Department of Biotechnology, University Institute of Engineering & Technology, Kurukshetra
University, Kurukshetra-136119, Haryana, India.
Corresponding author email: sunitakhatak2019@gmail.com
Article Publishing History
Received: 17/01/2023
Accepted After Revision: 29/03/2023
Nanotechnology has spawned a slew of new research and application opportunities. Bimetallic nanoparticles offer certain advantages over monometallic nanoparticles in magnetic and optical properties which have utmost requirement in medicinal industry for therapeutics and drug delivery system. The advantage of using a green synthesis technique results in increased stability and enhancement of many physical and chemical characteristics. Green synthesis has the benefit of being able to replace existing physical and chemical processes. Different monometallic nanoparticles have already been tested. Researchers are working on even trimetallic nanoparticles in present scenario.
In the present investigation research cum review article, we have randomly selected medicinal plants available in campus itself as lockdown conditions were prevalent all over the country. The bimetallic Cu-Zn nanoparticles were synthesized using mostly leaves as plant parts, from these selected plant materials. The Cu-Zn nanoparticles were confirmed by their synthesis, using visual color change along with UV visible spectroscopy techniques. The nanoparticles after confirmation were opted further for testing their efficacy against standard pathogens for plants viz Terminalia, Tecoma and Solanum nigrum.The results have been promising and significant giving zones bigger in size than 20 mm.The research is ongoing to reveal further the inherent potential of medicinal plants to act as alternative agents for antimicrobial potential.
Nanoparticles, Antimicrobial, Phytochemicals, Zone Ofinhibition.
Bhatti. L, Khatak. S, Gaur. A, Bagiyal M, Jain. P. Bimetallic Nanoparticles – Expanding Grassroots in Medical Health Care Through Enhanced Microbial Resistance. Biosc.Biotech.Res.Comm. 2023;16(1).
Bhatti. L, Khatak. S, Gaur. A, Bagiyal M, Jain. P. Bimetallic Nanoparticles – Expanding Grassroots in Medical Health Care Through Enhanced Microbial Resistance. Biosc.Biotech.Res.Comm. 2023;16(1). Available from: <ahref=”https://bit.ly/3Ly9CQY“>https://bit.ly/3Ly9CQY</a>
INTRODUCTION
From the birth of civilization, medicinal plants have been an integral element of human culture in the fight against illness.As a result, nanoparticles (NPs) can be described as particles with novel or better qualities to the bulk material from which they are made. It is among the most reliable fields in science these days. Numerous terminologies are used to describe nanotechnology; however the most notable often utilized terms are nanomaterial and nanoparticle, (Jeevarathinam et al., 2019 Umar et al., 2020). Nanoparticles have multifunctional properties and various applications with regards to different fields for example medication, energy and nutrition. The field of nanotechnology manages the particles present at nano-scale which will in general have interesting physio-chemical properties which render distinctive practical properties, (Abou-Okeil et al., 2012, Umar et al., 2020).
Progressed nano-biotechnology coupling biotechnology and nanotechnology has prompted the advancement of different methods used to create nanoparticles. Nanotechnology is a multidisciplinary scientific domain that has been used in chemical, physical, biological, pharmacological and material science domains. Because of the vast range of applications for metallic nanoparticles has led to a rise in global investment in nanotechnology-based advancement and research. Physical and chemical nanoparticle synthesis methods have been used in the past and they are based on ion sputtering, hydrothermal synthesis, microemulsion, and sol-gel procedures (Armendariz et al., 2004, Antony et al., 2011 & Argueta-Figueroa et al., 2014).
Plant-based nanoparticle synthesis (Phytonanotechnology) opens up new possibilities in the realm of NP synthesis since it is a green, easy, stable, fast, and low-cost technique that employs biologically acceptable solvents and little or no toxic chemicals. Additional to these benefits, phyto-nanotechnology creates biocompatible nanoparticles, the procedures are adaptable, and no hazardous substances are used as reductants. The all-purpose solvent, water, is widely employed as a medium and a reductant. Since the synthesis may be carried out more safely and easily without the use of any chemicals, it is also known as a green synthesis strategy (Ashishie et al., 2018).
The plant or portions of the plant can be extracted straight in a liquid medium in green synthesis that can be used in the formation of metal NPs as a reducing, capping or stabilizing agent. Numerous functional groups, such as phenolic or alcoholic groups and carboxylate groups, are used in the reducing, encapsulation, manufacturing, and preservation of NPs produced by the green synthesis process and so on. The limitations of microbial nanoparticles are readily defeated in green synthesis (Bai et al., 2006). Green synthesis tries to substitute harmful chemicals with biomolecules from plants that contain terpenoids, aldehydes, vitamins, alkaloids and polysaccharides that work as reducing, capping, and stabilizing agents in the production of desirable nanoparticles.
The characteristics of metals, as well as their applications, differ depending on the metal. Metal NPs have superior strength, malleability, and electrical-magnetic characteristics compared to their bulk counterparts. Magnetic metals (Fe, Co, Ni) (Beyth et al., 2015) and noble metals (Rh, Rd, Ag, Pd) (Brightson et al., 2010) are most commonly employed to make metal nanoparticles. The optical, electrical, plasmonic, thermal, magnetic, and catalytic characteristics of monometallic nanoparticles are altered by bimetallic nanoparticles synthesis in various forms, sizes, and compositions.
Bimetallic nanoparticles because of the synergistic impact of two metals hence increasing their electronic effects, lattice stress, bifunctional effects, and ensemble effects all contribute to the utility and applicability (Balamurugan et al., 2016). Nanoparticles may be categorized according to source, size, and structure. They can be natural or manufactured on the ground of source; 0,1,2 or 3 dimensional in terms of dimension; liposomes, dendrimers, carbon-based, or metal-based depending on their structure (Balamurugan et al., 2016).
Fe, Co, and Ni are late transition elements that can be added to noble elements to reduce the cost of the raw components. Several attempts were undertaken in the recent decade for the green and affordable production of metallic nanoparticles with broad applicability in all fields of research. Green synthesis of metal/metal oxide nanoparticles, fetched a great deal of attention in the past few years due to its growing uses in a variety of disciplines, as well as its low chemical use and simple experimental procedures. It is more efficient and practical. As a result, multiple attempts were made to use plant extracts to synthesize nanoparticles of various metals such as silver, gold, palladium, zinc, copper, iron, cobalt, nickel, and so on.
Both primary and secondary metabolites included in extracts were shown to decrease metal ions and aggregate them into nanoparticles. Carbohydrates (simple sugars and polysaccharides), proteins, and lipids are primary metabolites, while alkaloids, glycosides, terpenes, tannins, flavonoids, and acetogenins are secondary metabolites (Chowdhury et al., 2018). Bimetallic or alloying nanoparticles consist of two monometallic nanoparticles in various proportions, the final architectures of nanomaterial show versatility in their properties. The synthesis of required structural composition of alloys mainly depends on the composition metals, the synthetic route and the reaction conditions. Biosynthesis methods are more advantageous than any other classical procedures because of their accessibility to more biological substances.
The Biogenic synthesis utilizing organic concentrates are the expanding research consideration in nanotechnology, since it doesn’t require a lot of additional energy and is unable to produce highly toxic chemical byproducts. Often times the chemical method for the synthesis of nanoparticles results in the formation of toxic chemical substances that are absorbed on the surface, and have destructive effects in the medical application.Due to production of these chemical products, green synthesis has come under consideration. The utilization of plant extracts rather than traditional chemical toxic chemicals with the same procedure of synthesis of nanoparticles is at present under huge examination. Plant nanoparticles are more advantageous than that of microbial nanoparticles as plant nanoparticles are more stable and take less time to extract metal ions.
Moreover, they are not required to be kept or grown in a sterilizing environment like that of the microorganism. Additionally, they may be appropriately expanded for huge synthesis of nanomaterials (Devi & Singh 2016) (Faiyas et al., 2010). In general, there exist four structural possibilities regarding the pattern of bimetallic nanoalloys: Core-shell structure, sub-clusters, statistically mixed and multiple shell alloys. Bimetallic nanoparticles are made up of two different metals that combine to exhibit physio-chemical properties unique to each metal. They are therefore significant both industrially and technologically.
Bimetallic nanoparticles are more significant when contrasted with those of monometallic nanoparticles because of the presence of additional degrees of freedom. By combining nanoparticle gold with other metals like silver, nickel, cobalt, and so on, its catalytic characteristics can be upgraded positively in the greater amount (Gopalakrishnan et al., 2017). Bimetallic nanoparticles have a more prominent surface zone which expands their adsorption properties and therefore acts as an effective catalyst as compared to those of monometallic nanoparticles. Bimetallic nanoparticles contain a few useful highlights for example, the capacity to work in a wide scope of pH, temperatures, attractive properties, etc.
Biogenic/Ecofriendly Synthesis and Confirmation Techniques: Nanoparticle synthesis involves two key strategies: Top-down synthesis involves cutting through bulk materials to create nanoparticles, whereas bottom-up synthesis involves atoms accumulating into new compounds, which subsequently grow into bunches and produce nanoparticles (Hamid et al., 2013). A bottom-up method is used in the production of nanoparticles, where the two main reactions that take place are oxidation and reduction. The produced nanoparticles’ content, size, morphology, and crystalline phase are inferred using Raman, UV-Visible, XRD, FT-IR, DLS, and EDS spectroscopy. Bimetallic nanoparticles show excellent resistance to Gram-positive pathogen e.g. Staphylococcus aureus, Bacillus subtilis and Gram-negative bacteria e.g. Klebsiella pneumonia and Escherichia coli.
When these nanoparticle search out to the bacterial cells, they pass through their cell wall and cell membrane and immediately connect with the bacterial cell’s components including DNA, lysosomes, ribosomes and enzymes, leading to osmotic damage, diverse changes, variations in cellular uptake, problems with osmo-regulation, etc. Nanoparticles are more effective against Gram-positive pathogen because their Cell wall is composed of a peptidoglycan layer and teichoic acid consisting of ultra-small pores through which the nanoparticles can easily pass and hence causes damage to the cell wall resulting in the death of bacteria but the cell wall of Gram-negative pathogens is made up of lipopolysaccharides that only allow macromolecules to pass through it.
An emerging area of nanobiotechnology called “green synthesis” has advantages over conventional chemical and physical procedures in terms of both economy and the environment. Chemicals that are harmless, eco-friendly, and safe are used in this process. Plant extracts (Huang et al., 2007), cyclodextrin (Hyeon 2003), chitosan (Jamdagni 2018) and a variety of other natural resources have been investigated in the manufacturing of metal oxide nanoparticulate. The utilization of plant extracts in the green production of metal oxide nanoparticles has received a lot of interest as a simple method (Huang et al., 2007) & (Jeevarathinam et al., 2019). Metal oxide nanoparticles have been created synthetically from plant extracts by a number of researchers, (Huang et al., 2007) along with his coworkers were one of many who looked into the extracellular creation of Zn nanoparticles from seaweed. The authors hypothesized that fucoidan, a pigment that dissolves in water found in Sargassum myriocystum may reduce and stabilize the synthesis of Zn nanoparticles. Furthermore, because S. myriocystum is available throughout the year, it is possible to simply scale up the synthesis of Zn nanoparticles (Khatami et al., 2018 Jeevarathinam et al., 2019, Karupannan et al., 2020).
Jamdagni and associates in 2016 and Sithara and her co-researchers in 2017 improved the conditions for synthesizing Zn nanoparticles and Ag nanoparticles, respectively. Sithara and her coworkers optimized the concentration of the precursor, silver nitrate, the amount of plant extract and the temperature of the solution. The optimal concentration, volume, and temperature according to the results were 1.75 mM, 0.5 mL, and 500C, respectively. They also discovered that adding plant extract to silver nitrate changed the hue from colorless to brown, indicating that Ag nanoparticles were formed.
The absorption of the spectrum at 300-500 nm was measured with ultraviolet visible light to validate the manufacturing of nanoparticles. A band at 425 nm indicated the existence of Ag nanoparticles (Huang et al., 2007). The temperature has an essential influence in regulating the aspect ratio and relative numbers of gold nanotriangles and spherical nanoparticles during biogenesis. The form, size, and optical characteristics of anisotropic nanoparticles may be fine-tuned using temperature fluctuations in reaction conditions (Kitchens et al., 2005).
Magnolia kobus and Diopyros kaki leaf extracts were quickly transformed to gold nanoparticles at a reaction temperature of 95 °C, with over 90% of the extracts undergoing this transformation, implying reaction speeds greater or equivalent to those of chemical nanoparticle synthesis (Kumar et al., 2020). At higher reaction temperatures the size of gold nanoparticles was found to rise which was explained by an increase in micelle fusion efficiency, which dissipates super saturation (Kuppusamy et al., 2016). The size of nanoparticles is strongly influenced by the medium’s pH. In Avena sativa (Logeswari et al., 2015) the size of gold nanoparticles was regulated by changing the media’s pH. When the co-precipitation technique was used, the reaction process for generation of magnetite micro-particles was discovered as pH-dependent (Malapermal 2015, Kumar et al., 2020).
Other parameters, in addition to pH and temperature have a role in nanoparticle production. During hydrothermal synthesis using the Schikorr reaction (Malapermal et al., 2015) the size and crystallinity of 11 magnetite nanoparticles were shown to grow with increasing molar ratios of ferric/ferrous ions. With rising NaCl concentrations the size of gold nanoparticles shrinks (size ranges, 5-16 nm) than those made without the use of NaCl (size ranges 11-32 nm) (Mazhar et al., 2017).
Optical absorption spectroscopic technique Manjari et al., (2020) showed that the band gap energy in ZnS samples decreases with increasing dopant concentration.Nanoparticle production in plants is influenced by chloride, bromide, and iodide. Chloride enhances nanotriangles development, whereas iodide disrupts nano-triangles structure and leads to the creation of aggregated spherical nanoparticles (Minal & Prakash 2016). The production of diamond-shaped copper nanoparticles is caused by the chloride ion (Mizutani et al., 2008). Sun-dried Cinnamomum camphora leaf biomass generates triangular or spherical gold nanoparticles as well as silver nanoparticles (55-80 nm) when aqueous silver or gold precursors are kept at room temperature. The relative potential of protecting and reducing biomolecules from leaf extracts might explain by significant shape variation between gold and silver nanoparticles. The polyol and aqueous soluble heterocyclic compounds were primarily responsible for the reduction of silver ions or chloroaurate ions (Mohamad et al., 2011).
The visible color change of blue copper sulfate was used to confirm the presence of bimetallic Cu-Zn nanoparticles and colorless zinc nitrate solution to greenish colored solution on reduction using plant leaf extracts and as plant part offering dual benefit of capping and reducing effects (Fig-1). While UV visible spectrophotometer revealed two peaks one at 404nm and other at 408nm confirming nanoparticle synthesis. India is flooded with such bio resources and sacred plants like Tulsi, Baelpatra and Kadamba having medicinal and scientific logistics which need to furnish by developing drugs using ancient knowledge and Ayurveda medicinal flora.
Figure 1: Visual confirmation of Bimetallic Cu-Zn nanoparticles from leaf extracts of Terminalia(a) ,Tecoma(b) and c) Solanum

Nanoparticles as Antimicrobial agents: There are two sorts of applications for metallic nanoparticles that have been investigated; biological and non-biological applications. Nanoparticles have been exploited as antibacterial, antifungal, antiviral, anticancer, antidiabetic, and antioxidant properties in biological applications (Mokhena & Luyt 2017), (Mostaghnia et al., 2017) & Muralidharan et al., 2011). Harmful dyes including methylene blue, 4-nitrophenol and its derivatives utilized as pesticides, and usage in dye-sensitive solar cells (DSSCs) can all be photocatalyzed (Nava et al., 2017) and are non-biological uses. In the coming decade, numerous applications will result from the quick advancement in the production of bimetallic nanoparticles with distinctive physical, chemical and biological features. When two metals with different qualities are combined in a single nanosystem, it reveals that they can be used in a range of platforms thanks to its electrical, biological, thermal, chemical and mechanical properties (Nava et al., 2017).
Antimicrobial properties of bimetallic nanoparticles can supplement antibiotics in the fight against microorganisms. Also, antibacterial efficacy of Au-Ag bimetallic nanoparticles generated from Ocimum basilicum (Basil) flower and leaf extracts against Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Escherichia coli and other bacteria has been reported by researchers (Nazeruddin et al., 2014). Ag-Cu bimetallic nanoparticles have been found to have antimicrobial effects against the gram-positive Bacillus subtilis. As a result, antibacterial medicines can be created using their combined actions with antibiotics and sulfa drugs (PannareeSrinoi et al., 2018).
These nanoparticles can obstruct bacterial development by disrupting membranes or creating reactive oxygen species (ROS) which induce DNA degradation and obstruct mechanisms for protein activity (Porter & Youtie 2009). Ag-Au metallic produced from Gracilaria sp.show antimicrobial action against Staphylococcus aureus and Klebsiella (Joerger et al., 2000). Antimicrobial action of Ag doped ZnO nanoparticles against Staphylococcus aureus and Bacillus subtilis by changing the MIC (Minimum Inhibition Concentration) particularly for S. aureus has been reported (Ramakritinan et al., 2013).
Cu-Ni bimetallic nanoparticles have been found to exhibit bacteriostatic properties against microbes like E. coli, Staphylococcus aureus, and Staphylococcus mutans (Rane et al., 2018). Antifungal and antibacterial properties of Fe-Ag magnetic bimetallic nanoparticles have been demonstrated against a variety of harmful microorganisms. These antibacterial bimetallic nanoparticles might be employed in nanomedicine to create nanodrugs against human infections to fill in the gaps where antibiotics have failed to provide favorable outcomes.
RESULTS AND DISCUSSION
Antimicrobial Efficacy of Terminalia-Mediated Bimetallic Cu-Zn Nanoparticles
Terminalia chebula: Retz. family Combretaceaeis clearly depicts the applicability of its different plant parts in several ailments. It is found in Uttar Pradesh, Bengal, and North India’s forests and is very frequent in existence in Southern part of India. The plant is a huge tree, is found in China and other tropical and subtropical regions of Asia. Ailments like cough, gastroenteritis, diarrhea, fever, skin disease, urinary tract infection are cured using harad by Tribal people of Karnataka and TamilNadu (Rani et al., 2018). The plant parts are boon to nano-researchers as all type of nanoparticles can be synthesized using this plant as sole source.
We recommend researcher to work on different nanoparticles synthesis using terminalia as sole plant source as initial color development was observed in our preliminary experiments for all types (Cu, Zn, Ag, Ag-Cu and Cu-Zn) of nanoparticle synthesis. Zno and silver nanoparticles have extensive application in coating, painting industries, personal health care products, spin electronics, chemical sensors, light emitters and transparent electronics. Bimetallic Cu-Zn nanoparticles have more efficient systems to inhibit resistant strain of bacteria owing to better optical and magnetic characteristics which in turn is due to merging two metallic particles together. The zone size reported using bimetallic nanoparticles was above 20mm in size as compared to monometallic nanoparticles (Table-1), (Fig-2).
Table 1. Inhibition zone diameters (in mm) of copper-Zinc nanoparticles of T. chebula (leaf) against different pathogens.
| Sr. no. | Concentration of extracts(µl) | S. aureus | E. coli | P. aeruginosa | B. subtilis |
| 1. | 50 | 23 | 29 | 19 | 18 |
| 2. | 75 | 28 | 30 | 22 | 25 |
| 3. | 100 | 29 | 32 | 25 | 27 |
| PE | 100 | – | – | – | – |
Three volumes of extract were tested against four standard pathogens viz. E. coli, P. aeruginosa, S. aureus and B. subtilis. Significant zone of inhibition were observed as shown in Table-1 at all three volumes of extracts. The bimetallic nanoparticles resulted in zones of inhibition of size 23mm, 28mm and 29mm at three different volumes of 50, 75 and 100µl respectively while aqueous extract failed to generate any zone of inhibition. Similarly zones of size 29mm, 30mm and 32 mm were observed at three different volume of extract of 50, 75 and 100µl respectively. The zones show linear correlation to the volume concentrations used and validate the increase in volume extract resulting in increase in zone size even when there is minor difference of 25µl between different volumes of extract against E. coli a gram negative bacteria.
While testing against a gram negative strain P. aeruginosa significant zone of inhibition but lesser in diameter as compared to E. coli were observed resulting in zones of 19mm, 22mm and 25mm at three different volumes of 50, 75 and 100µl respectively. Similarly when tested against B. subtilis particular zones of 18mm,25mm and 27mm were observed as shown in figure-7 clearly at three different volumes of extract of 50,75 and 100µl respectively. In all cases there is positive correlation between zone size and volume of extract used. No contradiction was observed and further results obtained are promising in futuristic alternative to be used as antimicrobial agents. The bi functional nanoparticles exhibit cumulative effect which in turn is due to alteration in the geometric pattern and electronic properties resulting in enhanced activities as compared to monometallic nanoparticle synthesis (Patil & Kumbhar 2017). The atoms of two metals have parts of active catalytic sites which increase adsorption for more variable reactants or variable intermediates.
Figure 2: Zones of inhibition using Bimetallic Cu-Zn nanoparticles from Harad leaf plant extracts tested against four pathogens. a) P. aeruginosa b) E. coli. c) S. aureus d) B. subtilis.
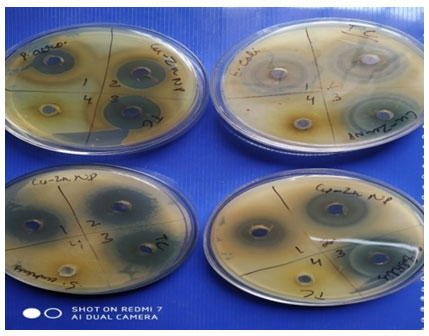
Antimicrobial Efficacy of Tecoma-Mediated Bimetallic Cu-Zn NanoparticlesTecoma stans: family Bignoniacaeaan ornamental plant is The Bahamas’ national flower. It is between 5-7.6 meters tall and is a perennial blooming shrub or small tree.Three volumes of extract were tested against four standard pathogens viz. E. coli, P. aeruginosa, S. aureus, and B. subtilis. A significant zone of inhibition was observed as shown in Table 2 at all three volumes of extracts.
Table 2. Inhibition zone diameters (in mm) of copper-Zinc nanoparticles of T. stans (leaf) against different pathogens.
| Sr. no. | Concentration of extracts(µl) | S. aureus | E. coli | P. aeruginosa | B. subtilis |
| 1. | 50 | 25 | 25 | 17 | 24 |
| 2. | 75 | 31 | 33 | 24 | 28 |
| 3. | 100 | 33 | 34 | 27 | 28 |
| PE(Aq) | 100 | — | – | – | – |
| Cu-Zn | 100 | 21 |
The bimetallic nanoparticles synthesized using Tecoma leaf resulted in zones of inhibition of size 23mm, 28mm and 29mm at three different volumes of 50, 75 and 100µl respectively, while aqueous extract failed to generate any zone of inhibition. Similarly zones of size 25mm, 31mm and 33 mm were observed at three different volume of extract of 50, 75 and 100µl respectively. The zones show linear correlation to the volume concentrations used and validate the increase in volume extract resulting in increase in zone size even when there is minor difference of 25µl between different volumes of extract against S. aureus a gram positive bacteria.
While testing against a gram negative strain P. aeruginosa significant zone of inhibition but lesser in diameter as compared to E. coli and S. aureus were observed resulting in zones of 17mm, 24mm and 27mm at three different volumes of 50, 75 and 100µl respectively. Similarly when tested against B. subtilis particular zones of 24mm,28mm and 28mm were observed as shown in Table -2 clearly at three different volumes of extract of 50,75 and 100µl respectively. Similarly zones of size 25mm, 33mm and 34 mm were observed at three different volume of extract of 50, 75 and 100µl respectively when tested against E. coli a gram negative bacteria. In all cases there is positive correlation between zone size and volume of extract used. No contradiction was observed and further results obtained are promising in futuristic alternative to be used as antimicrobial agents.
Antimicrobial Efficacy of Solanum Mediated Bimetallic Cu-Zn Nanoparticles: In the present study the zinc nanoparticles manufactured utilizing leaves of Solanum nigrum resulted in significant zones of inhibition against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans. Three concentrations of Cu-Zn nanoparticles were used, which are 50, 100 and 150 µl. Zone size of 19mm to 29mm was reported against Bacillus subtilis, while a minimum zone of 23mm and a maximum of 28mm were reported for Staphylococcus aureus. Against Escherichia coli and Pseudomonas aeruginosa the range of zone of inhibition (ZOI) were in the range of 19mm to 25mm and 24m to 26mm respectively. With Candida albicans it can be concluded that the three concentrations yielded fairly nearing ZOI with a minimum being 26mm and a maximum at 28mm as shown in Table 3 and Fig 3.
Table 3. Inhibition zone diameters (in mm) of copper-Zinc nanoparticles of Solanum (leaf) against different pathogens.
| Pathogen | Volume of Extract (µl) | Cu-Zn Np from leaves (mm) |
| Bacillus subtilis | 50 | 19 |
| 100 | 25 | |
| 150 | 29 | |
| Staphylococcus aureus | 50 | 23 |
| 100 | 27 | |
| 150 | 28 | |
| Escherichia coli | 50 | 19 |
| 100 | 22 | |
| 150 | 25 | |
| Pseudomonas aeruginosa | 50 | 24 |
| 100 | 25 | |
| 150 | 26 | |
| Candida albicans | 50 | 26 |
| 100 | 26 | |
| 150 | 28 |
Figure 3: Zones of inhibition using Bimetallic Cu-Zn nanoparticles from Solanum leaf plant extracts tested against pathogens. a) P. aeruginosa b) E. coli. c) S. aureus d) B. subtilis e) Candida Albicans

(Hamid et al.,2013) synthesized gold and silver-gold bimetallic nanoparticles via green route and aqueous extracts of sago pondweed resulting in spherical-shaped nanoparticles along with two different shapes of hexagon and nano triangles. The nanoparticles were dependant on ph and proteins and flavonoids present in phytochemical analysis proved to be major factor for nanoparticle synthesis. (Minala& Prakasha2019) reported larvicidal activity using Azadirachtaindica leaf extracts.
Finally,abimetallicananoparticlesaoutperformedamonometallicananoparticlesainatermsaofalarvicidalaactivity. (Devi & Singh, 2016) used ascorbic acid as a new green approach as a reducing and capping agent for the reduction of metal salts for the synthesis of monometallic copper and zinc along with bimetallic nanoparticle synthesis. Cu-Zn and Cu-Ni resulted in particle sizes of 43.47, 38.4 and 43.5nm for Cu, Cu-Zn and Cu-Ni nanoparticles respectively. The UV spectroscopy revealed that the solution is not just a mixture of solutions but is an alloy. Bimetallic nanoparticles were more potent in photo-degradation Cu-Zn(80.75) and Cu-Ni (80.4%) of methyl orangein 60 minutes as compared to monometallic nanoparticles (69.5%) due to bifunctional effects. (Mostaghni et al., 2017) manufactured Cobalt Iron Oxide powder utilizing Chenopodium album leaf extract.
X-ray diffraction techniques were used to know the structure of the synthesized material. The photocatalyst that was created was used to photodegrade methyl orange as a useful model contaminant. According to the findings, CoFe2O4 was highly effective at destroying germs when exposed to UV light, with a degrading ratio of 100 % after three hours of exposure. (Rani et al., 2018) exploited four plants namely Chenopodium album (JungliBathua), Mesua ferrea (Nagkesar), Syzygiumcumini (Jamun), and Cassia fistulav (Amaltas) to manufacture silver nanoparticlesand their antibacterial function was tested against Alternaria, Excrotium, and Fusarium oxysporum. These silver bio-nano particles were tested with phytopathogenic fungus for sustainable crop production. (Khatami et al.,2018) synthesized silver and zinc nanoparticles using Prosopsisfracta and Cofea arabica.
Standard confirmation via UV visible spectroscopy,XRD and SEM results in an average nanoparticle size 16 and 26nm respectively. further the nanoparticle were tested against Acinetobacter baummannii and P.aeruginosa culture and tested bandages which were dipped /impregnated with ,silver,zinc and also in mixed solution silver and zinc in equal proportionwere more efficient in healing. bandages dipped in both solutions were more efficient in curing,diabetes or burn injuries are particularly susceptible to infections. (Ashishie et al.,2018) synthesized silver monometallic and copper-silver bimetallic nanoparticles using Kigelia africana fruit extracts.
Aqueous plant extracts of fruits were characterized using standard techniques and methodology. The SEM images showed uniform spherical silver nanoparticles while silver copper was anisotropic in nature.the average size was reported as 10nm. The nanoparticles exhibit strong inhibitory capability against both gram + and gram – strain tested .it inhibited K. pneumonia and P. aeruginosa resulting in zones of 23 and 25mm in size. Using silver nanoparticles while bimetallic results in zones of 27mm against S. aureus. XRD analysis revealed crystalline particles. (Umar et al., 2020) investigated the green synthesis ofreduced graphene oxide nanoparticles utilizing Chenopodium album (Bathua in Hindi) a commonly found weed plant. Reduced graphene oxide (RGOX) from graphene oxide (GOX) was devised using vegetable extract as a reducing agent. When it came to antibacterial performance against both Gram-positive and Gram-negative microbes as well as antibiofilm action, the agar diffusion test outperformed GOX.
Nanoparticles, particularly metallic nanoparticles have piqued the curiosity of a variety oforganizations and professions including electronics, photonics, medicine and agriculture. Recent analysis of the manufacture of metallic nanoparticles utilizing biological organisms has been covered in this study. However, due to the variety of living organisms, from bacteria to plants, the majority of this issue is still understudied and unknown. The creation of nanoparticles from biological sources has the potential to open up new possibilities for the production of distinctive materials that are ecologically friendly, affordable, stable, and free of hazardous substances. Modern chemical and physical procedures usually involve risky compounds that can cause environmental toxicity and carcinogenic effects.
This green chemistry technique, which uses biological organisms, contrasts dramatically with such processes. Although many biological sources have been used to create nanoparticles, employing plants offers a straightforward, safe, non-toxic, and robust method that does not necessitate the additional culture preparation or isolation steps that are frequently needed for microbes and fungi-based approaches. Plant extracts are affordable, readily scaled up, and environmentally acceptable when used to synthesize nanoparticles of a specific size, form, and composition. Plant-derived nanoparticles have the possibility of replacing several currently used nanoparticle-based medical treatments, including fluorescent labeling in assay methods, therapeutically drug delivery, tumor killing by overheating (hyperthermia), and antimicrobial agents in bandages. Plant-derived nanoparticles, on the other hand, have the ability to transport anti-microbial chemicals as pesticides in agricultural crops. Furthermore, Agricultural crop wastes and wastes from the food sector are a great source of plant-based biochemicals that can be used to create metallic nanoparticles and other similar products.
Regardless of the ecological benefits of using eco-friendly chemistry-based biological production over conventional approaches, as mentioned in this article, some problems remain unresolved, such as the consistent properties of nanoparticles, reproducibility of the synthesis process, and comprehension of the factors underlying the production of metallic nanoparticles via living creatures. Nanoparticle production processes differ amongst plant species when it comes to plant extracts. As a result, to study and fully appreciate the scenario where plants are dependent, research is required. Nanomedicine has a strong impact being potential agents in disease diagnosis and treatment, developing surgical devices and other commercial health products. Green synthesis offers a remarkable progression over both physical and chemical approaches since it is affordable, environmentally friendly, and can be scaled up successfully for large-scale synthesis. Synthesis from plant extracts has many advantages, including sanitary workplace conditions, environmental and health protection, low waste, and extremely reliable products. Bimetallic nanoparticles produced using a green method has several uses in nanotechnology that are significant.
CONCLUSION
Nanotechnology has conquered almost all fields ranging from agriculture to tissue engineering. Utilizing plants for the green production of nanoparticles includes economic and environmental conservation. Thus, biosynthesized nanoparticles could be used in toothpaste, mouth wash and mouth fresheners to make them more effective. The antibacterial activity of clinical cotton bandages dipped in nanoparticle solutions showed good antimicrobial activities which open the door for antimicrobial bandages, tissues and diapers for babies in the future.
REFERENCES
Abou-Okeil, A., Amr, A., & Abdel-Mohdy, F. A. (2012). Investigation of silver nanoparticles synthesis using aminated β-cyclodextrin. Carbohydrate polymers, 89(1): 1-6.
Antony, J. J., Sivalingam, P., Siva, D., Kamalakkannan, S., Anbarasu, K., Sukirtha, R., & Achiraman, S. (2011). Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids and Surfaces B: Biointerfaces, 88(1): 134-140.
Argueta-Figueroa, L., Morales-Luckie, R. A., Scougall-Vilchis, R. J., & Olea-Mejía, O. F. (2014). Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu–Ni nanoparticles for potential use in dental materials. Progress in Natural Science: Materials International, 24(4): 321-328.
Armendariz, V., Herrera, I., Jose-Yacaman, M., Troiani, H., Santiago, P., & Gardea-Torresdey, J. L. (2004). Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. Journal of nanoparticle research, 6(4): 377-382.
Ashishie,P.B.;Anyama,C.A.;Ayi,A.A. Oseghale,C.O.; Adesuji,E.T.; and Labulo ,A.H. (2018). Green synthesis of silver monometallic and copper silver bimetallic nanoparticles using Kigelia Africana fruit extracts and evaluation of their antimicrobial activity. International J. of Physical sciences, 13(3): pp24-32.
Bai, H. J., Zhang, Z. M., & Gong, J. (2006). Biological synthesis of semiconductor zinc sulfide nanoparticles by immobilized Rhodobactersphaeroides. Biotechnology letters, 28(14): 1135-1139.
Balamurugan M , Kaushik S, Saravanan S. (2016). Green Synthesis of Gold Nanoparticles by Using Peltophorum Pterocarpum Flower Extracts. Nano Biomed Eng: 8(4): 213-218.
Balamurugan M, Kaushik S, Saravanan S. (2016). Green Synthesis of Gold Nanoparticles by Using Peltophorum Pterocarpum Flower Extracts. Nano Biomed Eng: 8(4): 213-218.
Beyth, N., Houri-Haddad, Y., Domb, A., Khan, W., & Hazan, R. (2015). Alternative antimicrobial approach: nano-antimicrobial materials. Evidence-based complementary and alternative medicine.
Brightson, M., Selvarajan, P., Vethanathan, J. K., Freeda, T. H., & Sundar, S. M. (2010). Investigations on the Effect of Manganese Ions on the Structural and Optical Properties of ZnS Nanoparticles Synthesized by Solvo-thermal Route. Recent Research in Science and Technology, 2(6).
Chowdhury, R., Mollick, M. M., Biswas, Y., Chattopadhyay, D., & Rashid, M. H. (2018). Biogenic synthesis of shape-tunable Au-Pd alloy nanoparticles with enhanced catalytic activities. Journal of Alloys and Compounds, 763, 399-408. doi:10.1016/j.jallcom.2018.05.343
Devi, H.S. and Singh, T.D.(2016).Cu-Zn and Cu-Ni bimetallic particles fabricated using ascorbic acid and its role in photodegradation of methyl orange.Asian Journal of Chemistry , 28(10):2255-2260.
Faiyas, A. P. A., Vinod, E. M., Joseph, J., Ganesan, R., & Pandey, R. K. (2010). Dependence of pH and surfactant effect in the synthesis of magnetite (Fe3O4) nanoparticles and its properties. Journal of Magnetism and Magnetic Materials, 322(4): 400-404.
Gopalakrishnan, R., Loganathan, B., Dinesh, S., & Raghu, K. (2017). Strategic green synthesis, characterization and catalytic application to 4-nitrophenol reduction of palladium nanoparticles. Journal of Cluster Science, 28(4): 2123-2131.
Hamid,A.A.A.;Ghobasy,M.A.; Fawzy,M.; Mohamed,M.B.; MottalebMohammed,M.S.A. (2013). Phytosynthesis of Au,Ag,Au-Ag bimetallic nanoparticles using aqueous extracts of Sago pondweed(Potamogetonpectinatus L.)ACS Sustainable chemistry and engineering 1:1520-1529.
Huang, J., Li, Q., Sun, D., Lu, Y., Su, Y., Yang, X.,& Chen, C. (2007). Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology, 18(10):105104.
Hyeon, T. (2003). Chemical synthesis of magnetic nanoparticles. Chemical communications, (8): 927-934.
Jamdagni, P., Khatri, P., & Rana, J. S. (2018). Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. Journal of King Saud University-Science, 30(2):168-175.
Jeevarathinam, C., Solomon, J. S., & Pandian, G. V. (2019). Cresceiaalata Flower Extract As Reducing Catalyst For Green Synthesis Of Au/Ag Bimetallic Nano Medicine And Its Antibacterial Activities. Journal of Applied Physical Science International, 11(4):170-182.Retrieved from https://ikprress.org/index.php/JAPSI/article/view/4786
Karupannan, S. K., Dowlath, M. J., & Arunachalam, K. D. (2020). Phytonanotechnology: Challenges and future perspectives. Phytonanotechnology, 303–322. https://doi.org/10.1016/b978-0-12-822348-2.00015-2
Khatami et al., (2018) application of green synthesized Ag,ZnO AND Ag/Zno nanoparticles for making clinical antimicrobial wound bandages, Sustainable chemistry and Pharmacy, 10: 9-15
Kitchens, C. L., McLeod, M. C., & Roberts, C. B. (2005). Chloride ion effects on synthesis and directed assembly of copper nanoparticles in liquid and compressed alkane microemulsions. Langmuir, 21(11): 5166-5173.
Kumar, H., Bhardwaj, K., Kuča, K., Kalia, A., Nepovimova, E., Verma, R., & Kumar, D. (2020). Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials, 10(4):766. doi:10.3390/nano10040766
Kuppusamy, P., Yusoff, M. M., Maniam, G. P., & Govindan, N. (2016). Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications – An updated report. Saudi Pharmaceutical Journal, 24(4):473-484. doi:10.1016/j.jsps.2014.11.013
Logeswari, P., Silambarasan, S., & Abraham, J. (2015). Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. Journal of Saudi Chemical Society, 19(3):311-317.
Malapermal, V. (2015). Biosynthesis Of Bimetallic Au-Ag Nanoparticles Using Ocimumbasilicum (L.) With Antidiabetic And Antimicrobial Properties. Advanced Materials Letters, 6(12):1050-1057. doi:10.5185/amlett.2015.5997
Malapermal, V., Mbatha, J. N., Gengan, R. M., & Anand, K. (2015). Biosynthesis of bimetallic Au-Ag nanoparticles using Ocimumbasilicum (L.) with antidiabetic and antimicrobial properties. Advanced materials letters (Online).
Manjari, G., Saran, S., Radhakrishanan, S., Rameshkumar, P., Pandikumar, A., & Devipriya, S. P. (2020). Facile green synthesis of Ag–Cu decorated ZnO nanocomposite for effective removal of toxic organic compounds and an efficient detection of nitrite ions. Journal of Environmental Management, 262:110282.doi:10.1016/j.jenvman.2020.110282.
Mazhar T, Shrivastava V and Tomar RS. (2017). Green Synthesis of Bimetallic Nanoparticles and its Applications: A Review”, Sci. &Res. . 9(2):102-110.
Minal S.P. and Prakash S., (2016) Cu-Zn and Ag-Cu bimetallic nanoparticles as larvicide to control malaria parasite vector: a comparative analysis, IEEE region 10 Humanitarian technology conference (R10-HTC), 1-6.
Mizutani, N., Iwasaki, T., Watano, S., Yanagida, T., Tanaka, H., & Kawai, T. (2008). Effect of ferrous/ferric ions molar ratio on reaction mechanism for hydrothermal synthesis of magnetite nanoparticles. Bulletin of Materials Science, 31(5): 713-717.
Mohamad, M. F., Kamarudin, K. S., Fathilah, N. N., & Salleh, M. M. (2011). The effects of sodium chloride in the formation of size and shape of gold (Au) nanoparticles by microwave-polyol method for mercury adsorption. International Journal of Chemical and Molecular Engineering, 5(2):176-180.
Mokhena, T. C., &Luyt, A. S. (2017). Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydrate polymers, 165:304-312.
Mostaghnia F, Daneshvar A, Sakhaieb M. (2017)Green synthesis of nano size CoFe2O4 using Chenopodium album leaf extract for photo degradation of organic pollutants. Iranian Journal of Organophysical Chemistry 8:181-189.
Muralidharan, G., Subramanian, L., Nallamuthu, S. K., Santhanam, V., & Kumar, S. (2011). Effect of reagent addition rate and temperature on synthesis of gold nanoparticles in microemulsion route. Industrial & engineering chemistry research, 50(14):8786-8791.
Nagarajan, S., & Arumugam Kuppusamy, K. (2013). Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. Journal of nanobiotechnology, 11(1):1-11.
Nava, O. J., Luque, P. A., Gómez-Gutiérrez, C. M., Vilchis-Nestor, A. R., Castro-Beltrán, A., Mota-González, M. L., & Olivas, A. (2017). Influence of Camellia sinensis extract on Zinc Oxide nanoparticle green synthesis. Journal of Molecular Structure, 1134:121-125.
Nazeruddin, G. M., Prasad, R. N., Shaikh, Y. I., & Shaikh, A. A. (2014). Synergetic effect of Ag-Cu bimetallic nanoparticles on antimicrobial activity. Der Pharmacia Lettre, 3, 129-36.
PannareeSrinoi, Chen Y.T, VitturV, Marquez M.D and Lee T.R, (2018). “Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications.
Porter, A. L., &Youtie, J. (2009). How interdisciplinary is nanotechnology? Journal of Nanoparticle Research, 11(5):1023–1041. https://doi.org/10.1007/s11051-009-9607-0
Joerger, T. Klaus, C.G. Granqvist. (2000). Biologically produced silver–carbon composite materials for optically functional thin-film coatings, Adv. Mater., 12: 407-409.
Ramakritinan, C. M., Kaarunya, E., Shankar, S., &Kumaraguru, A. K. (2013). Antibacterial effects of Ag, Au and bimetallic (Ag-Au) nanoparticles synthesized from red algae. In Solid State Phenomena 201: 211-230. Trans Tech Publications Ltd.
Rane, A. V., Kanny, K., Abitha, V. K., & Thomas, S. (2018). Methods for synthesis of nanoparticles and fabrication of nanocomposites. Synthesis of Inorganic Nanomaterials, 121–139. https://doi.org/10.1016/b978-0-08-101975-7.00005-1
Rani A, Kumar P, Singh R, Singh C, Chauhan N. (2018). Antifungal activity of biosynthesized silver nanoparticles. Present study is about bio-synthesis of silver nanoparticles and their antimicrobial activity against phytopathogenic fungi. Biotech Today 8(1):43-47.
S.P. Patil, S.T. Kumbhar. (2017). Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. LeavesBiochem. Biophys. Rep., 10:76-81, 10.1016/j.bbrep.2017.03.002
Semagina, N., & Kiwi‐Minsker, L. (2009). Recent advances in the liquid‐phase synthesis of metal nanostructures with controlled shape and size for catalysis. Catalysis Reviews, 51(2):147-217.
Shankar, S. S., Bhargava, S., & Sastry, M. (2005). Synthesis of gold nanospheres and nanotriangles by the Turkevich approach. Journal of nanoscience and nanotechnology, 5(10):1721-1727.
Sharma, J. K., Akhtar, M. S., Ameen, S., Srivastava, P., & Singh, G. (2015). Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. Journal of Alloys and Compounds, 632:321-325.
Sharma, N., Kumar, J., Thakur, S., Sharma, S., & Shrivastava, V. (2013). Antibacterial study of silver doped zinc oxide nanoparticles against Staphylococcus aureus and Bacillus subtilis. Drug Invention Today, 5(1): 50-54.
Sithara, R., Selvakumar, P., Arun, C., Anandan, S., &Sivashanmugam, P. (2017). Economical synthesis of silver nanoparticles using leaf extract of Acalypha hispida and its application in the detection of Mn (II) ions. Journal of advanced research, 8(6):561-568.
Solomon J.S, Jeevarathinam C, Pandian G.V, (2019). “ Cresceiaalata Flower Extract as Reducing Catalyst for Green Synthesis of Au/Ag Bimetallic Nano Medicine and its Antibacterial Activities”, Adalya, Vol.8.
Song, J. Y., Jang, H. K., & Kim, B. S. (2009). Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochemistry, 44(10): 1133-1138.
Srinoi, P., Chen, Y.-T., Vittur, V., Marquez, M., & Lee, T. (2018). Bimetallic nanoparticles: Enhanced Magnetic and optical properties for emerging biological applications. Applied Sciences, 8(7):1106. https://doi.org/10.3390/app8071106
Suriyakalaa, U., Antony, J. J., Suganya, S., Siva, D., Sukirtha, R., Kamalakkannan, S &Achiraman, S. (2013). Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids and Surfaces B: Biointerfaces, 102: 189-194.
Terminalia intro Dash et al.,1991.
Tomar, R. S., Chauhan, P. S., & Shrivastava, V. (2014). A critical review on nanoparticle synthesis: physicochemical v/s biological approach. World J. Pharm. Res, 4(1): 595-620.
Umar MF, Ahmad F, Saeed H, Usmani SA, Owais M, Rafatullah M. (2020) Bio-Mediated Synthesis of Reduced Graphene Oxide NPs from C. album: Their Antimicrobial and Anticancer Activities. Nanomaterials 10:1096.
Zhang, Z.-P., & Zhang, Y.-W. (2018). Introduction of bimetallic nanostructures. Bimetallic Nanostructures, 1–22. https://doi.org/10.1002/9781119214618.ch1.


