Department of Biological Sciences,College of Science,
King Abdulaziz University,Jeddah, Saudi Arabia.
Corresponding author email: maaalghamdi3@kau.edu.sa
Article Publishing History
Received: 21/06/2021
Accepted After Revision: 24/09/2021
Polycystic ovary (PCO) is a condition in which the secretion of specific hormones is disrupted. The adrenal glands are essential for the manufacture of certain hormones and the monitoring of steroid concentration, and they may be impacted in PCO situations. In a PCO-induced rat model, the researcher was aimed to investigate how administering exogenous estrogen, estradiol valerate affected on adrenal gland secretions and histology. The effects of Matricaria chamomilla flowers extract, and metformin will also be evaluated. 24 rats were divided into four equal groups: 1- control group, 2- PCO group, 3- metformin group, 4- Matricaria chamomilla group. PCO was induced by injecting 2 doses of estradiol valerate. The findings revealed a considerable increase in estrogen and ACTH levels in the PCO rats. The concentrations of cortisol and aldosterone were likewise higher in the PCO rats.
These findings were corroborated by histological investigation of adrenal sections (both H&E and PCNA staining). Matricaria chamomilla flowers extract reduced all the hormonal alterations linked to PCO, including estrogen, ACTH, cortisol, and aldosterone. Metformin similarly reduced estrogen and ACTH. On the other hand, Metformin did not affect the hormonal changes associated with this model in terms of adrenal gland hormones (cortisol and aldosterone). In conclusion both treatments are improved that the histological investigation of adrenal secretions (both H&E and PCNA staining). The antiestrogen activity of Matricaria chamomilla could clarify that all the hormonal modulation occurs in this PCO model.
Polycystic Ovary; Adrenal Glands; Cortisol; Aldosterone, Estrogen; Acth; Pcna.
Alahmadi A. A. Biochemical and Histological Remodeling of Adrenal Glands Associated with Estradiol Valerate-Induced Polycystic Ovary in Rats: Guarding Effects of Matricaria chamomilla. Biosc.Biotech.Res.Comm. 2021;14(3).
Alahmadi A. A. Biochemical and Histological Remodeling of Adrenal Glands Associated with Estradiol Valerate-Induced Polycystic Ovary in Rats: Guarding Effects of Matricaria chamomilla. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3khouVG“>https://bit.ly/3khouVG</a
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Polycystic ovary (PCO) is a condition in which the secretion of specific hormones is disrupted, which causing reproductive issues in women. Irregular menstruation, the development of ovarian cysts, and insulin intolerance are all common PCO problems. Estrogen is a hormone generated chiefly by the ovaries but also in minor amounts by the adrenal glands. It’s in charge of the formation of feminine sex traits.
Estrogen is necessary for the normal functioning of sexual organs, skeletal system preservation, menstrual cycle control, and pregnancy maintenance (Reed and Carr, 2000; Clarke and Khosla, 2010; Ndefo et al., 2013; Witchel et al., 2019; Delgado and Lopez-Ojeda, 2021).
Although estrogen levels vary naturally throughout the monthly cycle throughout a woman’s life, several women may experience estrogen dominance, in which their estrogen levels are more significant than average. Estrogen dominance, or raised estrogen concentrations, can occur in PCO ladies. PCO is the reasonable explanation of oligo-ovulation, and it’s supposed that estrogen dominance exerts a role. The absence of ovulation results in perpetual extraordinary estrogen levels and lacking progesterone (Hambridge et al., 2013; Dennett and Simon, 2015; Leon et al., 2021).
The adrenal glands are essential for the manufacture of certain hormones as well as the monitoring of steroid concentration in the body, and they may be impacted in PCO patients. Recently, a PCO model was established in our lab via injecting estradiol valerate in rats.
The model was characterized by an increase in blood estrogen levels. Women with PCO have widespread hypersecretion of adrenal gland cortical hormones, including pregnenolone, 17-hydroxypregnenolone, DHEA, androstenedione, 11-deoxycortisol, and cortisol both at basically and after stimulation with adrenocorticotropic hormone (ACTH) (Azziz et al., 1998; Dumitrescu et al., 2015; Rosenfield and Ehrmann, 2016; Alahmadi et al., 2020).
There are also subtle systemic steroid metabolic problems in PCO women, such as tendencies favoring increased estrogen and cortisol output. Oral estrogen replacement medication has already been demonstrated to raise total cortisol levels in women in various trials.
Some reports, on the other hand, did not reveal similar disparities. Most studies haven’t looked at cortisol levels after estrogen replacement medication (Tsilchorozidou et al., 2003; Tock et al., 2014). It’s uncertain whether the use of exogenous estrogen therapy will affect the levels of various adrenal hormones. Chamomile (Matricaria chamomilla L.) is a widespread herbal medicine in Southern and Eastern Europe. It is a member of the Asteraceae family.
Chamomile’s active components include flavonoids responsible for the pharmacological attributes of chamomile, mainly the anti-inflammatory and antioxidant benefits. Matricaria chamomilla extract has recently been shown to lower PCO-related estrogen increase ( Heidary et al., 2018; Alahmadi et al., 2020; Sotiropoulou et al., 2020).
The purpose of this study was to look at the effects of injecting exogenous estrogen, estradiol valerate, on adrenal gland secretions and histology in a PCO-induced rat model. Furthermore, the impact of Matricaria chamomilla flowers extract, and metformin were assessed.
MATERIAL AND METHODS
Chemicals: In this research, Matricaria chamomilla flowers was purchased from World of Herbs, Egypt, estradiol valerate purchased from Abcam Inc, USA, and metformin purchased from Sigma-Aldrich Co, USA.
Preparation of Matricaria chamomilla flowers extract: The extract was made by extracting the crushed Matricaria chamomilla flowers with ethyl alcohol (70 percent), then drying the extract under vacuum.
Animals: In the current study, twenty-four adult, virgin female Wistar rats were used. They obtained from the King Fahad Research Centre at King Abdullah University in Jeddah, Saudi Arabia. Their body weight varies between 186 and 208 grams. The research was conducted out under a conventional laboratory environment of temperature, humidity, and a 12:12 h light/dark cycle after a one-week acclimatization period. The animals were not subjected to any water or food restrictions. The research procedures were accepted by the Biomedical Ethics Research Council, Faculty of Medicine, KAU, Jeddah, SA, acceptance number, 168-19.
Induction of PCO: PCO was induced by administering two doses of estradiol valerate (0.2 mg each), one dose at the start and the other after six weeks of the experiment (Alzahrani et al., 2019; Farideh et al., 2010).
Experimental groups: The rats were divided into four equal groups (n = 6). Control group: corn oil-injected group, PCO group: estradiol valerate injected group, Metformin group: PCO rats treated with metformin (500 mg/kg) (Alahmadi et al., 2020), Matricaria chamomilla: PCO rats treated with Matricaria chamomilla flower extract (75 mg/kg) (Alahmadi et al., 2020). Corn oil, metformin, and Matricaria chamomilla were injected every day into the animals starting at the beginning of the experiment and remained for one month following PCO initiation.
Collection of serum and adrenal glands samples: A cardiac puncture was used to gather blood samples. Adrenal hormones (cortisol and aldosterone), anterior pituitary hormone (ACTH), and estrogen levels were measured after the serum was extracted and kept frozen at -80°C. Both adrenal glands were removed and preserved in 10% buffered formalin for histopathological evaluations and immunohistochemical proliferating cell nuclear antigen (PCNA) expression.
Measurement of serum cortisol, aldosterone, ACTH, and estrogen: Serum cortisol, aldosterone, ACTH, and estrogen were quantified in El-Safwa Laboratory, Tanta, Egypt, utilizing ADVIA Centaur automated competitive chemiluminescence immunoassay (Bayer HealthCare).
Evaluation of histopathological changes (light microscope): Adrenal glands were preserved in formalin, fixed in paraffin wax, sectioned at 3–5 mm, and stained with hematoxylin and eosin (H&E).
Evaluation of PCNA immune expression (light microscope): Immunohistochemical staining was performed employing the immune peroxidase (PAP, peroxidase/anti peroxidase) procedure utilizing anti PCNA antibodies from Lab Vision (Fremont, CA. USA) diluted at 1/100.
Statistical interpretation of findings: GraphPad Prism version 5 was used to analyse the results, which included an ANOVA test and a Tukey’s multiple comparison test. At p 0.05, the results were considered statistically significant.
RESULTS AND DISCUSSION
Effect of Matricaria chamomilla flowers extract on serum ACTH and estrogen levels measured in estradiol valerate-induced PCO in rats: PCO-induced rats showed significantly increased serum ACTH and estrogen levels compared to the control group. Treatment of PCO-induced rats with Matricaria chamomilla and metformin significantly decreased serum ACTH and estrogen levels compared to the PCO group. Matricaria chamomilla significantly decreased serum ACTH level compared to the metformin group (Table 1).
Table 1. Effect of Matricaria chamomilla flowers extract on serum ACTH and estrogen levels measured in estradiol valerate-induced PCO in rats.
| Experimental group | ACTH (pmol/L) | Estrogen (ng/mL) |
| Control | 55.33 ± 1.15 | 99.47 ± 1.13 |
| PCO | 89.83 ± 4.29a | 339.27 ± 20.33a |
| Metformin | 70.50 ± 3.55b | 92.42 ± 2.98 b |
| Matricaria chamomilla | 58.83 ± 2.41b | 95.80 ± 2.36 b |
Results are expressed as mean ± SE (n=6). asignificant difference compared to the control group. bsignificant difference compared to the PCO group.
Effect of Matricaria chamomilla flowers extract on serum aldosterone and cortisol levels measured in estradiol valerate-induced PCO in rats: PCO-induced rats showed significantly increased serum aldosterone and cortisol levels compared to the control group. Treatment of PCO-induced rats with Matricaria chamomilla significantly decreased serum aldosterone and cortisol levels compared to the PCO group. There was no difference between the metformin group and PCO group concerning serum aldosterone and cortisol levels (Figure 1).
Figure 1: Effect of Matricaria chamomilla flowers extract on serum aldosterone and cortisol levels measured in estradiol valerate-induced PCO in rats.
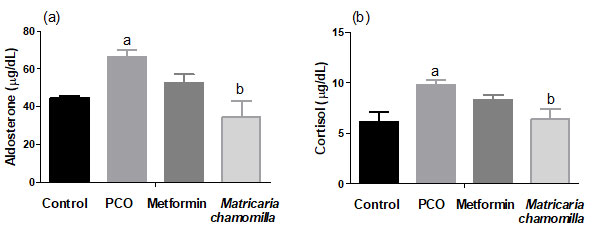
Results are expressed as mean ± SE (n=6). asignificant difference compared to the control group. bsignificant difference compared to the PCO group.
Effect of Matricaria chamomilla flowers extract on adrenal glands histopathological changes: The examination of H & E-stained adrenal glands sections of PCO-induced rats revealed a potential increase in cortical zone thickness, an increase in thickness of zona glomerulosa, increase in cellularity of both zona glomerulosa and zona fasciculata. Treatment of PCO group with either metformin or Matricaria chamomilla decreased cortical zone and zona glomerulosa thickness, besides normal cellular features of zona fasciculata (Figure 2).
Figure 2: Effect of Matricaria chamomilla flowers extract on adrenal glands histopathological changes.
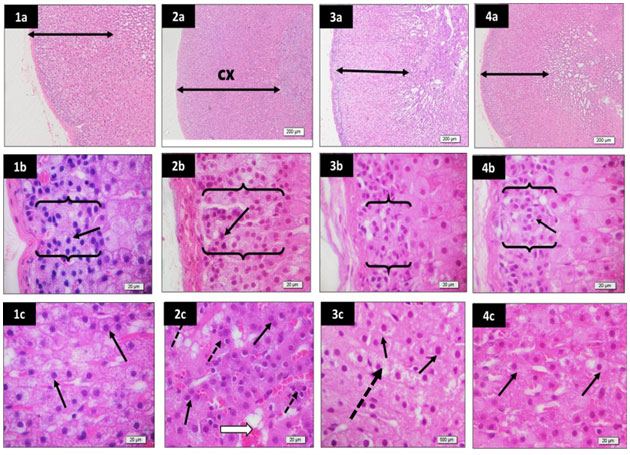
Sections from rat adrenal glands (low power x40) and higher magnification (x400) of zona glomerulosa and zona fasciculata stained by H&E to show a potential increase in cortical zone (cx) thickness in PCO group (2a) compared to control (1a). Besides, an increase in thickness of zona glomerulosa of PCO group (brackets) (2b) compared to control (1b) is observed. The cellularity of both zona glomerulosa (2b) and zona fasciculata (2c) of the PCO group is increased compared to control (1b, and 1c). Mild dilation of sinusoids (white arrow) and presence of lymphocytes (dotted arrows) are also evident in the zona fasciculata of the PCO group (2c). The metformin group showed decreased cortical zone (3a) and zona glomerulosa (3b) thickness compared to the PCO (2a and 2b) and the control groups (1a and 1b). Besides, moderate return to normal cellular features (black arrows) of zona fasciculata is observed in the metformin group (3c), although frequent cells showed unstained cytoplasm (dotted arrow). The Matricaria chamomilla group show relatively normal cortical zone thickness (4a), zona glomerulosa thickness (4b), and cellular features of zona fasciculata (4c) compared to the PCO (2a and 2b) and the control groups (1a and 1b).
Effect of Matricaria chamomilla flowers extract on adrenal glands zona glomerulosa PCNA immune expression: Sections of PCO-induced rats were showed relative increase in zona glomerulosa thickness, besides, increased number of nuclei expressing PCNA. A mild or moderate decrease in nuclei expressing PCNA is observed in the metformin and Matricaria chamomilla treated rats (Figure 3).
Figure 3: Effect of Matricaria chamomilla flowers extract on adrenal glands zona glomerulosa PCNA immune expression.
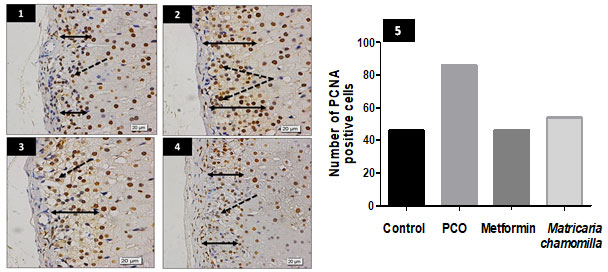
Sections from rat adrenal gland zona glomerulosa stained for PCNA show relative increase in zona thickness (double head arrows) in the PCO group (2) compared to the control group (1). Besides, the number of nuclei expressing PCNA looked to be more numerous (dotted arrows) (2). A mild or moderate decrease in nuclei expressing PCNA is observed in the metformin and Matricaria chamomilla groups (dotted arrows) (3 and 4). (5) A bar chart shows the number of PCNA positive cells in all groups.
Effect of Matricaria chamomilla flowers extract on adrenal glands zona fasciculata PCNA immune expression: Sections of PCO-induced rats show relative increase in zona fasciculata, the number of nuclei expressing PCNA. A mild or moderate decrease in nuclei expressing PCNA is observed in the metformin and Matricaria chamomilla treated rats (Figure 4).
Figure 4: Effect of Matricaria chamomilla flowers extract on adrenal glands zona fasciculata PCNA immune expression.
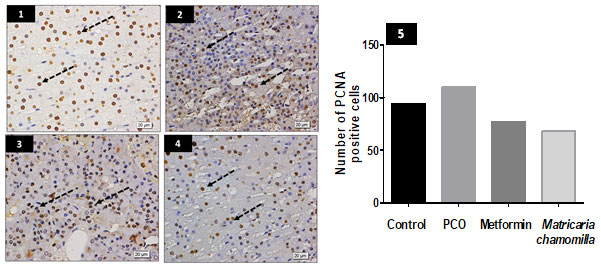
Sections from rat adrenal gland zona fasciculata stained for PCNA show increasing nuclei expressing the marker in the PCO group (2) compared to the control group (1) (dotted arrows). PCNA immuno-expression is still high in the metformin group (3) (dotted arrows). PCNA immuno-expression is nearly normal in Matricaria chamomilla group (4). (5) A bar chart shows the number of PCNA positive cells in all groups.
The current study results showed a significant increase in the level of estrogen in the PCO model developed by exogenous estrogen injection. There was also a significant increase in the level of the ACTH in the PCO rats. It was also clear that the adrenal gland cortisol and aldosterone concentrations were increased in the PCO group. The histological examination (both H&E and PCNA staining) of adrenal sections confirmed these findings. Similar to this study findings, previous research showed an overproduction of various adrenal gland hormones, including cortisol, in PCO women (Azziz et al., 1998).
The researchers attributed this to the increased sensitivity of adrenal glands to the ACTH, 7α-hydroxylase, and increased cytochrome P450c17α. According to previous research, ladies with PCO were observed to have enhanced peripheral cortisol metabolism, which was linked to raised ACTH production in order to maintain optimal cortisol levels.
The possible causes for elevated adrenal hormones levels in PCO ladies incorporate changes in adrenocortical biosynthesis, increased responsiveness of the adrenal to ACTH stimulation, or abnormalities in adrenal product metabolism (Stewart et al., 1990; Bhathena, 2005; Yildiz and Azziz, 2007; Baskind and Balen, 2016; Alahmadi et al., 2020).
Numerous probable theories can describe how ACTH and cortisol levels were raised after estradiol valerate therapy. One probability is that estrogen treatment directly or indirectly impacted ACTH production from the pituitary gland, which encouraged cortisol release from the adrenal gland. In pregnant ladies, it is affirmed that the hypothalamic-pituitary-adrenal axis is activated, and hypercortisolism is recognized.
Different probability is that high estradiol suppressed transcription of 11-β hydroxysteroid dehydrogenase (11-β HSD), which is identified to decrease cortisol synthesis. It is likely that if local cortisol concentration is reduced in the pituitary and/or hypothalamus, ACTH concentration is raised by the low cortisol concentration (Carr et al., 1981; Ho et al., 2007; Chapman et al., 2013; Anno et al., 2019; Sotiropoulou et al., 2020).
Previously reported data indicated that estrogen impacts 11B-HSD, however, it persists questionable how ACTH and cortisol concentrations were raised following estradiol valerate therapy. This study results showed that Matricaria chamomilla flowers extract modified all the hormonal changes associated with this PCO model including, estrogen, ACTH, cortisol, and aldosterone. Similarly, metformin significantly decreased estrogen and ACTH in PCO rats. On the other hand, metformin did not affect the hormonal changes associated with this model concerning the hormones of the adrenal glands (cortisol and aldosterone) (Low et al., 1993; Pepe et al.,2001; Anno et al., 2019; Sotiropoulou et al., 2020).
The histological examination (both H&E and PCNA staining) of adrenal sections confirmed these findings. The active phytoestrogen ingredients of Matricaria chamomilla extract are coumarins, which may inhibit estrogen production. Moreover, phytoestrogens interfere with the action of cytochrome P450 enzymes, preventing cholesterol from being converted to pregnenolone and reducing estrogen synthesis. The chamomile flower, Matricaria chamomilla, has traditionally been used to relieve tension (Brueggemeier et al., 2001; Löfgren et al., 2004; Ronis, 2016; Witchel et al., 2019; Delgado and Lopez- Ojeda, 2021).
Furthermore, apigenin, the main ingredient in chamomile, lowers cortisol levels in the blood. An earlier study found that breathing chamomile oil vapor alleviated restriction stress in ovariectomized rodents by lowering plasma ACTH levels. In bovines, Matricaria chamomilla CH12 reduced stress by inhibiting cortisol secretion. According to the current study’s findings, Matricaria chamomilla extract may be responsible for the modification of adrenal gland hormones by lowering the pituitary hormone ACTH by restoring estrogen levels to their normal levels.
However, it appears that there is a mechanism behind the impact of Matricaria chamomilla other than regulating estrogen and ACTH, as metformin changes estrogen and ACTH but does not reduce cortisol and aldosterone levels, implying another mechanism that has to be investigated further (Yamada et al., 1996; Reis et al., 2006; Sotiropoulou et al., 2020; Leon et al., 2021).
CONCLUSION
The PCO model produced by estradiol valerate was linked to higher serum ACTH and estrogen levels. The PCO model also showed that cortisol and aldosterone levels of the adrenal glands were also increased. The hormonal alterations associated with this PCO model were altered by Matricaria chamomilla flowers extract, including estrogen, ACTH, cortisol, and aldosterone. These findings were corroborated by histological investigation of adrenal sections (both H&E and PCNA staining).
ACKNOWLEDGEMENTS
The author acknowledges Prof. Soad Shaker, Prof. of Histology, KAU, and Assiut University, Egypt, for reviewing the histological part of this manuscript.
Conflict of Interest: Authors declares no conflicts of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of College of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
REFERENCES
Alahmadi AA, Alzahrani AA, Ali SS, Alahmadi BA, Arab RA and El-Shitany NA (2020). Both Matricaria chamomilla and metformin extract improved the function and histological structure of thyroid gland in polycystic ovary syndrome rats through antioxidant mechanism. Biomolecules 10(88). DOI: 10.3390/BIOM10010088.
Alzahrani AA, Alahmadi AA, Ali SS, Alahmadi BA, Arab RA, Wahman LF and El-Shitany NA (2019). Biochemical and histological evidence of thyroid gland dysfunction in estradiol valerate model of the polycystic ovary in Wistar rats. Biochemical and Biophysical Research Communications 514(1): 194–199. DOI: 10.1016/j.bbrc.2019.04.126.
Anno T, Kawasaki F, Shigemoto R, Irie S, Mune T, Kaku K and Kaneto H (2019). Alteration of ACTH and cortisol levels after estradiol valerate treatment in a male subject with gender dysphoria: a case report. Frontiers in Endocrinology 10: 571. DOI: 10.3389/fendo.2019.00751.
Azziz R, Black V, Hines G, Fox LM and Boots LR (1998). Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. The Journal of clinical endocrinology and metabolism 83(7): 2317–2323. DOI: 10.1210/JCEM.83.7.4948.
Baskind N and Balen A (2016). Hypothalamic–pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Practice & Research Clinical Obstetrics & Gynaecology 37: 80–97. DOI: 10.1016/J.BPOBGYN.2016.03.005.
Bhathena R (2005). Polycystic ovary syndrome: a guide to clinical management. Journal of Family Planning and Reproductive Health Care 31(4): 328–328. DOI: 10.1783/jfp.31.2.328.
Brueggemeier R, Gu X, Mobley J, Joomprabutra S, Bhat AS and Whetstone JL (2001). Effects of phytoestrogens and synthetic combinatorial libraries on aromatase, estrogen biosynthesis, and metabolism. Annals of the New York Academy of Sciences 948: 51–66. DOI: 10.1111/J.1749-6632. 2001.TB03986. X.
Carr B, Parker C, Madden J, MacDonald PC and Porter JC (1981). Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. American Journal of Obstetrics & Gynecology 139(4): 416–422. DOI: 10.1016/0002-9378(81)90318-5.
Chapman K, Holmes M and Seckl J (2013). 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiological reviews 93(3): 1139–1206. DOI: 10.1152/PHYSREV.00020.2012.
Clarke B and Khosla S (2010). Female Reproductive System and Bone. Archives of biochemistry and biophysics 503(1): 118. DOI: 10.1016/J.ABB.2010.07.006.
Delgado B and Lopez-Ojeda W (2021). Estrogen. Encyclopedia of Stress. StatPearls Publishing: 951–954. Available at: https://www.ncbi.nlm.nih.gov/books/NBK538260/b
Dennett C and Simon J (2015). The Role of Polycystic Ovary Syndrome in Reproductive and Metabolic Health: Overview and Approaches for Treatment. Diabetes Spectrum: A Publication of the American Diabetes Association 28(2): 116–120. DOI: 10.2337/DIASPECT.28.2.116.
Dumitrescu R, Mehedintu C, Briceag I, Purcarea VL and Hudita D (2015). The Polycystic Ovary Syndrome: An update on metabolic and hormonal mechanisms. Journal of Medicine and Life 8(2): 142–145. Available at: /pmc/articles/PMC4392092
Farideh Z, Bagher M, Ashraf A, Akram A and Kazem M (2010). Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. Journal of reproduction & infertility 11(3): 169–74. Available at: https://pubmed.ncbi.nlm.nih.gov/23926485
Hambridge H, Mumford S, Mattison D, Ye A, Pollack AZ, Bloom MS, Mendola P, Lynch KL, Wactawski-Wende J and Schisterman EF (2013). The influence of sporadic anovulation on hormone levels in ovulatory cycles. Human reproduction (Oxford, England) 28(6): 1687–1694. DOI: 10.1093/HUMREP/DET090.
Heidary M, Yazdanpanahi Z, Dabbaghmanesh M, Parsanezhad ME, Emamghoreishi M and Akbarzadeh M (2018) Effect of chamomile capsule on lipid- and hormonal-related parameters among women of reproductive age with polycystic ovary syndrome. Journal of research in medical sciences 23(4). DOI: 10.4103/JRMS.JRMS_90_17.
Ho J, Lewis J, O’Loughlin P, Bagley CJ, Romero R and Dekker GA (2007). Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clinical endocrinology 66(6): 869–877. DOI: 10.1111/J.1365-2265.2007.02826. X.
Leon L, Anastasopoulou C and Mayrin J (2021). Polycystic ovarian disease. Encyclopedia of Genetics, Genomics, Proteomics and Informatics. StatPearls Publishing: 1528–1528. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459251/ (accessed 10 September 2021).
Löfgren S, Hagbjörk AL, Ekman S, Fransson-Steen R and Terelius Y (2004). Metabolism of human cytochrome P450 marker substrates in mouse: A strain and gender comparison. Xenobiotica 34(9): 811–834. DOI: 10.1080/00498250412331285463.
Low S, Assaad S, Rajan V, Chapman KE, Edwards CR and Seckl JR (1993). Regulation of 11 beta-hydroxysteroid dehydrogenase by sex steroids in vivo: further evidence for the existence of a second dehydrogenase in rat kidney. The Journal of endocrinology 139(1): 27–35. DOI: 10.1677/JOE.0.1390027.
Ndefo U, Eaton A and Green M (2013). Polycystic Ovary Syndrome: A Review of Treatment Options with a Focus on Pharmacological Approaches. Pharmacy and Therapeutics 38(6). MediMedia, USA: 336. Available at: /pmc/articles/PMC3737989.
Pepe G, Burch M and Albrecht E (2001). Estrogen regulates 11 beta-hydroxysteroid dehydrogenase-1 and -2 localization in placental syncytiotrophoblast in the second half of primate pregnancy. Endocrinology 142(10): 4496–4503. DOI: 10.1210/ENDO.142.10.8434.
Reed B and Carr B (2000). The Normal Menstrual Cycle and the Control of Ovulation. MDText.com, Inc. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279054/ (accessed 10 September 2021).
Reis L, Pardo P, Oba E, Kronka SN and Frazatti-Gallina NM (2006). Matricaria chamomilla CH12 decreases handling stress in Nelore calves. Journal of Veterinary Science 7(2): 189. DOI: 10.4142/JVS.2006.7.2.189.
Ronis M (2016). Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug metabolism reviews 48(3): 331–341. DOI: 10.1080/03602532.2016.1206562.
Rosenfield R and Ehrmann D (2016). The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocrine Reviews 37(5): 467–520. DOI: 10.1210/ER.2015-1104.
Sotiropoulou N, Megremi S and Tarantilis P (2020). Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L.) and Sage (Salvia officinalis L.) Prepared at Different Temperatures. Applied Sciences 10 (7): 2270. DOI: 10.3390/APP10072270.
Stewart P, Shackleton C, Beastall G and Edwards CR (1990). 5 alpha-reductase activity in polycystic ovary syndrome. Lancet 335(8687): 431–433. DOI: 10.1016/0140-6736(90)90664-Q.
Tock L, Carneiro G, Pereira A, Tufik S and Zanella MT (2014). Adrenocortical production is associated with higher levels of luteinizing hormone in nonobese women with polycystic ovary syndrome. International Journal of Endocrinology 2014. DOI: 10.1155/2014/620605.
Tsilchorozidou T, Honour J and Conway G (2003). Altered cortisol metabolism in polycystic ovary syndrome: insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates. The Journal of clinical endocrinology and metabolism 88(12): 5907–5913. DOI: 10.1210/JC.2003-030240.
Witchel S, Oberfield S and Peña A (2019). Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. Journal of the Endocrine Society 3(8): 1545–1573. DOI: 10.1210/JS.2019-00078.
Yamada K, Miura T, Mimaki Y and Sashida Y (1996). Effect of inhalation of chamomile oil vapour on plasma ACTH level in ovariectomized- rat under restriction stress. Biological and Pharmaceutical Bulletin 19(9): 1244–1246. DOI: 10.1248/bpb.19.1244.
Yildiz B and Azziz R (2007). The adrenal and polycystic ovary syndrome. Reviews in Endocrine and Metabolic Disorders 8(4): 331–342. DOI: 10.1007/S11154-007-9054-0.


