Department of Life Sciences, University of Calicut, Kerala, India 673635
Corresponding author Email: kfzuhra@gmail.com
Article Publishing History
Received: 03/01/2019
Accepted After Revision: 26/03/2019
Bile salt hydrolase (BSH) is a highly significant enzyme involved in bile acid alteration in the gastrointestinal tract of humans and animals. Bile salt hydrolase belongs to the chologlycine hydrolase enzyme family and is normally associated with the gastrointestinal bacteria of both human and animals. This enzyme is responsible for the hydrolysis of conjugated bile acids into free bile acid and amino acid residue. Today, BSH is considered as an upcoming pharmacologically important enzyme since it has the ability to lower cholesterol levels in hypercholesterolemic patients because high cholesterol levels are found as an important reason of atherosclerosis which results in cardiovascular diseases (CVD’s). This review discusses about the incidence of BSH enzyme among bacteria and the role and potential application of this highly signifi cant enzymes on the host.
Hydrolase, Potent Enzyme, Cholesterol
Rajan A. B, Abhini K N, Zuhara K F. Bile Salt Hydrolase, a Potent Enzyme Capable of Removing Cholesterol Present in Bacteria: A Review. Biosc.Biotech.Res.Comm. 2019;12 (1).
Rajan A. B, Abhini K N, Zuhara K F. Bile Salt Hydrolase, a Potent Enzyme Capable of Removing Cholesterol Present in Bacteria: A Review. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2XcewXT
Copyright © Rajan et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Cholesterol, though is regarded as an important substance in human body, high levels of serum cholesterol may lead to atherosclerosis which in turn can end in cardiovascular diseases (CVD’s) ( Jones et al., 2004, Tsai et al., 2014). There is tremendous increase in the number of people suffering from cardiovascular diseases in the developing countries like India. During the past fi ve decades, rate of coronary artery diseases among urban population have risen from 4% to 11% due to modernization and stressful life styles. Many clinical and epidemiological studies indicate that a correlation exists between the elevated serum cholesterol levels and coronary heart disease (Pereira & Gibson, 2002). According to the recent reports of World Health Organization (WHO), the CVD’s are regarded as number 1 cause of death globally and responsible for 31% of all global deaths (WHO, 2017). On 22 September, 2016 WHO has launched “Global Hearts”, a new initiative to beat back the global threat of cardiovascular diseases.
Nowadays, a number of non-pharmacologic methodologies such as dietary management, regular exercise and drug therapies are commonly used for lowering cholesterol levels (Dunn‐Emke et al., 2001). But most of the drugs in use, though may effectively reduce the cholesterol level, are expensive and known to have side effects (Bliznakov, 2002). Hence the importance of using enzymatic deconjugation by bile salt hydrolase (BSH), both to lower serum cholesterol levels in hypercholesteremic patients and to prevent hypercholesteremia in normal people is increasing day by day (Sridevi et al., 2009). In this regard, bile salt hydrolase can be considered as an alternative therapy for lowering serum cholesterol levels.Bile salt hydrolase, a highly biologically significant enzyme, belongs to the family chologlycine hydrolase (EC 3.5.1.11). This enzyme has been classifi ed as N-terminal nucleophilic (Ntn) hydrolase which is involved in the deconjugation of conjugated bile acids resulting in the formation of free bile acids and amino acids (Kumar et al., 2006, Chand et al., 2017).
Bile
Bile is a yellow-green aqueous solution which typically consists of bile acids, cholesterol, phospholipids and the pigment biliverdin. In addition to the trace amounts of mucus, tocopherol and immunoglobulin A (IgA) are also present which prevent the bacterial growth and oxidative damage to the epithelium (De Smet et al., 1998, Schiff et al., 2002) . Bile is synthesized in the pericentral hepatocytes of the liver in many mammals and is stored and concentrated in gallbladder followed by the release of this into the duodenum just after the food intake.The bile acids play important role as a biological detergent which emulsifies and solubilizes lipids, thereby enhancing the absorption and digestion of fats. Under normal conditions, the conservation of bile acids are done by a process called enterohepatic recirculation (Ridlon et al., 2006, Russell, 2009).
Enterohepatic circulation
The cholesterol metabolism, in humans and animals, leads the formation of C24 acid steriods possessing a carboxyl group at the end of the side chain called as bile acids. The synthesis of C24 acid steriods in liver from cholesterol is followed by their conjugation with amino acids such as taurine or glycine at the C24 position of the steroid nucleus, catalysed by the enzyme N-acyltransferase (Appleby and waters 2014, Schapp et al 2014, Camilleri & Gores 2015). The ratio of conjugation of bile acids with the amino acids glycine or taurine, depends upon the relative abundance of these amino acids, which may not have any functional or regulatory consequences (Ridlon, 2006). These conjugated bile salts are amphipathic in nature with enhanced solubility which makes them impermeable to cell membranes. At physiological pH, the carbon at the terminal position of glycocholic acid or oxygen atom of taurocholic acid bonded with sulphur gets ionized. The ionized oxygen atom along with planar structure of bile acids and the hydroxyl groups present in their rings make them highly amphipathic in nature. In the case of glycocholic acid, the conjugation of aminoacid glycine with cholic acid results in the reduction of pKa of cholic acid from 6.4 to 4.4 units thereby enhancing the bile acids to get completely ionized and highly soluble (Alrefai et al., 2007).
The intake of lipids initiates the secretion of bile salts through the common duct into the duodenum, hence results in the association with dietary lipids and various digestive products (Begley, 2005). The conjugated bile acids are ionized molecules which are resistant to deamidation by pancreatic and mucosal carboxypeptidases. Instead they move to the distal ileum, where they get absorbed by an active transport system known as ileum bile acid transporter (IBAT) and the members of the ATP binding cassette (ABC) family of transporters (Lack & Weiner, 1966, Nicoloau et al., 2012). About 95% of the bile salt mixture is re-absorbed and returns back to the liver by hepatic portal circulation and this process is known as enterohepatic circulation (fi g.1). Approximately 600 to 800 ml of bile is being produced daily and the total circulating bile acid pool is about 1.7 to 40 g. The recirculation of entire bile acid pool is about 6 to 15 times per day and a total of 0.2 to 0.5 g is excreted via feces thereby enhancing the synthesis of bile acid by de novo pathway (Kumar et al., 2012). The bile acid that eludes the absorption is subjected to bacterial metabolism including reduction and epimerization of their OH groups from α to β conformation (Chiang, 2009). One such important transformation by indigenous intestinal bacteria is deconjugation of bile acids which is catalyzed by the enzyme called bile salt hydrolase (BSH) (Kim and Lee, 2005)
Role of BSH
The function of BSH still remains unclear and several hypotheses have been put forward regarding the expression and function of the BSH gene present in the bacteria of the human gastrointestinal tract.
Nutritional role
BSH confer nutritional advantage for the BSH positive strains as they utilize the hydrolysed amino acid moieties (Begley et al., 2005). The deconjugation process results in the release of two amino acids such as glycine, which may be further metabolized to ammonia and carbon dioxide and taurine, which may be metabolized to ammonia, carbon dioxide and sulphate. Evidences supporting these hypotheses, proposed by Huijghebaert et al. in 1982 and Van Eldere et al. in 1996, stated that certain bile salt deconjugating Clostridium strains were able to utilize the released taurine as an electron acceptor and the growth rates of these strains were improved in the presence of taurine and taurine-conjugated bile salts (Huijghebaert et al.,1982; Van Eldere et al., 1996).
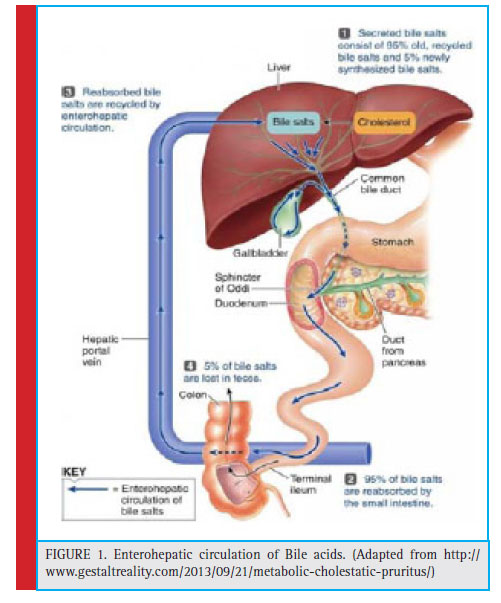 |
Figure 1: Enterohepatic circulation of Bile acids. (Adapted from http:// www.gestaltreality.com/2013/09/21/metabolic-cholestatic-pruritus/) |
Modification of Membrane characteristics
Most of the friendly intestinal bacteria play a crucial role in maintaining host gut health. The hydrolytic enzymes, lysozyme, phospholipase A2 and α-defensins, produced by these bacteria in host gut provide a defense mechanism in the intestine. The extent of damage by host defenses on these bacterial membranes is determined by the composition, fluidity, permeability, hydrophobicity and net charge of the membrane (Peschel, 2002). Studies have revealed that the cholesterol or bile incorporation into the bacterial membranes has been facilitated by BSHs (Dambekodi & Gilliland, 1998; Taranto et al., 2003; Taranto et al., 1997), which may increase the membrane potency by forming BSH- mediated lipid intermolecular hydrogen bonding (Boggs, 1987) or could change its fluidity or charge. The resultant cell surface modification by BSH activity could offer safety against perturbation of the structure and bacterial membranes integrity by the immune system and such resistance mechanisms ensures in establishing prolonged persistence. This mechanism may strongly confer commensals, possessing BSH enzyme, the capacity to dominate over the BSH- negative pathogens or other transients (Patel et al., 2010).
Bile detoxification
BSH activity of microorganisms might be detoxification action and these enzymes make the strains bile tolerant, thereby making positive environment in the gastrointestinal tract for survival. Many investigators have disproved this hypothesis of correlation between bile tolerance and BSH activity (Ahn et al, 2003; Moser & Savage, 2001;). Although ambiguity exists between the correlation of bile salt hydrolase and bile tolerance, several studies have been undertaken to study this relation.
Studies by four independent groups using wild-type and bsh mutant combinations provide a connection between BSH activity and bile tolerance. The study using Lactobacillus amylovorus mutant, with partly reduced BSH activity, displayed reduced growth rates in the presence of bile salts (Grill et al., 2000). Also BSH mutated Listeria monocytogenes made the cells more sensitive to bile and bile salts (Begley et al., 2005; Dussurget et al., 2002) and Lactobacillus plantarum (De Smet et al., 1995). The actual mechanism by which BSH positive strains exhibit bile tolerance is not yet fully known. However it was proposed that the toxicity may be exhibited through the intercellular interface by the protonated form of bile salts and BSH positive cells may protect themselves by the weaker unconjugated counterparts. Hence this mechanism helps in dropping the pH and making suitable environment for bringing back and exporting the co-transported proton (De Smet et al., 1995). The unconjugated bile acids resulting from the enzymatic deconjugation of BSH active strains have an inhibitory effect on bacteria but the study of De Smeth et al suggested that the BSH active strains may be capable of detoxifying these effects or they may be associated with 7α-dehydroxylating bacteria which will dehydroxylate unconjugated bile acids (De Boever & Verstraete, 1999).
Application of Bile Salt Hydrolase enzyme Hypocholesterolemic effect
An elevated level of cholesterol in blood can lead to hypercholesterolemia which in turn becomes a threat for the development of coronary heart diseases. Pharmacologic agents such as statins, bile acid sequestrants etc. and food products are being formulated continuously to control the serum cholesterol levels in these patients. Recently many studies have shown that probiotics with bile salt hydrolase activity have the ability of cholesterol lowering. These have given much attention of using BSH positive probiotic strains in animal models and human subjects. In 1995 De Smet et al proposed that the BSH active probiotic strains or cultured products containing them result in the reduction of serum cholesterol levels by interacting with the bile salt mechanism of the host. The studies of Sukling et al in 1991 revealed that the proposed mechanism of cholesterol lowering effect by De Smet et al (1995) is comparable with pharmacological agents (sequestrants) which prevent the bile salts from being reabsorbed by binding with them (Suckling et al., 1991). In addition to this Liong and shah (2005) and Parvez et al (2005) found that the strains Bifi dobacterium and Bifi dobacterium bifi dum NRRL 1976 was also able to remove cholesterol by bacterial assimilation and precipitation. Studies of Dong et al (2012) has also demonstrated that bsh positive Lactobacillus plantarum BBE7 was capable of removing cholesterol in vitro.
Human studies
The hypocholesterolemic effects of probiotic strain, first discovered by Mann and Spoerry in 1974, showed that the Maasai tribes in Africa who consumed large amount of milk fermented by Lactobacillus sps resulted in the reduction of serum cholesterol levels (Mann & Spoerry, 1974). In 1979 Hepner reported that the consumption of either pasteurized or non-pasteurized milk showed an effective reduction of serum cholesterol levels which was higher than that expressed by those who consumed 2% butterfat milk after 1 week (Hepner et al.,1979). Moreover, the studies of Sarkar in 2003 revealed that the cholesterol reducing abilities of six strains of L. acidophilus was either due to the assimilation of cholesterol or attachment of cholesterol to the surface of L. acidophilus cells. Since this bacterium is a natural inhabitant of intestine with bile salt hydrolase activity, they can be utilized for the production of acidophilus milk which can bring out hypocholesterolemic effect. As part of the study he has also proposed that the efficacy of acidophilus milk to lower serum cholesterol level can be infl uenced by a number factors such as the milk type employed for the manufacture and also the age, sex, food habits and initial cholesterol concentration of the tested subjects (Sarkar, 2003).
Lactobacillus sps have been most widely used since there are several reports supporting the hypocholesterolemic effects of this strain. It was in 1989 Lin et al experimented with 23 human subjects, who received tablets containing 3×107 CFU L. acidophilus (ATCC 4962) and Lactobacillus delbrueckii ssp bulgaricus (ATCC 33409) daily for 16 weeks, which resulted in reduction of serum cholesterol level in an experimental group from 5.7 to 5.4 mmol/L, while the control group remained the same (Lin et al., 1989). Another study with the consumption of buffalo milk, fermented with specifi c strain of L. acidophilus, for a month resulted in the reduction of serum cholesterol by 12 to 20% (Khedkar et al.,1993). Even a small reduction in serum cholesterol of 1% can reduce risk of coronary heart disease by 2-3% (Gilliland, Nelson, & Maxwell, 1985; Manson et al., 1992). A number of placebo controlled studies have been carried out to study these effects. In one such study 30 volunteers consumed yoghurt enriched with specifi c strain L. acidophilus and it resulted in lowering of cholesterol levels in serum by 0.23 mmol/L (Schaafsma et al, 1998).
Another study with L. Plantarum 229 from the food product Pro-Viva has revealed that the cholesterol levels were affected in humans with moderately elevated serum cholesterol. For this study Bukowska et al., 2001 utilized a randomized-placebo design where 30 healthy men who consumed 200 mL/day of Pro-viva for 6 weeks showed a significant decrease in total cholesterol, LDL cholesterol and fi brinogen levels(Bukowska et al.,1998).
The fi ndings of Harrison and Peat, 1975 stated that a decrease in serum cholesterol level was visible in bottle- fed babies whereas the count of L. acidophilus in their stool was increased (Harrison & Peat, 1975). Similarly the consumption of yogurt has also resulted in the reduction of serum cholesterol levels in humans (Hepner et al., 1979; Mann, 1977).
Studies with Bifi dobacterium sp have also shown potential hypocholesterolemia effects. Rasic et al in 1992 had found that consumption of B. bifidum can lead to the assimilation of cholesterol by in vitro experiments (Rašic´ et al., 1992). It was also able to reduce the serum cholesterol concentration in human subjects with hypercholesterolemia. In 2003 Xiao et al proposed that milk fermented with B. longum BL1 resulted in the reduction of serum total cholesterol, LDL cholesterol and triglycerides in hypercholesterolemia patients as well as in rats (Xiao et al., 2003).
Animal studies
Animal models such as rats, mice, hamsters, guinea pigs and pigs have been widely used due to their similarities with respect to digestive anatomy and physiology, nutritional requirements and various other metabolic processes including cholesterol and bile acid metabolism, distribution of plasma lipoprotein and regulation of hepatic cholesterol enzymes. Several studies have been conducted to compare the effect of milk and milk products on cholesterol concentrations in animal models. Gilliland et al in 1975 showed that L. acidophilus RP32, capable of assimilating cholesterol in vitro, was able to inhibit the increase of serum cholesterol levels of pigs fed on a high-cholesterol diet (Gilliland, Speck, & Morgan, 1975). In addition, the same author in 1985 had also reported the cholesterol lowering capability of L. casei P47 in pigs (Gilliland et al., 1985). Another study conducted by Mahrous et al., 2011 reported that consumption of yogurt, fermented by L. acidophilus, by mice signifi cantly decreased the cholesterol content in the serum and increased bile acid content in the feces (Mahrous, Shaalan, & Ibrahim, 2011). Chiu et al., 2006 had showed that intake of probiotic fermented foods results in the reduction of total serum cholesterol levels in hamsters with high blood cholesterol levels (Chiu, Lu, Tseng, & Pan, 2006). Moreover, Sindhu and Khetarpaul in 2003 had studied the effect of probiotic fermented foods in 20 young Swiss mice, where there was reduction in the total serum cholesterol (Sindhu & Khetarpaul, 2003).
Studies with L. plantarum PH04 from infant feces showed a significant reduction in the serum cholesterol level and triglycericides when compared with the control (Nguyen, Kang, & Lee, 2007). The effi cacy of buffalo milk-yogurts containing B. longum Bb-46 was determined by administering it to 48 hypercholesterolemic male albino rats for 35 days and signifi cant reduction of total cholesterol, LDL cholesterol and triglycerides were obtained when compared to the control (El-Gawad et al., 2005). L. acidophilus fermented rice bran showed signifi cant improvement of lipid profile in hypercholesterolemic male Fischer rats (Fukushima et al., 1999). In a study by Lee 2007, on the anti-obesity activity of Trans-10, cis-12 conjugated linoleic acid produced by L. plantarum PL62, an observable reduction was obtained in the weight of epididymal, inguinal, mesenteric and perirenal white adipose tissues in mice (Lee et al., 2007). Similarly the intake of L. gasseri BNR17 also exhibited a reduction in weight, hip and waist circumference without any change in behavior or diet (Jung et al., 2013).
Until now, several studies have been conducted to evaluate the effects of consumption of probiotics or fermented products and they have given variable data (Taylor and Williams, 1998). In most of the cases cholesterol lowering effect was observed only during the intake of very high doses of the product whereas the normal consumption of probiotics failed to deliver such effects. Such contradictory results obtained may be due to the design of the experiment, lack of statistical power, inadequate sample sizes, improper nutrient intake and expenditure of energy during the experiments and variation in the baseline levels of blood lipids (Pereira & Gibson, 2002).
BSH a desirable trait in probiotics
In 2002 World Health Organization (WHO) has recommended BSH activity as one of the main characteristics for considering the strains Lactobacillus and Bifi dobacteria as probiotics, along with their ability to resist the harsh environment of the gut and to colonize in gastrointestinal epithelia (WHO, 2002). Overall, several studies have strongly supported the hypothesis that BSH positive strains are capable of detoxifying bile salts there by increasing the intestinal survival. Hence BSH activity can be considered as a desirable factor for the probiotics. The BSH activity may result in the accumulation of large amounts of deconjugated bile salts and hence cause undesirable effects in the human host which may arise concerns over the safety administration of BSH positive strains. However, the probiotic strains Lactobacillius and Bifi dobacterium are not capable of dehydroxylating deconjugated bile salts (Ahn et al., 2003; Gilliland & Speck, 1977; Takahashi & Morotomi, 1994) and hence majority of the breakdown products may be precipitated or excreted through feces which may vary from person to person depending upon the colonic pH and intestinal transit time (Thomas et al., 2000; Thomas et al., 2001).
Studies by two other groups have proposed that the intestinal bacteria, except certain strains of Clostridium and Eubacterium, the only strains that possess dehydroxylating activity, (Dawson et al., 1996; Doerner et al, 1997) inhibit further modifi cation of deconjugated products. First, Kurdi et al in 2003 proposed that BSH positive probiotic strains Lactobacillus and Bifi dobacterium are able to accumulate cholic acid, the main free bile acid produced in the intestine by BSH activity, as long as the bacteria were energized (Kurdi et al., 2003). Second, studies by Jones et al in 2004 revealed that microencapsulated BSH positive L. plantarum can hydrolyze bile salts in vitro and the deconjugated products were trapped within the membrane which makes these products less bioavailable. In addition to this the encapsulation of strain would protect them from the harsh gut environment and persistence of the strain (Jones et al., 2004).
Present scenario
Various studies have been carried out with BSH positive probiotics to illustrate the cholesterol lowering capacity with the same concept based on the interruption of enterohepatic circulation. So far the hypocholesterolemic effects of probiotics have been confirmed in both humans (Anderson & Gilliland, 1999) as well as animals(Gilliland et al., 1975; Mahrous et al., 2011; Sridevi et al., 2009). It was observed and reported that Bile salt hydrolase enzyme was associated with only microbiota in gastrointestinal tract and autochthous intestinal bacteria such as Listeria monocytogenes (Dussurget et al., 2002) and Xanthomonas maltophila (Dean et al., 2002).
Future perspectives
Recently this enzyme is reported to be present in thermophilic bacteria, Brevibacillus borstelensis isolated from hot water springs, near Konkan, Maharashtra, India (Sridevi & Prabhune, 2009). Also, a potent producer of BSH, Staphylococcus saprophyticus ZABR2, was isolated from the soil samples collected from the dumping sites of fi sh waste (Rajan & Fathimathu Zuhara, 2018). These fi ndings reveal that microorganisms from other sources may also possess the highly significant enzyme BSH and hence it needs to be explored more for the isolation of the potent producers.
References
Ahn, Y. T., Kim, G. B., Lim, K. S., Baek, Y. J., & Kim, H. U. (2003). Deconjugation of bile salts by Lactobacillus acidophilus isolates. International Dairy Journal, 13(4), 303–311.
Alrefai, W. A., and R. K. Gill. 2007. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharmaceutical Research 24: 1803-1823.
Anderson, J. W., & Gilliland, S. E. (1999). Effect of Fermented Milk (Yogurt) Containing Lactobacillus Acidophilus L1 on Serum Cholesterol in Hypercholesterolemic Humans. Journal of the American College of Nutrition, 18(1), 43–50.
Appleby, RN and Walters, JR (2014). The role of bile acids in functional GI disorders. Neurogastroenterology & Motility 26: 1057–1069
Begley, M., Sleator, R. D., Gahan, C. G. M., & Hill, C. (2005). Contribution of Three Bile-Associated Loci, bsh, pva, and btlB,to Gastrointestinal Persistence and Bile Tolerance of Listeria monocytogenes. Infection and Immunity, 73(2), 894–904.
Beher, W. T., Lin, G. J., & Bajraszewski, F. (1984). The determination and excretion of individual human fecal bile acids. Steroids, 44(6), 539–547.
Bliznakov, E. G. (2002). Lipid-lowering drugs (statins), cholesterol, and coenzyme Q10. The Baycol case–a modern Pandora’s box. Biomedicine & Pharmacotherapy= Biomedecine & Pharmacotherapie, 56(1), 56–59.
Boggs, J. M. (1987). Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. BBA – Reviews on Biomembranes, 906(3), 353–404.
Bortolini, O., Medici, A., & Poli, S. (1997). Biotransformations on steroid nucleus of bile acids. Steroids, 62(8–9), 564–577.
Bukowska, H., Pieczul-Mroz, J., Jastrzebska, M., Chelstowski, K., & Naruszewicz, M. (1998). Decrease in fi brinogen and LDLcholesterol
levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol [1]. Atherosclerosis, 137(2), 437–438.
Camilleri, M and Gores, GJ (2015). Therapeutic targeting of bile acids. American Journal of Physiology-Gastrointestinal and Liver Physiology 309:G209–G215.
Chand, D., Avinash, V. S., Yadav, Y., Pundle, A. V., Suresh, C. G., & Ramasamy, S. (2017). Molecular features of bile salt hydrolases and relevance in human health. Biochimica et Biophysica Acta (BBA)-General Subjects, 1861(1), 2981-2991.
Chiu, C. H., Lu, T. Y., Tseng, Y. Y., & Pan, T. M. (2006). The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Applied Microbiology and Biotechnology, 71(2), 238–245. https://doi.org/10.1007/s00253-005-0145-0
Dambekodi, P. C., & Gilliland, S. E. (1998). Incorporation of Cholesterol into the Cellular Membrane of Bifi dobacterium longum. Journal of Dairy Science, 81(7), 1818–1824.
Dawson, J. A., Mallonee, D. H., Bjorkhem, I., & B, P. (1996). 4 bile acid 12708 in Escherichia coli k &. Journal Of Lipid Research, 37, 1258–1267.
De Boever, P., & Verstraete, W. (1999). Bile salt deconjugation by Lactobacillus plantarum 80 and its implication for bacterial toxicity. Journal of Applied Microbiology, 87(3), 345–352.
De Smet, I., De Boever, P., & Verstraete, W. (1998). Cholesterol lowering in pigs through enhanced bacterial bile salt hydrolase activity. British Journal of Nutrition, 79, 185–194.
De Smet, I., Van Hoorde, L., Vande Woestyne, M., Christiaens, H., & Verstraete, W. (1995). Signifi cance of bile salt hydrolytic activities of Lactobacilli. Journal of Applied Bacteriology, 79(3), 292–301.
Dean, M., Cervellati, C., Casanova, E., Squerzanti, M., Lanzara,V., Medici, A., … Bergamini, C. M. (2002). Characterization of cholylglycine hydrolase from a bile-adapted strain of Xanthomonas maltophilia and its application for quantitative hydrolysis of conjugated bile salts. Applied and Environmental Microbiology, 68(6), 3126–3128.
Doerner, K. C., Takamine, F., LaVoie, C. P., Mallonee, D. H., & Hylemon, P. B. (1997). Assessment of fecal bacteria with bile acid 7-dehydroxylating activity for the presence of bai-like genes. Applied and Environmental Microbiology, 63(3), 1185–1188.
Dong, Z., Zhang, J., Lee, B., Li, H., Du, G., & Chen, J. (2012). A bile salt hydrolase gene of Lactobacillus plantarum BBE7 with high cholesterol-removing activity. European Food Research and Technology, 235(3), 419-427.
Dunn‐Emke, S., Weidner, G., & Ornish, D. (2001). Benefi ts of a Low‐Fat Plant‐Based Diet. Obesity Research, 9(11), 731.
Dussurget, O., Cabanes, D., Dehoux, P., Lecuit, M., Buchrieser, C., Glaser, P., & Cossart, P. (2002). Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Molecular Microbiology, 45(4), 1095–1106.
El-Gawad, I. A. A., El-Sayed, E. M., Hafez, S. A., El-Zeini, H. M., & Saleh, F. A. (2005). The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing bifi dobacteria in rats fed on a cholesterol-enriched diet. International Dairy Journal, 15(1), 37–44.
Franklund, C. V, de Prada, P., & Hylemon, P. B. (1990). Purifi – cation and characterization of a microbial, NADP-dependent bile acid 7 alpha-hydroxysteroid dehydrogenase. Journal of Biological Chemistry, 265(17), 9842–9849.
Fukushima, M., Yamada, A., Endo, T., & Nakano, M. (1999). Effects of a mixture of organisms, Lactobacillus acidophilus or Streptococcus faecalis on 6-desaturase activity in the livers of rats fed a fat-and cholesterol-enriched diet. Nutrition, 15(5), 373–378.
Gilliland, S. E., Nelson, C. R., & Maxwell, C. (1985). Assimilation of cholesterol by Lactobacillus acidophilus. Applied and Environmental Microbiology, 49(2), 377–381.
Gilliland, S. E., & Speck, M. J. (1977). Deconjugation of bile acids by human intestinal bacteria. Applied and Environmental Microbiolog, 33, 15–18.
Gilliland, S. E., Speck, M. L., & Morgan, C. G. (1975). Detection of Lactobacillus acidophilus in feces of humans, pigs, and chickens. Applied Microbiology, 30(4), 541–5.
Grill, J. P., Cayuela, C., Antoine, J. M., & Schneider, F. (2000). Isolation and characterization of a Lactobacillus amylovorus mutant depleted in conjugated bile salt hydrolase activity: Relation between activity and bile salt resistance. Journal of Applied Microbiology, 89(4), 553–563.
Harrison, V. C., & Peat, G. (1975). Serum cholesterol and bowel fl ora in the newborn. The American Journal of Clinical Nutrition, 28(12), 1351–1355.
Hepner, G., Fried, R., St Jeor, S., Fusetti, L., & Morin, R. (1979). Hypocholesterolemic effect of yogurt and milk. The American Journal of Clinical Nutrition, 32(1), 19–24. Retrieved from Huijghebaert, S. M., Mertens, J. a, & Eyssen, H. J. (1982). Isolation of a bile salt sulfatase-producing Clostridium strain from rat intestinal microfl ora. Applied and Environmental Microbiology, 43(1), 185–192.
Jones, M. L., Chen, H., Ouyang, W., Metz, T., & Prakash, S. (2004). Microencapsulated genetically engineered Lactobacillus plantarum 80 (pCBH1) for bile acid deconjugation and its implication in lowering cholesterol. Journal of Biomedicine and Biotechnology, 2004(1), 61–69.
Jung, S. P., Lee, K. M., Kang, J. H., Yun, S. Il, Park, H. O., Moon, Y., & Kim, J. Y. (2013). Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: A randomized, double-blind clinical trial. Korean Journal of Family Medicine, 34(2), 80–89.
Khedkar, C. D., Garge, R. D., Mantri, J. M., Kulkarni, S. A., & Khedkar, G. D. (1993). Effect of feeding acidophilus milk on serum cholesterol in human volunteers. J Dairy Foods Home Sci, 12, 33–38.
Kim, G and Lee, BH (2005) Biochemical and molecular insights into bile salt hydrolase in the gastrointestinal microflora-a review. Asian Australasian Journal of Animal Sciences 18: 1505
Kumar, R. S., Brannigan, J. A., Prabhune, A. A., Pundle, A. V.,Dodson, G. G., Dodson, E. J., & Suresh, C. G. (2006). Structural and functional analysis of a conjugated bile salt hydrolase from Bifi dobacterium longum reveals an evolutionary relationship with penicillin V acylase. Journal of Biological Chemistry. M. Kumar, R. Nagpal, R. Kumar (2012). Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases, Experimental Diabetes Research, vol. 2012, Article ID 902917, 14 pages, 2012.
Kurdi, P., Tanaka, H., van Veen, H. W., Asano, K., Tomita, F., & Yokota, A. (2003). Cholic acid accumulation and its diminution by short-chain fatty acids in bifi dobacteria. Microbiology, 149(8).
Lack, L., & Weiner, I. M. (1966). Intestinal bile salt transport: structure-activity relationships and other properties. American Journal of Physiology-Legacy Content, 210(5), 1142–1152.
Lee, K., Paek, K., Lee, H. Y., Park, J. H., & Lee, Y. (2007). Antiobesity effect of trans‐10, cis‐12‐conjugated linoleic acid‐producing Lactobacillus plantarum PL62 on diet‐induced obese mice. Journal of Applied Microbiology, 103(4), 1140–1146.
Liong, M.T. and Shah, N.P. (2005). Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. International Dairy Journal 15, 391-398
Lin, S. Y., Ayres, J. W., Winkler, W., & Sandine, W. E. (1989). Lactobacillus Effects on Cholesterol: In Vitro and In Vivo Results1. Journal of Dairy Science, 72(11), 2885–2899.
Macdonald, I. A., Bokkenheuser, V. D., Winter, J., McLernon, A. M., & Mosbach, E. H. (1983). Degradation of steroids in the human gut. Journal of Lipid Research, 24(6), 675–700.
Mahrous, H., Shaalan, U. F., & Ibrahim, A. M. (2011b). The role of some probiotic lactic acid bacteria in the reduction of cho lesterol on mice. International Research Journal of Microbiology,2(7), 242–248.
Mann, G. V., & Spoerry, A. (1974). Studies of a surfactant and cholesteremia in the Maasai. American Journal of Clinical Nutrition, 27(May), 464–469.
Mann, G. V. (1977). A factor in yogurt which lowers cholesteremia in man. Atherosclerosis, 26(3), 335–340.
Manson, J. E., Tosteson, H., Paul M, R., Satterfi eld, S., Herbert, P., O’Conner, G. T. &Hennekens, C. H. (1992). The Primary prevention
of Myocardial infraction. The New England Journal of Medicine, 326, 1406–1416.
Moser, S. A., & Savage, D. C. (2001). Bile Salt Hydrolase Activity and Resistance to Toxicity of Conjugated Bile Salts are Unrelated Properties in Lactobacilli. Applied and Environmental Microbiology, 67(8), 3476–3480. https://doi.org/10.1128/AEM.67.8.3476-3480.2001
Nicolaou, M., Andress, E. J., Zolnerciks, J. K., Dixon, P. H., Williamson, C., & Linton, K. J. (2012). Canalicular ABC transporters and liver disease. The Journal of pathology, 226(2),300- 315.
Nguyen, T. D. T., Kang, J. H., & Lee, M. S. (2007). Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. International Journal of Food Microbiology, 113(3), 358–361.
Owen, R. W., Thompson, M. H., & Hill, M. J. (1984). Analysis of metabolic profi les of steroids in faeces of healthy subjects undergoing chenodeoxycholic acid treatment by liquid-gel chromatography and gas-liquid chromatography-mass spectrometry. Journal of Steroid Biochemistry, 21(5), 593–600.
Parvez, S., Malik, K.A., Kang, S.AH. & Kim, H.K. (2005). Probiotics and their fermented food products are beneficial for health. Journal of Applied Microbiology ISSN 1364-5072.
Patel, A. K., Singhania, R. R., Pandey, A., & Chincholkar, S. B. (2010). Probiotic bile salt hydrolase: Current developments and perspectives. Applied Biochemistry and Biotechnology. Pereira, D. I. A., & Gibson, G. R. (2002). Cholesterol Assimilation by Lactic Acid Bacteria and Bifi dobacteria Isolated from the Human Gut. Applied and Environmental Microbiology, 68(9), 4689–4693.
Peschel, A. (2002). How do bacteria resist human antimicrobial peptides? Trends in Microbiology. Rajan, A. B., & Fathimathu Zuhara, K. (2018). Isolation screening and identification of bile salt hydrolase producing bacteria from waste samples, 11(1), 104–108.
Raši´c, J. L., Vujiˇci´c, I. F., Škrinjar, M., & Vuli´c, M. (1992). Assimilation of cholesterol by some cultures of lactic acid bacteria
and bifi dobacteria. Biotechnology Letters, 14(1), 39–44.
Ridlon, JM, Kang, D-J and Hylemon, PB (2006). Bile salt biotransformations by human intestinal bacteria. Journal of Lipid Research 47: 241–259.
Russell, DW (2009). Fifty years of advances in bile acid synthesis and metabolism. Journal of Lipid Research 50: S120–S125 Sarkar, S. (2003). Potential of acidophilus milk to lower cholesterol. Nutrition & Food Science, 33(6), 273–277
Schaafsma, G., Meuling, W. J., van Dokkum, W., & Bouley, C. (1998). Effects of a milk product, fermented by Lactobacillus acidophilus and with fructo-oligosaccharides added, on blood lipids in male volunteers. European Journal of Clinical Nutrition, 52(6), 436–40.
Schaap, FG, Trauner, M and Jansen, PL (2014). Bile acid receptors as targets for drug development. Nature Reviews Gastroenterology & Hepatology 11: 55–67
Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff’s Diseases of the Liver. Philadelphia: Lippincott, Williams & Wilkins; 2002. pp. 135–165.
Sindhu, S. C., & Khetarpaul, N. (2003). Effect of feeding probiotic fermented indigenous food mixture on serum cholesterol levels in mice. Nutrition Research, 23(8), 1071–1080.
Smet, I. De, Hoorde, L. Van, Saeyer, N. De, Woestyne, M. Vande, & Verstraete, W. (1994). in vitro study of bile salt hydrolase (BSH) activity of BSH isogenic Lactobacillus plantarum 80 strains and estimation of cholesterol lowering through enhanced BSH activity. Microbial Ecology in Health and Disease, 7(6), 315–329.
Sridevi, N., & Prabhune, A. A. (2009). Brevibacillus sp: A novel thermophilic source for the production of bile salt hydrolase. Applied Biochemistry and Biotechnology, 157(2), 254–262.
Sridevi, N., Pradnya, V., & Prabhune, A. (2009). Hypocholesteremic effect of bile salt hydrolase from Lactobacillus buchneri ATCC 4005. Food Research International, (42), 516–520.
Suckling, K. E., Benson, G. M., Bond, B., Gee, A., Glen, A., Haynes, C., & Jackson, B. (1991). Cholesterol lowering and bile acid excretion in the hamster with cholestyramine treatment. Atherosclerosis, 89(2–3), 183–190.
Takahashi, T., & Morotomi, M. (1994). Absence of cholic acid 7 alpha-dehydroxylase activity in the strains of Lactobacillus and Bifi dobacterium. J Dairy Sci, 77(11), 3275–3286.
Taranto, M. P., Fernandez Murga, M. L., Lorca, G., & De Valdez, G. F. (2003). Bile salts and cholesterol induce changes in the lipid cell membrane of Lactobacillus reuteri. Journal of Applied Microbiology, 95(1), 86–91.
Taranto, M. P., Sesma, F., Pesce De Ruiz Holgado, A., & De Valdez, G. F. (1997). Bile salts hydrolase plays a key role on cholesterol removal by Lactobacillus reuteri. Biotechnology Letters, 19(9), 845–847.
Thomas, L. A., Veysey, M. J., Bathgate, T., King, A., French, G., Smeeton, N. C. & Dowling, R. H. (2000). Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology, 119(3) 806–815.
Thomas, L. A., Veysey, M. J., Murphy, G. M., & Dowling, R. H. (2001). Infl uence of pH on the phase distribution of nascent deoxycholic acid in fresh human cecal aspirates. American Journal of Physiology-Gastrointestinal and Liver Physiology, 281, G371–G374.
Tsai, C.-C., Lin, P.-P., Hsieh, Y.-M., Zhang, Z., Wu, H.-C., & Huang, C.-C. (2014). Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. The Scientifi c World Journal, 2014.
Van Eldere, J., Celis, P., De Pauw, G., Lesaffre, E., & Eyssen, H. (1996). Tauroconjugation of cholic acid stimulates 7 alphadehydroxylation
by fecal bacteria. Applied and Environmental Microbiology, 62(2), 656–61.
Xiao, J. Z., Kondo, S., Takahashi, N., Miyaji, K., Oshida, K., Hiramatsu, A &Hosono, A. (2003). Effects of Milk Products Fermented by Bifi dobacterium longum on Blood Lipids in Rats and Healthy Adult Male Volunteers. Journal of Dairy Science, 86(7), 2452–2461.


