1,2Departement of Fisheries Science, Faculty of Agriculture, Madura Islamic University, Pamekasan 69351, Indonesia
Corresponding author email: yonosugiono78@yahoo.co.id
Article Publishing History
Received: 17/10/2019
Accepted After Revision: 15/12/2019
Brown algae has a fucoidan and alginate bioactive components with different characteristics. Brown algae has a great potential as feedstock for biorefinery alginate and fucoidan extraction which is integrated. Alkaline extraction process parameters in the sequential fucoidan and alginate extraction are integrated affected to the characteristics of alginate and fucoidan from brown alga Sargassum cristaefolium. This study aims to understand the effect of the parameters of the alkaline extraction process on multiple responses alginate and fucoidan yields, and determine the optimal alkaline extraction process in the integrated alginate and fucoidan sequential extraction processes according to the concept of industrial biorefinery. Box Bhenken Design from the response surface method was used to understand the effect of process parameters temperature, time and Na2CO3 levels on the multiple responses alginate (yield, intrinsic viscosity, molecular weight) and fucoidan yield. The results showed that the alkaline extraction process parameters significantly affected on the multiple responses alginate and fucoidan yield with quadratic pattern. The optimal conditions occur at a temperature of 57.02 oC, time of 123.96 min, and Na2CO3 concentration of 2.66%. Under the optimal point, the yield of alginate was 33.93%, intrinsic viscosity was 404.73 ml/g, molecular weight of alginate was 191.38 kDa and fucoidan yield was 1.92%.
Fucoidan, Alginate, Biorefinery, Alkaline-Treatment, Sargassum cristaefolium
Sugiono S, Ferdiansyah D. Biorefinery Sequential Extraction of Alginate By Conventional and Hydrothermal Fucoidan from the Brown Alga, Sargassum cristaefolium. Biosc.Biotech.Res.Comm. 2019;12(4).
Sugiono S, Ferdiansyah D. Biorefinery Sequential Extraction of Alginate By Conventional and Hydrothermal Fucoidan from the Brown Alga, Sargassum cristaefolium. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2DPmuOF
Copyright © Sugiono and Ferdiansyah This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Brown algae has a fucoidan and alginate bioactive components with different characteristics (Rioux et al., 2007; Torres et al., 2007; Draget and Taylor, 2011). The function of alginates works as thickener and gelling agent, while alginate gels are thermostable (Rahelivao et al., 2013; Sellimi et al., 2015). Alginate is widely used in non-food and food industries (Poncelet et al., 1999; Gomez et al., 2009), in the field of pharmaceutical, alginate is used as a slow release of drugs (pharmaceuticals) and antitumor compounds (Sousa et al., 2007; Moebus et al., 2012; Wang et al., 2010; Jensen et al., 2012). The fucoidan works as an anti-inflammatory, anti-tumor, anti-cancer, and immunomodulatory compound (Asker et al., 2007; Ye et al., 2008; Kim et al. 2010; Ale et al., 2011; Costa et al., 2011). Brown algae has great potential as a feedstock process of bio-refinery alginate extraction and integrated fucoidan (Jung et al., 2013; Ruiz et al., 2013), however this potential cannot be optimally utilized to produce alginate and fucoidan to meet domestic needs.The problem of the fucoidan and alginate sequential extraction on bio-refinery process is low fucoidan yield. This is because fukoidan extraction with acid treatment is carried out at low temperatures and when done at high temperatures, the results show degradation of the alginate polymer chains that indicates this method is not effective (Sugiono and Ferdiyansah, 2018).
Therefore, an alternative extraction is necessary for more effective methods that can produce high yield fucoidan with good quality, namely the conventional alginate extraction bio-refinery process and algae residues as feedstock for hydrothermal extraction of fucoidan.
Alginates works as polar and in sodium carbonate solution, alginate extraction with sodium carbonate solution can produce alginates with high yield and viscosity . While fucoidan is polar soluble in acidic solutions and water. Hydrothermal fucoidan extraction can produce fucoidan with low molecular weight and high yield (Quitain et al., 2013). Low fucoidan molecular weight has higher bioactive properties than large fucoidan molecular weight . This is the basis for the development of the conventional alginate extraction bio-refinery process and hydrothermal fucoidan extraction. However, the effect of the alkaline treatment conditions on multiple responses of alginate and fucoidan through an integrated process is not yet known.
Some researchers have used alkaline treatment for sequential extraction of alginate and fucoidan to characterize (Rioux et al., 2007), but it does not refer to the perspective of industrial bio-refinery. Therefore, it is essentially needed for optimal alkaline treatment conditions in the bio-refinery extraction process of alginate and fucoidan from brown algae so that it can produce high yields and good quality. In this study optimization parameters of Na2CO3, temperature and time of conventional alginate extraction and integrated fucoidan hydrothermal will be carried out.
MATERIALS AND METHODS
Materials and reagents
Brown algae Sargassum cristaefolium was obtained from Poteran Island in Sumenep, Madura, and collected in Maret 2019. Chemicals (distilled water, ethanol 99.8%, Na2CO3) for extraction and analysis were analytical grade.
Sample preparation
The Brown algae washed with fresh water until it is clean, then dried under the sun to reach 13% moisture content. Dried brown algae are processed with a coffee grinder and filtered with a 60 mesh filter (Lorbeer et al. 2015). Brown algae were immersed in a 96% Etoh for overnight to remove phenol and protein components, washed and dried at 45 oC for 6 hours (Ale et al. 2012).
Sequensial extraction alginate and fucoidan:Alginate extraction
10 g of brown algae were added with Na2CO3 solution with a concentration of 1-5%, solvent ratio 1:20 (w/v). Alginate is extracted conventionally with a water temperature of 30-90 oC for 60-180 min (Lorbeer et al., 2015). Then cooled and filtered with a filter press cloth so that the residue A and filtrate are obtained. The alginate filtrate was added with a 96% ethanol ratio of 1: 2 (v / v) left for 2 hours and filtered. Alginate is washed twice with 70% and 96% of ethanol then filtered and pressed, the alginate is dried in an oven at 45 oC for 24 hours and milled on 60 mesh.
Fucoidan extraction
Residue A of alginate extraction was dissolved in 1:60 (w/v) ratio distilled water, and hydrothermally extracted using ECOPAN VITA + Smart Pressure Cooker (90 KPa) for 3 hours. Then the residue and filtrate are separated. Fucoidan filtrate added with ethanol 96% ratio of 1: 2 (v/v) was left overnight, fucoidan were separated by centrifugation speed of 7000 rpm for 10 minutes. The fucoidan are collected and dried with a vacuum dryer at 45 oC for 16 hours (Ale et al., 2012).
Experimental design and statistical analysis
The experimental design used in this study is the Box Behnken Design from the response surface method. The parameters and levels studied were temperature (30, 60, 90 oC), duration (60, 120, 180 min) and Na2CO3 levels (1, 3, 5%) coded +1, 0, and -1 (Table 1). Actual variables and codes with 3 central point replications are presented in Table 2, the total number of experiments was 15 treatments (Montgomery, 2005). The central point chosen in this study is the result of previous studies. The yield of alginate extract (%), intrinsic viscosity (ml/g), molecular weight (kDa) and fucoidan yield (%) of the BBD design were analyzed response surface regression and the accuracy of the polynomial model (eq. 1).
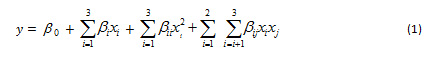
Table 1. Coded and actual of independent variables
| Independent variables | Symbols | Variables | |
| Coded | Actual | ||
| Temperature (oC) | x1 | -1 | 30 |
| 0 | 60 | ||
| +1 | 90 | ||
| Time (min) | x2 | -1 | 60 |
| 0 | 120 | ||
| +1 | 180 | ||
| Na2CO3 (%) | x3 | -1 | 1 |
| 0 | 3 | ||
| +1 | 5 | ||
Table 2. Box-Behnken Design from RSM and responses
| No | Actual variables | Responses | |||||
| Temperature
(°C) |
Time
(min) |
Na2CO3 | Fucoidan yield
(%) |
Alginate yield
(%) |
Intrinsic viscosity (ml/g) | Molecular weight
(kDa) |
|
| 2 | 60 | 180 | 1 | 1.92 | 30.12 | 140.15 | 65.14 |
| 3 | 90 | 120 | 1 | 0.75 | 28.90 | 135.73 | 63.05 |
| 4 | 30 | 180 | 3 | 1.72 | 28.21 | 260.21 | 122.18 |
| 6 | 90 | 120 | 5 | 0.20 | 40.60 | 65.92 | 29.83 |
| 7 | 30 | 120 | 1 | 1.50 | 27.10 | 149.61 | 69.61 |
| 8 | 60 | 60 | 5 | 0.22 | 27.32 | 113.45 | 52.55 |
| 9 | 90 | 60 | 3 | 0.27 | 29.80 | 198.61 | 92.84 |
| 10 | 30 | 60 | 3 | 0.25 | 27.48 | 267.92 | 125.86 |
| 12 | 30 | 120 | 5 | 0.26 | 29.13 | 112.55 | 52.130 |
| 13 | 60 | 180 | 5 | 1.23 | 36.74 | 97.32 | 44.97 |
| 14 | 90 | 180 | 3 | 1.78 | 35.98 | 195.40 | 91.32 |
| 15 | 60 | 60 | 1 | 1.54 | 29.85 | 287.42 | 135.17 |
| 1 | 60 | 120 | 3 | 1.81 | 35.80 | 407.21 | 192.59 |
| 5 | 60 | 120 | 3 | 1.91 | 34.54 | 412.06 | 194.93 |
| 11 | 60 | 120 | 3 | 1.72 | 33.21 | 392.56 | 185.55 |
| Pred. | 57.02 | 123.99 | 2.66 | 1.92±0.17a | 33.12±0.82b | 404.73±18.55c | 191.38±8.81d |
| Valid | 57.02 | 123.99 | 2.66 | 1.81±0.06a | 34.51±0.87b | 409.72±7.59c | 194.08±3.65d |
Yield
Yield was calculated based of ratio between alginate or fucoidan weight over to initial wight of brown algae, and then multiplied 100 % (Lorbeer et al., 2015).
Intrinsic viscosity
Intrinsic viscosity of alginate was determined by using capillary viscometer Ubbelohde (Canon, USA), capillary diameter of 0.56 and employer at temperature 25 oC. 30 mg alginate was diluted in 10 ml aquabides, stirred for 5 hour at room temperature (25 oC), and then made serial concentration of 0.05-0.3 g/dL. Flow time of solution (t) relative to flow time of solvent (t0). The intrinsic viscosity was determined by extrapolating from equation ɳsp/c (eq. 5) until zero concentration (Chee et al., 2011)
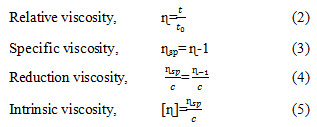
Molecular weight
Alginate molecular weight was calculated based relationship between averaged intrinsic viscosity and molecular weight by using Mark-Houwink equation (eq. 6), where k=0.023 dL/g and a=0.984 (Clementi et al., 1998).
![]()
Where [ɳ] is intrinsic viscosity in dL/g, Mw = molecular weight in kDa
RESULT AND DISCUSSION
Alginate yield
The results showed that the extraction process parameters (temperature, time, concentration of Na2CO3) on the yield of alginate was obtained yield of alginate within a range between 27.1-40.6% (Table 2). The higher the temperature, extraction time and concentration of Na2CO3, the yield of alginate tends to increase with a higher temperature, extraction time, and concentration of Na2CO3. The cell walls of brown algae become softer and expand with increasing temperature, time and concentration of Na2CO3 so that alginate extractability increases. Fertah et al. (2014) states that the higher the extraction process parameters (temperature and time), the higher the yield obtained until it reaches the optimal point after it decreases. Brown algal cell walls are increasingly porous as a result of higher temperatures and extraction times (Hernadez-Carmona et al., 1999; Torres et al., 2007).
The results of the study (Figure 1) showed that the extraction process parameters had a significant effect (P <0.05) on alginate yield. Alginate yield is higher with the increasing temperature, time and concentration of Na2CO3 with quadratic pattern. The highest alginate yield of 40.6% occurred at 90 oC, 120 min and 5% Na2CO3 of concentration, while the lowest alginate yield was 27.1% at 30 oC, 180 min and 1% Na2CO3 of concentration. The results of this study are aligned with the mentioned literature (Rahelivao et al., 2013; Lorbeer et al., 2015; Sugiono et al., 2019b).
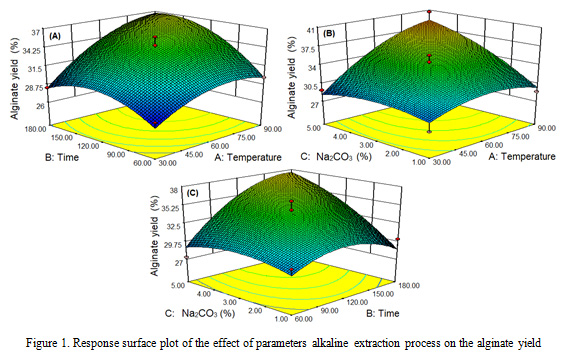 |
Figure 1: Response surface plot of the effect of parameters alkaline extraction process on the alginate yield |
Intrinsic viscosity
The parameters of the extraction process temperature, time and concentration of different Na2CO3 obtained an intrinsic viscosity of alginate that increased at 60 oC, 120 min and concentration of 3% then decreased at 90 oC, 180 min, and 5% of Na2CO3 concentration. The highest intrinsic viscosity is obtained for 412.06 ml/g at a temperature of 60 oC, 120 minutes and a concentration of 3%. The lowest intrinsic viscosity at 90 oC, 180 minutes, and 5% of concentration is 65.92 ml/g. The results of this study are in accordance with that reported by Rahelivao et al. (2013), Fenorodosa et al. (2010) and Torres et al. (2009).
The parameters of 1-5% Na2CO3 concentration, temperature of 30-90 oC and extraction time of 60-180 min had a significant effect (P <0.05) with a quadratic pattern on the intrinsic viscosity of Sargassum cristaefolium alginate (Figure 2). Intrinsic viscosity of alginate increases with the higher extraction parameters and then decreases after reaching optimal points. This matter can be explained by several phenomena: first, increasing the process parameters causes the algae cell wall to expand and soft so that the extraction of alginate of large molecular weight increases. Second, the low intrinsic viscosity at 1% Na2CO3 concentration because of the long chain polymer alginate is not extracted, so that the viscosity is low. Third, the decrease in intrinsic viscosity of alginate at 5% Na2CO3 concentration is produced as a result of the degradation of the alginate polymer chain (Smidsrod et al., 1969; Haug et a1., 1967). The degradation of the main chain of alginate polymers increases rapidly at 90 oC and 180 minutes (Hernandez-Carmona et al., 1999; Lorbeer et al., 2015; Silva et al., 2015; Sugiono et al., 2019a).
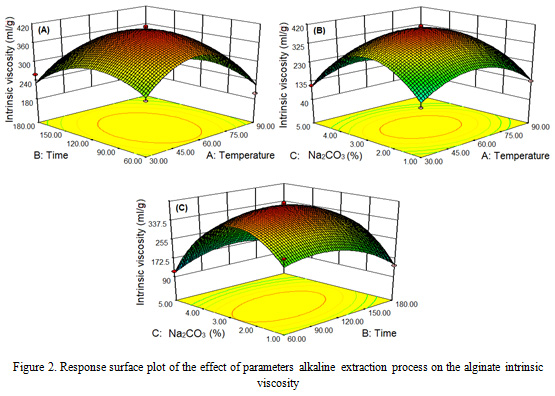 |
Figure 2: Response surface plot of the effect of parameters alkaline extraction process on the alginate intrinsic viscosity |
Molecular weight
Biorefinery process parameters extraction on temperature, time and concentration of Na2CO3 to the molecular weight of Sargassum cristaefolium, obtained alginate molecular weight with a range of 29.83-194.93 kDa (Table 2). The highest molecular weight of alginate occurs at 60 oC, 120 min and 3% of Na2CO3 concentration, while the lowest alginate molecular weight occurs at 30 oC, 120 min and 5% of Na2CO3 concentration. The parameters of the extraction process on temperature, time and concentration of Na2CO3 have a positive effect on the molecular weight of alginate up to 60 oC, 120 min and 3% Na2CO3, decreasing at 90 oC and 5% of concentration within 60-180 min. The results of this study are consistent with the reported by Fertah et al. (2014), Hernandez-Carmona et al. (1999), and Sugiono et al. (2018).
The effect of single factor extraction process of different temperature, time and Na2CO3 concentration significantly affected (P<0.05) alginate molecular weight with quadratic pattern (Figure 3). The higher the extraction process parameters, the alginate molecular weight increases, then decreases after reaching the optimal point. This can be explained by several phenomena. First, the higher the parameters process, it will cause the algae cell walls to become soft so that long chain alginates are extracted. Second, at 5% Na2CO3 concentration, the alginate molecular weight is low due to the beta elimination reaction and the degradation of the alginate polymer chain (Hernandez-Carmona et al., 1999; Smidsrod et al., 1969). Third, at low concentrations of Na2CO3 alginate polymer, degradation occurs due to hydrolysis catalyzed by protons (Smidsrod et al., 1963). Alginate extraction from brown algae in a solution of pH 12 Na2CO3 obtained low molecular weight alginate as a result of the degradation of the alginate polymer chain, this is proven by the low intrinsic viscosity of alginate (Sugiono et al., 2019b).
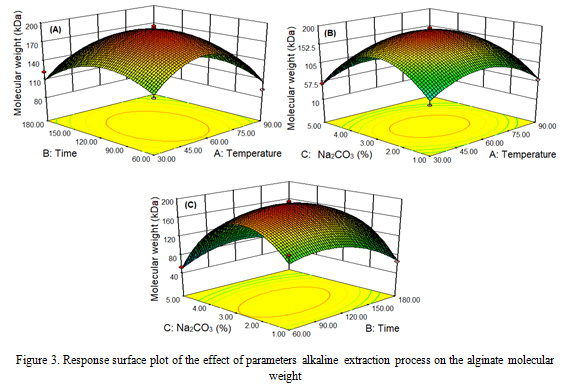 |
Figure 3: Response surface plot of the effect of parameters alkaline extraction process on the alginate molecular weight. |
Fucoidan yield
The results showed that the effect of different extraction parameters of temperature, time and concentration of Na2CO3 on the alginate and fucoidan sequential extraction processes of fucoidan yields, it is obtained fucoidan yield ranged from 0.2-1.96%. The highest fucoidan yield occurred at 60 oC, 120 minutes and 3% concentration, while the lowest fucoidan yield occurred at 90 oC, 120 minutes and 5% Na2CO3 concentration. The results of this study are consistent with those mentioned in the literature (Ale et al., 2012; Sugiono et al., 2014; Lorbeer et al., 2015).
The different extraction process parameters (temperature, time and concentration of Na2CO3) had a significant effect (P<0.05) on the yield of fukoidan (Figure 4). The higher the extraction process parameters, the higher the fucoidan yield obtained, then it decreases after reaching the optimal point. The results of this study are in accordance with that reported by Qiao et al. (2009), Rodriquez et al. (2010), and Lorbeer et al. (2015). The Increase of the temperature parameters and the concentration of Na2CO3 causes the algae cell walls to become brittle, in that case fucoidan is easily extracted. At 90 oC and Na2CO3 concentration 5%, fucoidan yield decreased because it was suspected that some of the fucoidan was extracted and dissolved as impurities during alginate extraction. Sugiono et al. (2019a) reported that the increasing Na2CO3 concentrations in the alginate extraction process would cause the algae cell wall to expand and soft, while the fucoidan is also extracted and dissolved as a impurity
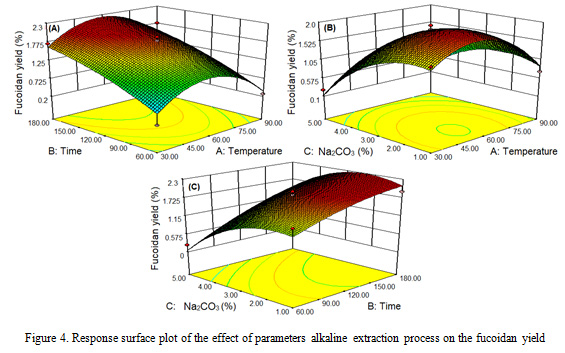 |
Figure 4: Response surface plot of the effect of parameters alkaline extraction process on the fucoidan yield |
Fitting models
Box Behnken Design (BBD) from the response surface method with three central point replications used to test the effect of three variables (temperature, time and Na2CO3) sequential extraction of alginate and fucoidan against alginate yield, intrinsic viscosity, alginate molecular weight and fucoidan yield. The second order polynomial model of multiple responses alginate and fucoidan yield is presented in Table 3.
Table 3. Polynomial models, significance codes and fitting models
| Coefficient | Fucoidan yield
(%) |
Alginate yield
(%) |
Intrinsic viscosity
(ml/g) |
Molecular weight
(kDa) |
| Intercept | ||||
| β0 | +1.81 | +31.65 | +403.94 | +191.02 |
| Linear | ||||
| valueβ1 | -0.091ns | +2.92* | -24.33ns | -11.59ns |
| β2 | +0.55** | +2.07ns | -21.79ns | -10.35ns |
| β3 | -0.48** | 2.23 ns | -40.46* | -19.19* |
| Quadratic | ||||
| β11 | -0.68** | -1.86 ns | -108.52** | -51.89** |
| β22 | – 0.13ns | -2.29 ns | -64.89* | -31.09* |
| β33 | -0.46* | -1.22 ns | -179.47** | -85.48** |
| Cross product | ||||
| β12 | +1.00ns | +1.36ns | +1.13ns | +0.54ns |
| β13 | 0.17ns | +2.42* | -8.19 ns | -3.94 ns |
| β23 | 0.16ns | +2.29 ns | +32.78 ns | +15.61ns |
| Fitting model | ||||
| P value | 0.0145* | 0.0205* | 0.0578* | 0.0022** |
| Lack of Fit | 0.0611ns | 0.2778ns | 0.7914ns | 0.059ns |
| R2 | 0.9393 | 0.9296 | 0.9723 | 0.9725 |
Equation of the type Y= β0+ βx1+ βx2+ βx3+ βx1x2+ βx1x3+ βx2x3+ βx1x1+ βx2x2+ βx3x3
Significance codes: ***= P <0.001
** = 0.001<P<0.01
* = 0.01<P<0.05
ns = P>0.05
Evaluation of the accuracy of the quadratic model of alginate yield response, intrinsic viscosity, alginate molecular weight and fucoidan yield based on parameters of model significance, correlation coefficient and lack of fit are presented in Table 3. The model compatibility has a significance value of P < 0.05, R2 ≥ 0.8 and Lack of fit > 0.1 (Montgomery, 2005). The fitting model based on these parameters show that, the second-order of all response polynomial model is high adequate, P value in all the multiple response alginate and fucoidan yield is < P=0.05, there is no significance lack of fit because lack of fit value is more than 0.1 in all response and R2 value more than 80% in all response.
Optimization and verification
Based on the results of the design expert version 7 program analysis, the optimal conditions for fucoidan and alginate sequential extraction occurred at 57.02 oC, 123.96 min, and 2.66% Na2CO3 concentration. The response of prediction value under optimal conditions is 33.93% alginate yield, 404.73 ml/g intrinsic viscosity, alginate molecular weight of 191.38 kDa and fucoidan yield 1.92% with desirability value of 0.989. The desirability value is close to 1 indicates that the predicted value of the Design Expert program has a high level of validity (Ale et al., 2012; Sugiono et al., 2019b).
The optimal process parameter conditions as predicted by the design expert program version 7 (57.02 oC, 123.96 min, Na2CO3 2.66%) were conducted with 3-replication verification experiments. The optimal point verification results obtained alginate yield 34.51 ± 0.87%, intrinsic viscosity 409.72 ± 7.59 ml/g, alginate molecular weight 194.08 ± 3.65 kDa and fucoidant yield 1.81 ± 0.06%. The results of verification of multiple responses alginate and fucoidan yield are in the range of predicted 95% PI high and 95% PI low. The paired t-test results found that between the predicted values of the program and the validation experiments were not significantly (P > 0.05), this indicates that the results of the validation experiments supported the results of the program analysis.
CONCLUSION
The bio-refinery of sequential alginate extraction through conventional and hydrothermal fucoidan has been succeeded to develop. This processes produces two products which are alginate and fucoidan. The temperature extraction, duration and Na2CO3 concentration process significantly affect to fucoidan yields and alginate multiple response. The optimal condition of temperature and Na2CO3 concentration of bio-refinery extraction occurs at the temperature of 57.02 oC, 124.01 min and Na2CO3 concentration of 2.66%, with the value of fucoidan yield is 1.92 ± 0.034%, alginate yield 33.93 ± 2.13%, intrinsic viscosity 404.73 ± 37.47 ml/g, and alginate molecular weight of 191.38 ± 17.80 kDa.
ACKNOWLEDGMENT
Authors wish to acknowledge the funding support from Ministry of Research, Technology, and Higher Education of Rep. of Indonesia for financial support with contract number 055/SP2H/LT/K7/2019.
CONFLICTS OF INTEREST
The author state that in this study there were no conflict of interest.
REFERENCES
Ale, M. T., Mikkelsen, J. D. and Meyer, A. S. (2011). Review: Important determinants for fucoidan bioactivity: A Critical Review of Structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Marine Drugs. 9: 2106-2130
Ale, M.T., Mikkelsen, J. D. and Meyer, A.S. (2012). Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. Journal Applied Phycolology, doi:10.1007/s10811-011-9690-3.
Asker, M. S., Mohamed S. F., Osama and Ali F. M. (2007). Chemical structure and antiviral activity of water-soluble sulfated polysaccharides from Sargassum latifolium. Journal Applied Sciences Research. 3(10): 1178-1185.
Chee, S.Y., Wong, P.K., and Wong, C.L. (2011). Extraction and characterisation of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. Journal Applied Phycology, 23:191–196
Clementi, F., Mancini, M., Moresi, M. (1998). Rheology of alginate from Azotobacter vinelandii in aqueous dispersions. Journal Food Engineering, 36: 51-62
Costa, S.L., 1,2, Telles, C.B.S., Oliveira, R. M., Leonardo, T.D.B.N., Nednaldo, D.D.S., Rafael, B.G.C., Mariana, S.S.P.C., Jailma, A.L., Raniere, F.M.S., Ivan R.L., Albuquerque, Edda, L.l. and Hugo A.O.R. (2011). Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Marine Drugs, 9: 952-966.
Draget, Kurt I., and Taylor, C. (2011). Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocolloids, 25(2): 251–56.
Fenoradosoa, T.A., Ali, G., Delattre, C., Laroche, C., Petit, E., Wadouachi, A., and Michaud, P. (2010). Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. Journal Applied Phycology, 22: 131–137
Fertah, M., Belfkira, A., Dahmane, E.M., Taurirte, M., Brouillette, A., and Taurirte, M. (2014). Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arabian Journal of Chemistry, 5(3): 1878-1888
Gomez, C.G., Lambrcht, M.V.P., Lozano, J.E., Rinaudo, M. and Villar, M.A. (2009). Influence of the extraction-purification condition on final properties of alginates obtained from brown algae (Macrocystis pyrifera). International Journal of Biological Macromolecules, 44: 365-371
Hernandez-Carmona, G., McHugh, D.J., Arvizu-Higuera, D.L., and Rodriguez-Montesinos, Y.E. (1999). Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1. Effect of pre-extraction treatments on yield and quality of alginate. Journal of Applied Phycology, 10: 507-513
Jensen, M.G., Knudsen, J.C., Viereckb, N., Kristensen, M., and Astrup, A. (2012). Functionality of alginate based supplements for application in human appetite regulation. Food Chemistry, 132: 823–829
Jung, K.A., Lim, S.R., Kim, Y., Park, J.M. (2013). Potentials of macroalgae as feedstocks for biorefinery. Bioresource Technology, 135: 182–190
Kim, W.J., Koo ,Y.K., Jung, M.K., Moon, H.R., Kim, S.M., Synytsya, I., Yun-Choi, H., H.S.Y., Kim, Y.S., Park, J.K., Park, Y. (2010). Anticoagulating Activities of Low-Molecular Weight Fuco-Oligosaccharides Prepared by Enzymatic Digestion of Fucoidan from the Sporophyll of Korean Undaria pinnatifida. Arch Pharmaceutical Research, 33(1): 125-131.
Lorbeer, A. J., Lahnstein, J., Bulone, V., Nguyen, T., and Zhang, W. (2015) Multiple-response optimization of the acidic treatment of the brown alga Ecklonia radiata for the sequential extraction of fucoidan and alginate. Bioresource Technology, 197: 302-309
Moebus, K, Siepmann, J., and Bodmeier, R. (2012). Novel preparation techniques for alginate–poloxamer microparticles controlling protein release on mucosal surfaces. European Journal of Pharmaceutical Sciences, 45: 358–366
Montgomery, D.C. (2005). Response surface methods and designs. New York. USA: John Willy and Sons. Inc.
Poncelet, D., Babak , V., Dulieu, C., and Picot, A. (1999). A physico-chemical approach to production of alginate beads by emulsification-internal ionotropic gelation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 155: 171–176
Qiao, D., Hu, B., Gan, D., Sun, Y., Ye, H,, Zeng, X. (2009). Extraction optimized by using response surface methodology, purification and preliminary characterzation of polysaccharide from Hyriopsis cumingi. Carbohydr Polym. 76:422–429.
Quitain, A.T., Kai, T., Sasaki, M., Goto, M. (2013). Microwave– hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled Undaria pinnatifida. Industrial and Engineering Chemistry Research., 52(23): 7940-7946
Rahelivao, Pascaline, M., Andriamanantoanina, H., Heyraud, A., and Rinaudo, M. (2013). Structure and Properties of Three Alginates from Madagascar Seacoast Algae. Food Hydrocolloids 32(1): 143–46.
Rioux, L.E., Turgeon, S.L., and Beaulieu, M. (2007). Characterization of polysaccharides extracted from brown seaweeds. Carbohydrates polymers, 69. 530-537.
Rodriguez-Jasso, R.M., Mussatto, S.I., Pastrana, L., Aguilar, C.N. and Teixeira, J.A. (2011). Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydrate Polymers, 86: 1137-1144
Ruiz, H. A., Rosa, M., Rodrıguez-Jasso, Bruno D., Fernandes, Antonio, A., Vicente, Jose A., Teixeira. (2013). Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renewable and Sustainable Energy Reviews, 21: 35–51
Sellimi, S., Younes, I., Ayed, H.B., Maalej, H., Montero, V., Rinaudo, M., Dahia, M., Mechichi, T., Hajji, M., Nasri, M. (2015). Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. International Journal of Biological Macromolecules, 72: 1358–1367
Silva, M., Gomes, F., Oliveira, F., Morais, S., and Delerue-matos, C. (2015). Microwave-Assisted Alginate Extraction from Portuguese Saccorhiza polyschides – Influence of Acid Pretreatment, World Academy of Science, Engineering and Technology. International Journal of Chemical, Nuclear. Materials and Metallurgical Engineering, 9(1): 30–33
Smidsrod, O., Haug, A., Larsen, B. (1963). Degradation of alginate in the presence of reducing compounds. Acta Chemica Scandinavica, 17(10): 2628-2637
Smidsrod, O., Larsen, B., Painterand T. Haug, A. (1969). The role of intramolecular autocatalysis in the acid hydrolysis of polysaccharides containing 1.4-linked hexuronic acid. Acta Chemica Scandinavica, 23: 1573-1580
Sousa, Alves de, A.P., Torres, M.R., Pessoa, C., Moraes, M.O.D., Filho, F.D.R., Alves, A.P.N.N., Costa-Lotufo, L.V. (2007). In vivo growth-inhibition of Sarcoma 180 tumor by alginates from brown seaweed Sargassum vulgare. Carbohydrate Polymers, 69: 7–13
Sugiono, S., Ferdiansyah, D. (2018). Fucoidan and alginate sequential biorefinery extraction: Effect of Pre-extraction Acid-treatment Against Intrinsic viscosity of alginate from brown alga Sargassum cristaefolium. Food Science and Technology Journal, 1(2): 44-51
Sugiono, S., Masruri, M., Estiasih, T., Widjanarko S.B. (2019a). Optimization of extrusion-assisted extraction parameters and characterization of alginate from brown algae (Sargassum cristaefolium). Journal of Food Science and Technology, 56 (8): 3687-3696
Sugiono, S., Masruri, M., Estiasih, T., Widjanarko S.B. (2019b). Structural and Rheological Characteristics of Alginate from Sargassum cristaefolium Extracted by Twin Screw Extruder, Journal of Aquatic Food Product Technology, 28 (9): 944-959
Sugiono, S., Masruri, M., Estiasih, T., Widjanarko, S.B. (2018). Multiple-response optimization of the acidic pre-treatment of the brown alga Sargassum cristaefolium for the alginate extraction using twin screw extruder. Bioscience research, 15(2): 683-693
Sugiono, Widjanarko, S.B., Adisoehono, L. (2014). Extraction Optimization by Response Surface Methodology and Characterization of Fucoidan from Brown Seaweed Sargassum polycystum. International Journal of ChemTech Research, 6(1): 195-205
Torres, M.R., Saosa, A.P.A., Filho E.A.T.S., Melo D.F., Feitosa J.P.A., Paula R.C.M.D., and Lima M.G.S. (2007). Extraction and physochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydrate Research, 342: 2067-2074
Wang, J., Zhang Q., Zhang Z., Song H. and Li P. (2010). Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. International Journal of Biological Macromolecul, 46: 6–12.
Ye, H., Wang K., Zhou C., Liu J. and Zeng X. (2008). Purification, antitumor and antioxidant activities in vitro of polysaccharide from the Brown Seaweed Sargassum pallidium. Food Chemistry, 111: 428-432.


