldana-Cruz, A. M. & Flores-Martínez, S. E. (2013). Association Analysis Between–308g/A And–238g/A Tnf-Alpha Gene Promoter Polymorphisms And Insulin Resistance In Mexican Women With Gestational Diabetes Mellitus. Journal Of Investigative Medicine, 61, 265-269.
Ihsan, I., Tehreem, A. & Rasool, S. (2018). Significance Of Tnf-Alpha And Insulin Resistance In Women With Polycystic Ovarian Syndrome. Pakistan J Med Health Sci, 12, 459-463.
Khan, I. A., Jahan, P., Hasan, Q. & Rao, P. (2019). Genetic Confirmation Of T2dm Meta-Analysis Variants Studied In Gestational Diabetes Mellitus In An Indian Population. Diabetes Metab Syndr, 13, 688-694.
Khan, I. A., Kamineni, V., Poornima, S., Jahan, P., Hasan, Q. & Rao, P. (2015). Tumor Necrosis Factor Alpha Promoter Polymorphism Studies In Pregnant Women. Journal Of Reproductive Health And Medicine, 1, 18-22.
Korhonen, S., Romppanen, E.-L., Hiltunen, M., Mannermaa, A., Punnonen, K., Hippeläinen, M. & Heinonen, S. (2002). Lack Of Association Between C-850t Polymorphism Of The Gene Encoding Tumor Necrosis Factor-Α And Polycystic Ovary Syndrome. Gynecological Endocrinology, 16, 271-274.
Lentscher, J. A., Slocum, B. & Torrealday, S. (2021). Polycystic Ovarian Syndrome And Fertility. Clinical Obstetrics And Gynecology, 64, 65-75.
Ma, Y., Ma, L., Cao, Y. & Zhai, J. (2021). Construction Of A Cerna-Based Lncrna-Mrna Network To Identify Functional Lncrnas In Polycystic Ovarian Syndrome. Aging (Albany Ny), 13, 8481.
Milner, C., Craig, J., Hussey, N. & Norman, R. (1999). No Association Between The–308 Polymorphism In The Tumour Necrosis Factor Α (Tnfα) Promoter Region And Polycystic Ovaries. Molecular Human Reproduction, 5, 5-9.
Mohamadin, A. M., Habib, F. A. & Elahi, T. F. (2010). Serum Paraoxonase 1 Activity And Oxidant/Antioxidant Status In Saudi Women With Polycystic Ovary Syndrome. Pathophysiology, 17, 189-196.
Pan, M.-L., Chen, L.-R., Tsao, H.-M. & Chen, K.-H. (2015). Relationship Between Polycystic Ovarian Syndrome And Subsequent Gestational Diabetes Mellitus: A Nationwide Population-Based Study. Plos One, 10, E0140544.
Tatkare, H. U. (2021). The Relationship Between Obesity, Visceral Adiposity And Polycystic Ovarian Syndrome In Adult Women Of Childbearing Age. California Baptist University.
Thathapudi, S., Kodati, V., Raj, A. Y., Addepally, U., Katragadda, A. & Hasan, Q. (2014). Role Of Tnf Α In The Etiopathogenesis Of Pcos: A Clinical, Biochemical And Molecular Genetic Study. Molecular Cytogenetics, 7, 1-1.
Vural, P., Değirmencioğlu, S., Saral, N. Y. & Akgül, C. (2010). Tumor Necrosis Factor Α (− 308), Interleukin-6 (− 174) And Interleukin-10 (− 1082) Gene Polymorphisms In Polycystic Ovary Syndrome. European Journal Of Obstetrics & Gynecology And Reproductive Biology, 150, 61-65.
Wu, H., Yu, K. & Yang, Z. (2015). Associations Between Tnf-Α And Interleukin Gene Polymorphisms With Polycystic Ovary Syndrome Risk: A Systematic Review And Meta-Analysis. Journal Of Assisted Reproduction And Genetics, 32, 625-634.
Yun, J.-H., Choi, J.-W., Lee, K.-J., Shin, J.-S. & Baek, K.-H. (2011). The Promoter-1031 (T/C) Polymorphism In Tumor Necrosis Factor-Alpha Associated With Polycystic Ovary Syndrome. Reproductive Biology And Endocrinology, 9, 1-6.


