1Institute of Microbiology, Madurai Medical College, Madurai, India.
2Department of Microbiology, Vinayaka Mission’s Medical College and
Hospital, Vinayaka Mission Research Foundation, Karaikal, India.
Corresponding author email: drsmohan1971@gmail.com
Article Publishing History
Received: 14/07/2021
Accepted After Revision: 20/09/2021
Candidal vulvovaginitis is accountable for a third of all cases of vulvovaginitis in reproductive-aged women, and 70% of women report having or had candidal vulvovaginitis at certain point in their lifespans. Vulvovaginal Candidiasis (VVC) is a recurrent, multifaceted and unwieldy illness that can cause corporeal and mental distress to the individual. Candida albicans was stated as the greatest common cause of VVC yet it appears that we are newly facing changes in the configuration of Candida species in VVC. In the present study we measured diverse species of Candida isolated from patients with VVC. This research was a descriptive analytical cross-sectional study. Candida Sps were isolated from females aged 20 – 60 years, who existing erythema and itching of vulva, vagina, or both and unpleasant vaginal exoneration.
Biofilm formation of the isolates were evaluated using crystal violet staining and their drug resistance pattern were evaluated using standard antifungal antibiotic discs. Biofilm production could act as one of the factors in reducing the penetrability of the antifungal agents and also increasing the virulence nature of invasive candidiasis. Biofilm development test was done on all the 85 samples. All sample was prepared in triplicate and the average was determined. Light microscopic biofilm imaging showed the biofilm formation of the budding yeast cells from tiny micro-colonies (2-4 h). After four hours, the budding cells started to divide, and formed pseudo hyphae and ultimately true hyphae. We report that this extremely contagious yeast has the capability to form antifungal resistant biofilms sensitive to the antifungal agent in vitro.
Biofilms, Candida Albicans, Vulvovaginal Candidiasis
Rammurugan, S. Mohan. Biofilm-Mediated Drug Resistance in Candida Species Isolated from Vulvovaginal Infections: A Descriptive Cross-Sectional Study. Biosc.Biotech.Res.Comm. 2021;14(3).
Rammurugan, S. Mohan. Biofilm-Mediated Drug Resistance in Candida Species Isolated from Vulvovaginal Infections: A Descriptive Cross-Sectional Study. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/37sI7mD“>https://bit.ly/37sI7mD</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
In the last decade, fungal infections were presented as a serious problem in hospitals, especially in ICUs that are epicentres in Candidemia and invasive Candida infections (ICI). Candida infections are the leading opportunistic fungal pathogen, with C. albicans responsible for most of this infection significantly increasing world-wide. These diseases are highly morbid and deadly, which also affect healthcare costs. (i.e., increased hospital length of stay, high costs for antifungal therapy). They are generally associated with many therapies, such as parenteral feeding, pre-exposure to antibacterial therapy, chemotherapy, and dialysis, and intravascular equipment (mainly CVC and urinary catheters) (Pristov and Ghannoum 2019; Fakhim et al. 2020).
A septic epidemiology study found that, between (1979) and (2000), the rate of fungal sepsis was triple. In 80 percent of cases, Candida is the liable agent in about 8% of all nosocomial infections. Different Candida species may cause these challenging infections. Candida albicans, preceded by Candida glabrata, is the most common pathogen responsible for Candida’s infections. In urinary tract infections, Candida tropicalis is especially relevant, while Candida parapsilosis is also present in the skin of healthy hosts, and is the causative substance of catheter infections. A number of factors such as phenotype flipping, dimorphic transitions between the hyphae and yeast, and the secretion of proteases and phospholipases may be caused by virulence of C. albicans (Blostein et al. 2017; Fakhim et al. 2020).
Albicansare capable of dimorphating between two principal types, a circular budding yeast and a long, parallel, real hypha (with an intermediary, pseudohyphae form consisting of stretched ellipsoid cells), is the source of the germ tube test used in most hospitals to recognise C. albicans from other Candida species, C. dubliniensis being the exception as it can form true hyphae. Another key feature of C. albicans pathogenesis is biofilm production (Shukla et al. 2019; Willems et al. 2020). Biofilm is a group of cells that are attached to a surface, are surrounded by an extracellular matrix and have characteristics which differ from its free-floating counterparts. A biofilm is an extracellular matrix (Kalaiarasan et al. 2017; Kannan et al. 2020).
This phenomenon lets Candida to attach to mucosal cells and to plastic surfaces of medical devices such as catheters and dentures leading to device associated infections and eventually spreading nosocomial infections (Rosati et al. 2020). The cells forming biofilms are distinct from that of floating cells, since they are embedded in a 3D matrix and will proliferate inside the host’s immune system in stable individuals that are more resistant to antimicrobial medicines. The connection between drug resistance and C. albicans virulence is, however, still little understood (Rosati et al. 2020). This study obtained 85 isolates in two major hospitals between March and December 2019 in patients with vulvovaginal candidiasis. API, germ tunnel, CHROM agar and ITS sequencing were used to identify isolates. Furthermore, antifungal susceptibility testing against four antifungal drugs was performed, and the isolates were additionally tested for biofilm formation to determine the adherence mediated virulence of the isolates.
MATERIAL AND METHODS
This research was a descriptive analytical cross-sectional study. The members were married females aged 20 – 60 years, who existing erythema and itching of vulva, vagina, or both and unpleasant vaginal exoneration. The patients filled out a consent form to participate in the research. Concomitant with each obtained specimen, a questionnaire was completed for each patient enquiring about their age, marital status, and duration of symptoms, comorbidities, signs and symptoms of current condition, methods of pregnancy prevention, prior parturitions, and history of antibiotic consumption (Hosseini et al. 2020).
Sterilised speculum moistened with sterile water was positioned in the vagina (in case of being married), and posterior fornix where excretions are hoarded was experimented by introducing two swabs concomitantly. The swabs were located in a tube comprising 1 ml of sterile PBS (Phosphate buffer saline) and then the samples were transported to laboratory. Then the swabs were inoculated on HI Chrome Candida agar plates, incubated at 35°C for 4 days, aerobically. All dishes were assessed for the fungal growth and colony shade on a daily basis. Direct microscopy slides were prepared from each colony for yeast confirmation. All yeast isolates were cautiously sub cultured on Sabraud dextrose agar (SDA, Merck, Germany) plates and ‘incubated at room temperature for future mycological analyses (Hefzy et al. 2021).
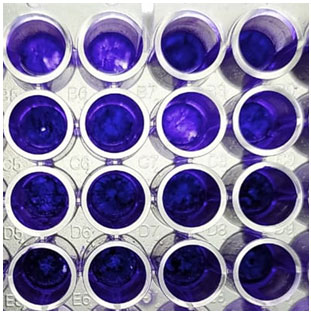
Biofilm development test was done on all the 85 samples. All sample was prepared in triplicate and the average was determined. Three to four colonies were suspended in YNB (Yeast Nitrogen Base, Fluka, Switzerland) and incubated overnight with moderate pulsating. The optical density of each of the suspensions was adjusted to 0.1. 0.5 mL of the suspension was added to a flat-bottomed 96-well microtiter plates at 4°C and placed in a shaker at 37°C for 3 h to allow for preliminary grip. Plates were then eroded with 0.5 mL PBS and another 0.5 mL of the cell suspension was added. Following 48 h incubation at 37°C, cells were washed with 1 mL PBS and fixed using 0.2 mL of 99 % methanol for 15 min. Plates were then allowed to air-dry for 20 min. Staining was performed by adding 0.2 % crystal violet, removed after 20 min, and followed by 0.75 mL of 33% acetic acid. The absorbance was immediately measured using a spectrometer (Thermo Spectronic) at 590 nm. C. albicans strain SC5314 was used as a reference strain (Yassin et al. 2021).
Penetration of antifungal agents over biofilms was measured by an alteration of the strainer disk technique defined before for bacterial biofilms. After biofilm development on film sieves, smaller polycarbonate membrane filters (diameter, 13 mm; pore size, 0.2 μm; Whatman) existed sterilized by disclosure to UV radiation for 15 min on both sides and were then cautiously located on top of the 48-h-old biofilms. Paper concentration disks (diameter, 6 mm; Becton Dickinson) were also decontaminated by introduction to UV radiation for 15 min per side and then moisturised with growth medium before settlement on top of the membranes.
Often a slightly higher or lower medium volume was essential for saturating the disks because of a difference in the disk thickness. Biofilms crammed amongst the membranes and moisturized disks were transported to antifungal agent-containing agar medium (Said et al. 2020). To regulate statistical significance of the biofilm experiment, both a -test and a post hoc ANOVA test were conceded. For the ANOVA test isolates were assembled into 3 clusters: those with biofilm competences below the reference strain, those comparable to the reference strain, and those overhead the reference strain. Statistical significance with the reference strain group was observed for both groups containing isolates above and below the reference strain (data not shown). p value below 0.05 was deemed significant.
RESULTS AND DISCUSSION
Sample collection and Laboratory diagnosis of Candida Sps: Candidiasis is among the most common fungal diseases that can contribute to systemic and life-threatening diseases, such as vaginitis. C. albicans is an opportunistic pathogen that is now one of the main causes. Candida is one of the most common presentations of genital involvement in women (Tits et al. 2020).
Thirty three out of 97 patients were identified with Candida infection, among whom 32 isolates of Candida were obtained. The mean patients’ age was 36.5 years. The most commonly described symptoms were vaginal discharge (99%), vulvovaginal itching (52.3%), vulvovaginal burning sensation (32.7%) and dysuria (3.2 %). The risk factors were including previous VVC infections (n = 14), consumption of antibiotics (n = 7) and antifungal agents (n = 3), pregnancy (n = 9), diabetes mellitus (n = 13), RVVC (n = 4), and intrauterine contraceptive device (IUCD) usage (n = 2). No patients were positive for the human immunodeficiency virus (HIV). Of these isolates, 28 were germ tube positive and presented a green colony colour on the chromogenic medium that were classified as C. albicans complex species. Vulvovaginal candidiasis consequences to irregular progress of Candida in the genital tract mucosa and has augmented intensely in the latest existences (Tits et al. 2020).
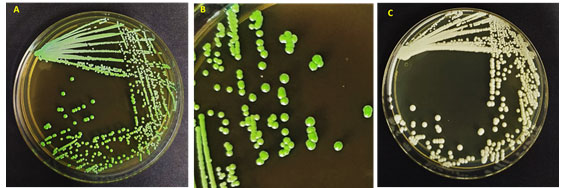
This contamination is a universal health problem and disturbs a lot of women, yearly. Candida albicans was stated as the most mutual agent of VVC yet it appears that we are recently encountering changes in the pattern of Candida species in VVC (Ramage et al. 2006; Tits et al. 2020). The microscopic study of the genital specimens revealed the presence of yeasts and a small number of lactobacilli. A culture of the vaginal specimen on Sabraud Dextrose agar (Difco, Becton, Dickinson and Co., Sparks, MD) yielded a single type of yeast colony (Figure 1). The latter was morphologically compatible with Candida spp. showing a light creamy dull colour on SDA. The yeast isolate grew better at 30°C than at 37°C and was unable to grow at 40°C. In some cases, the organism developed slowly settled turquoise blue colonies, resembling those of Candida dubliniensis (Paiva et al. 2020).
Figure 1: Morphology of Candida albicans strains (A, B) in HiChrome Candida agar, (C) In SDA agar medium
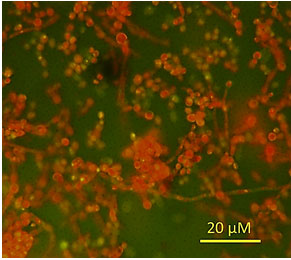
Microscopically, the fungal isolate showed ovoid to elongate cells, singly or in pairs and the development of pseudohyphae (Figure 2). It formed germ tubes in horse serum after 3 h of incubation at 37°C, but it did not produce chlamydospores on Corn-meal agar after 10 days of incubation at 30°C.
Figure 2: Microscopic examination of C. albicans after acridine orange staining.
In vitro biofilm formation by C. albicans: The fact that this organism has a high tendency to bind to a rank of surfaces such as tissues living substratum and biomaterials is partly linked to these results. Subsequently, they form spatially ordered populations of phenotypically diverse sessile cells that are significantly different from their planktonic components (Bandara et al. 2020).
Cells like this are known to be micro-biofilm communities and are normally known to have anti-microbial and immune resistant (Kuhn et al. 2002). Maximum illnesses instigated by C. albicans are related with the development of biofilms on abiotic or host surfaces (Bandara et al. 2020). More considerably, C. albicans is proficient at stick to catheters and numerous medical implants, and is presently classified by the Centres for Disease Control and Prevention, United States, as the third most commonly isolated bloodstream pathogen in hospitalized patients with a mortality rate of up to 50% (Goel et al. 2021).
Light microscopic biofilm formation parallel analyses showed how the biofilm existed most of the budding yeast cells from tiny micro-colonies (2-4 h). After four hours, the budding cells started to divide, and formed pseudo hyphae and ultimately true hyphae. After 8 hours hyphae from the surrounding micro-colonies, made up mainly of formation cells of yeast, fused in an inventive network of filamentous spatially scattered forms which formed a cohesive monolayer of woven frameworks. As biofilm maturing happened (24 and 48 h growth), the difficulty of the biofilm augmented into a multi-layered biofilm matrix with all fungal morphologies being present in the ending biofilm construction (Kannan et al. 2021).
The kinetics of adherence and subsequent biofilm formation by C. albicans on the surface of polystyrene wells over 48 h, as determined by the crystal violet assay, are showed in Figure 3. The production of the soluble colour crystal violet from sessile cells, a direct reflection of cellular metabolic activity, increased over time with the increased sessile cellular density. The biofilms were highly metabolically active in the first 8 h, but as the biofilm matured and the complexity increased (24 to 48 h) the metabolic activity reached a plateau, but remained high probably reflecting the increased number of cells that constituted the mature biofilm. Experiments were performed in sets of eight replicates on three separate occasions, with similar results obtained in all experiment (Perira et al. 2020).
Figure 3: Biofilm formation by the strains isolated
Susceptibility testing of C. albicans biofilms against clinically used antifungal agents: There exist a problem with antimycotic treatment, which may be due to several factors, leading to clinical resistance (Kannan et al. 2020). The in vitro activity of clinically used fluconazole and amphotericin B against pre-formed C. albicans biofilms was assessed using the modified biofilm penetration assay. Experiments revealed the increased resistance of sessile C. albicans cells compared to their planktonic counterparts.
The antifungal agents tested showed less activity against 48 h biofilms compared to planktonic MIC’s, generally much greater than the concentration of antifungal required to inhibit planktonic cells. Data revealed that C. albicans biofilms were intrinsically resistant to fluconazole (MICs >1700 µg/ml), and the activity of this azole derivative against biofilms was reduced up to 325 times compared with its activity against planktonic cultures. Previous reports were stated the high resistance rate for C. albicans and C. tropicalis from animal origin and the fact that the antifungal against C. parapsilosis sensu lato from animals (Paiva et al. 2020).
Amphotericin B demonstrated certain activity against C. albicans biofilms, as indicated by 18 µg/ml, but this concentration is generally regarded as resistance already, due to the high toxicity displayed by this drug. Importantly, complete killing of cells within the biofilms was never achieved, as reflected by residual metabolic activity of biofilms at concentrations up to 25µg/ml. In few latest published reports, we observed this lack of inhibitory properties of and fluconazole resistance when delivered to C. albicans biofilms rather than the free-floating cells (Tummanapalli et al. 2021).
Few literatures indicate fungal biofilm science and antimicrobial resistance. An understanding of the complexities and phenotypic properties of C. albicans biofilm will enable one to improve antifungal agents and treatment methods to eradicate and avoid Candida biofilm, which reduce the occurrence of C. albicans infection. In line with this outcomes, recent studies have also shown an increase in C. albicans antifungal resistance in C. albicans, P. aeruginosa mixed species biofilms via upregulated C. albicans proteins associated with drug resistance and virulence (Tita et al. 2020).
Studies on increasing resistance of vaginal candida isolates to mainstay antibiotics are a worry, and there is sign that for vaginal disease this resistance translates into worse clinical outcomes (Pereira et al. 2020). Novel antibiotics are being established, but not by large pharmaceutical companies and mostly in university research laboratories and smaller biotech companies (Castelo et al. 2020).
CONCLUSION
The findings of the present study show the occurrence of biofilms may trait to the development of drug resistance in the clinical isolates. Henceforth, testing for the analysis of biofilm development is suggested in the routine laboratory diagnostic practices. In addition, the contour of treatment in recurring and drug resistant VVC cases should aim both treating the planktonic cells as well as extinguishing the biofilms formation of the Candida sp. Advanced study of biofilm mediated molecular genetic markers and procurement of evidence on the association of the genetic and phenotypic properties of Candida spp., as well as features distressing gene expression, will make it possible to improve diagnostics for the timely recognition of resistant strains and the rational selection of therapy.
ACKNOWLEDGEMENTS
This study was supported by Vinayaka Mission Research Foundation (Deemed to be University) and Vinayaka Mission Medical College, Karaikal. Authors acknowledge both for the facilities provided by them to complete this research.
Conflict of interests: Authors declare no conflicts of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of Madurai Medical College, Madurai Tamil Nadu 625020, India.
REFERENCES
Bandara, H.M.H.N., Wood, D.L.A., Vanwonterghem, I., et al. (2020). Fluconazole resistance in Candida albicans is induced by Pseudomonas aeruginosa quorum sensing. Scientific reports, 10(1), 1-17.
Blostein, F., Levin-Sparenberg, E., Wagner, J. et al. (2017). Recurrent vulvovaginal candidiasis. Annals of Epidemiology, 27(9), 575-582.
Castelo-Branco, D.D.S.C.M., Paiva, M.D.A.N., Teixeira, C.E.C., et al. (2020). Azole resistance in Candida from animals calls for the One Health approach to tackle the emergence of antimicrobial resistance. Medical mycology, 58(7), 896-905.
Fakhim, H., Vaezi, A., Javidnia, J., et al. (2020). Candida africana vulvovaginitis: Prevalence and geographical distribution. Journal de mycologie medicale, 30(3), 100966.
Goel, N., Fatima, S.W., Kumar, S., et al. (2021). Antimicrobial resistance in biofilms: Exploring marine Actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnology Reports, e00613.
Hefzy, E.M., Khalil, M.A., Amin, A.A.I., et al. (2021). Bacteriocin-Like Inhibitory Substances from Probiotics as Therapeutic Agents for Candida Vulvovaginitis. Antibiotics, 10(3), 306.
Hosseini, S.S., Joshaghani, H., Shokohi, T., et al. (2020). Antifungal activity of ZnO nanoparticles and nystatin and downregulation of SAP1-3 genes expression in fluconazole-resistant Candida albicans isolates from vulvovaginal candidiasis. Infection and drug resistance, 13, 385.
Kalaiarasan, K., Singh, R. and Chaturvedula, L., (2017). Fungal profile of vulvovaginal candidiasis in a tertiary care hospital. Journal of clinical and diagnostic research: JCDR, 11(3), DC06.
Kannan, S., Balakrishnan, J. and Govindasamy, A., (2020). Listeria monocytogens-Amended understanding of its pathogenesis with a complete picture of its membrane vesicles, quorum sensing, biofilm and invasion. Microbial Pathogenesis, 104575.
Kuhn, D.M., George, T., Chandra, J., et al. (2002). Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrobial agents and chemotherapy, 46(6), 1773-1780.
Paiva, M.D.A.N., Teixeira, C.E.C., Caetano, É.P., et al. (2020). Azole resistance in Candida from animals calls for the One Health approach to tackle the emergence of antimicrobial resistance.
Pereira, R., dos Santos Fontenelle, R.O., de Brito, E.H.S. et al. (2020). Biofilm of Candida albicans: Formation, regulation and resistance. Journal of Applied Microbiology.
Pristov, K.E. and Ghannoum, M.A., (2019). Resistance of Candida to azoles and echinocandins worldwide. Clinical Microbiology and Infection, 25(7), 792-798.
Ramage, G., Martínez, J.P. and López-Ribot, J.L., (2006). Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS yeast research, 6(7), 979-986.
Rosati, D., Bruno, M., Jaeger, M., et al. (2020). Recurrent vulvovaginal candidiasis: an immunological perspective. Microorganisms 8: 144.
Said, M.M., Watson, C. and Grando, D., (2020). Garlic alters the expression of putative virulence factor genes SIR2 and ECE1 in vulvovaginal C. albicans isolates. Scientific reports, 10(1), 1-9.
Shukla, A. and Sobel, J.D., (2019). Vulvovaginitis caused by Candida species following antibiotic exposure. Current infectious disease reports, 21(11), 1-6.
Tits, J., Cools, F., De Cremer, K., et al. (2020). Combination of miconazole and domiphen bromide is fungicidal against biofilms of resistant Candida spp. Antimicrobial Agents and Chemotherapy, 64(10).
Tummanapalli, S.S. and Willcox, M.D., (2021). Antimicrobial resistance of ocular microbes and the role of antimicrobial peptides. Clinical and Experimental Optometry, 104(3), 295-307.
Willems, H.M., Ahmed, S.S., Liu, J., Xu, Z. and Peters, B.M., (2020). Vulvovaginal candidiasis: a current understanding and burning questions. Journal of Fungi, 6(1), 27.
Yassin, M.T., Mostafa, A.A., Al-Askar, A.A. et al. (2020). In vitro antifungal resistance profile of Candida strains isolated from Saudi women suffering from vulvovaginitis. European journal of medical research, 25(1), 1-9.


