Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
Corresponding author email: rmakki@kau.edu.sa
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 23/09/2020
Arabidopsis thaliana is the most popular plants in scientific fields whereas it is used as molecular genetics plant model because it can be manipulated easily and has a relatively small genome of approximately 135 mega base pairs. This plant possessed many known mechanisms of DNA repair, producing an unusual pattern of inheritance. Because of the unique characteristics of the plant, A. thaliana has considered as a model plants for many studies. It is also extensively studied as a model for flower development. Importantly, the integral sequence of the Arabidopsis genome allowed the swift discovery of the molecular basis of recognizing mutant plant, which made it convenient and a powerful to recognize genes that are involved in many aspects of the plant life cycle. This review deals with the unique importance and characteristics that make A. thaliana a better choice for scientists and researchers interested in the study of living things, particularly those with interest in molecular plants.
Arabidopsis thaliana, Gene Expression, Pollen, Anther, Mutants.
Makki R. M. Arabidopsis thaliana a Medicinal Plant as a Genetic Model System in Crop Science And Scientific Fields. Biosc.Biotech.Res.Comm. 2020;13(3).
Makki R. M. Arabidopsis thaliana a Medicinal Plant as a Genetic Model System in Crop Science And Scientific Fields. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/30fTeN5
Copyright © Makki This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Although the Arabidopsis Thaliana is currently found in different parts of the world, the plant is thought to have existed in Europe and Asia at first. As biologists and other scientists developed an interest in Arabidopsis Thaliana plant, Arabidopsis thaliana went through several stages under which it was given different and unique names. At some point, the plant was identified as Pilosella siliquata by a biologist called Johannes before the name changed to Conringia thaliana, Pilosella thaliana, and Sisymbrium thalianum. Much later, the name was changed to Arabidopsis thaliana a code name that inferred the plants usefulness in the study of genes (Provart et al., 2016, Yang et al., 2017, James 2018, Neetu 2019).
After decades of research, studies revealed that Arabidopsis thaliana was a perfect model organism that could be used in the study of genes. As a model organism, Arabidopsis thaliana has crucial characteristics. It takes a short duration time to grow, tolerate high temperatures, need no fertilization and it can achieve the same growth milestones when planted indoors. Moreover, it has small seeds and genome of 132 Mbp and grows perfectly under tough conditions such as under turbulent wind exposures.
The Genome Sequence of Arabidopsis Thaliana:The Arabidopsis thaliana genome is often used as a model for other plants following a series of studies and apparently, Arabidopsis thaliana was reliable for genome sequencing. Out of the required 125 megabases that define genome regions, the Arabidopsis thalianahas more than 115.4 megabases (Quilichini et al., 2015). Nonetheless, the plant has numerous types of proteins with diversity which making up the plant as the best choice for hybridization and crop development, ( James 2018, Dennis 2018, Neetu 2019).
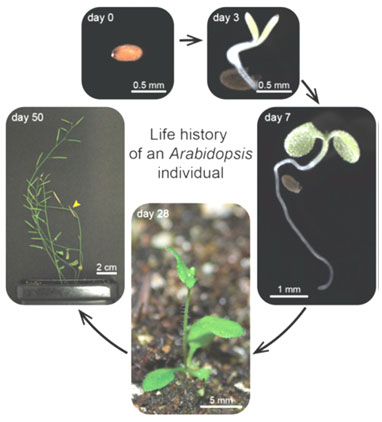
Figure 1: The life history of the Arabidopsis thaliana from seed to maturity after 50 days (Boyes et al., 2001).
Gene Expression of Arabidopsis thaliana:Arabidopsis thaliana enables researchers to review the various stages of life, which is paramount for the analysis of gene expression. This is in view that the regulatory regions of the plant genes are significantly more stable compared to those of animals (Yamada et al. 2003, Birnbaum et al. 2003, Becker et al., 2005). Nonetheless, Arabidopsis thaliana genes are similar to any other plant when it comes to pollens and flowers among other aspects, as seen in Figure 2.
Figure 2: Arabidopsis thaliana genes (Yamada, et al. 2003).
Effect of 5’UTR Introns on Gene Expression in Arabidopsis thaliana: Intron refers to the nucleotide sequence found in genes, which is removed by RNA splicing at the maturation of the final RNA product, was first discovered in 1977 (Achard et al., 2004). The two researchers and scientists came up with the name introns rather than exons that code for gene products. According to subsequent studies, particularly plant expression studies in chimeric RNA, the intron sequence can reinforce the level of protein expression, a phenomenon called Intron-Medicated Enhancement (Callis et al., 1987). Thus, introns within the 5’UTR exhibit specific features that make them different from the introns found within the coding sequence and the 3’UTR. Pertaining to the EF1α-A3 gene, the presence of a long intron in the 5’UTR is sufficient to enhance gene expression in plants.
The Arabidopsis thaliana Chloroplast Proteome and Protein Functions: In photosynthesis, the process through which plants manufacture food, chloroplast proteome organelles pick up energy from sunlight, convert, and store it in energy storage molecules while releasing oxygen from the plant and algal cells. Chloroplasts are of cyanobacterial origin although their autonomy during evolution and development (Anthony and Frank, 2005). They usually transfer part of their genes to the nucleus. Apparently, part of the genetic information found on proteome constitutes the chloroplast and the metabolic functions that define the protein complement of Arabidopsis thaliana plastids.
Linking Genes with Ecological Strategies in Arabidopsis thaliana: From the oncological perspective, Arabidopsis thaliana exhibits a collection of numerous diverse genotypes defined by a complex population structure. Its phenotypic variation makes it a dependable plant for the study of genes. Importantly, it is applicable in studies because it has high-adaptation capabilities when subjected to varying environmental conditions (Callis et al., 1987). Owing to the phenotypic change capacity, Arabidopsis thaliana copes with environmental differences as may be necessary when conducting studies (Anthony and Frank, 2005). Co-variations between the plant traits are a replication of evolution, trait syndromes, and are a revelation of biodiversity.
Protein Oxidation in Arabidopsis thaliana: In Arabidopsis thaliana, protein oxidation is an irreversible procedure that involves the modification of side chains in a few native amino acids such as histidine, cysteine, and lysine. Often, the oxidation process results increase in the levels of carbonyl and a dysfunctional protein during the first few days of the Arabidopsis thaliana life cycle (Provart et al., 2016). Particularly, experiences a significant reduction in protein carbonyls prior to bolting and flower growth over the first 20 days after germination (Becker et al., 2003). However, the model of the plant is best utilized if kept under optimal growth conditions during the first 20 days. After fertilization, and as the seed matures, the plant’s somatic tissues usually become more senescent. Apparently, leaf senescence defines the last step of leave development considered as a vital step towards the death of the plant’s life cycle. With consideration of the carbonylation of Arabidopsis thaliana during the various stages of the plant life cycle, it is clear that the plant is a unique life form to consider and use in scientific studies.
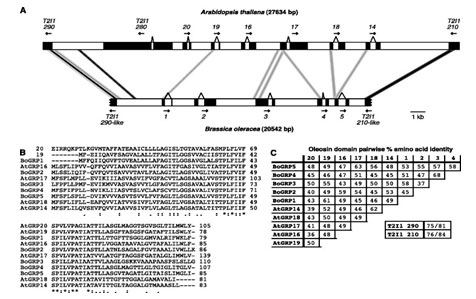
Gene Families Defining Arabidopsis thaliana Pollen Coat Proteome: Arabidopsis thaliana pollens are comprised of protein intermediate species and composition needed for effective pollination. The pollens include over 10 kilo Daltons of proteins and genomic clusters that correspond with genes (Birnbaum et al., 2003). With lipids, the proteins are utilized by flowering plants much as they are coated with sophisticated extra-cellular pollens. These interact selectively with receptive female stigma cells. Apparently, the coating makes it possible for plants with dry stigmas to connect since they have a functionally similar lipid-rich exudate on the surface of stigmas. Previous studies have revealed significant evidence of synteny between Arabidopsis thaliana and Brassica oleracea clusters as observed from the comparison of oleosin clusters and flanking DNA. These differences are observed in black boxes, exons, arrows, and the direction of transcription, regions of BLASTN value of E, and the black connecting bars (Birnbaum et al., 2003). Additional aspects that show variations include the grey connecting bars and regions of Value E at 10220 as well as the oleosin domains.
Figure 3: Intraspecific Genetic Variations, Fitness Cost and Benefit of RPW8, A Disease Resistance Locus in Arabidopsis thaliana (Orgil et al., 2007).
Arabidopsis thaliana features a unique RPW8-encoding class of genes with its origin in ancient land plants. Over the years, the genes evolved through processes such as domain fission, fusion, and duplication to confer the resistance needed counter pathogens such as powdery mildew. Essentially, two homologous genes, particularly, RPW8-1 and RPDW8-2 best illustrated in the following images, define this locus.
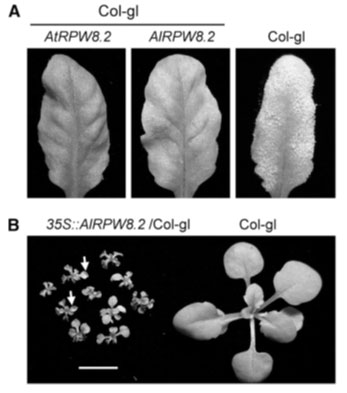
Figure 4: AlRPW8-1 and AlRPW8-2 (Hudson and Kaplan, 1985, Xiu-Tang et al., 2012).
In the experiment, a 2.3 kb genomic fragment with AIRW8-2 and its promoter were introduced in Col-gl background with a transgenic plant inoculated with cichor-acearum USCS1. Additionally, disease phenotypes were recorded and typically infected leaves photographed after 10 days of exposure in post-inoculation. Notably, the researchers expressed the AIRPW8-2 gene under the 35S promoter and Co-gl background. It emerged that plants with up to 10% transgenic lines exhibited SHL whereas the 4-week-old T3 plants showed a single line (Achard et al., 2004). The most severe SHL was seen in the wild type. Arrows were interpreted as signs of dead or dying leaves. Speculatively, the mutation would appear at frame shift and truncation of up to 34 amino acids at the C-termini or reduced proteins (Hudson and Kaplan, 1985).
Regulation of the developmental processes of Arabidopsis thaliana: Plant growth and development is depended on a wide range of genetic factors that trigger the production of the hormones needed for. Arabidopsis thaliana stands out as an important plant for biological studies because the plant exhibits variable hormones such as the gibberellin (GA) that coordinates the development process of different plant parts (Yang et al., 2017). Ordinarily, plants grow under abiotic stress that they must cope with for survival. To cope with the stress, plants require morphological adaptation capabilities that are primarily facilitated by hormones (Smalle et al., 1997). For instance, plant hormones regulate and mediate the morphological response of roots under adverse soil conditions. Particularly, plants rely on jasmonic acid for mediation of stress response among other developmental processes, such as metabolism and biosynthesis (Stefanie et al., 2015). In Arabidopsis thaliana, the auxin hormone regulates the development of seeds, a process that involves cotyledon formation, pollen grains formation, and hypocotyl cell elongation among other key processes. Hence, Arabidopsis thaliana is considered an important model to use in biological studies.
Pertaining to the maintenance of a unique R gene locus, there are two key observations to note. Firstly, genetic variation is noted in high levels of RPW8, a sign of zero selective sweeps for the locus. Secondly, benefits and costs are observed in the RPW8 expression although depending on the fitness of individual plants, which is in turn influenced by exposures to pathogens as well as the development of anthers (Baesso et al., 2018). This is in view that the Arabidopsis LFR Gene is a crucial requirement in the formation process of Anther Cell Layers. As usual with any other process, genes play a key role in anther development; the molecular technique behind the transcriptional regulation of associated genes is indefinite (Zoe et al., 2011). The leaf and flower relationship are deducible, which is a reaffirmation that all genes are a crucial component of the genetic network that modulates all the plant processes.
In Arabidopsis thaliana, the receptor-like protein kinase-2 (RPK-2) plays an important role in the control of anther development. Ordinarily, the receptor-like kinases (RLK) falls in a large gene family within the Arabidopsis genome and plays a central role in plant growth and development. It is an important determinant of plant response to hormonal changes and stress, which makes it a key regulator of anther development in Arabidopsis (Becker et al., 2003). Ostensibly, defects in anther dehiscence and pollen maturation trigger enhanced shoot growth and male sterility. Rpk-2 anthers, which are one of the primary insertional mutants, tend to develop into three cell layers around the male gametophyte (Cecchetti et al., 2008). The middle layer is not necessarily differentiated from inner parietal cells.
The pollen mother cells can often afford meiosis although subsequent differentiation might be inhibited by tapetum hypertrophy. The resultant pollen grains tend to exhibit aggregated morphologies. Besides, the presence of microspores and tetradson anthers, as might be noticed during microspore formation, are a sign of developmental homeostasis (Claudia et al., 2004). Often, anther locules crush without necessarily undergoing stomium breakage, a phenomenon largely presumed to be a consequence of lignification and inadequate thickening of the endothelium (Birnbaum et al. 2003). Based on results of microarray analysis, most genes encoding metabolic enzymes, such as those involved in metabolic processes on the cell walls and lignin biosynthesis, tend to downgrade throughout the anther development process. Therefore, RPK-2 controls the tapel cell fate by invoking subsequent tapetum degradation. Mutating RPK-2 impairs pollen maturation as well as the anther dehiscence, which is a result of disruptions on key metabolic processes.
GAMYB-like genes, particularly MYB33 and MYB65, as they are found in Arabidopsis are microRNA-regulated genes that facilitate anther development redundantly. Previous studies have not revealed any significant information about the gene encoding for R2R3 MYB domain proteins as found in Arabidopsis (Achard et al., 2004). However, closely related genes MYB33 and MYB65 found in Arabidopsis thaliana have been shown to have great sequence similarity with the GAMYB gene found in barley, (Haseneyer et al., 2008). In an earlier study where T-DNA insertional mutants were isolated, findings showed that a myb33 and myb65 double mutant was defective in anther development. Ostensibly, it emerged that tapetum undergoes hypertrophy within the cell stage of the pollens, which results in a pre-meiotic abortion of the pollen development process. However, sterility is conditional in that fertility increases under higher light conditions as well as when Arabidopsis thalianais placed in lower temperature conditions (Zoe et al., 2011). MYB33 and MYB65, therefore, are not necessarily essential for the development of anthers although the genes seem to facilitate the process.
In a manner consistent with functional redundancy, promoter–β-glucuronidase (GUS) fusions of MYB33 and MYB65 give identical expression patterns in flowers. The impact is noticeable in kin sepals, receptacle, and anther filaments (Claudia et al., 2004). It is not necessarily limited to anthers although it is traceable in shoot apices and root tips as well. In relatively young anthers, the expressions of MYB33 genes is consistent with the male-sterile phenotype and no staining as a result of shoot meristems. From a micro-RNA sequence, however, the fusion between MYB33 and GUS results in an expansive expression pattern and tissues similar to the promoter-GUS lines. The interpretation is that the micro-RNA target sequence is a restriction of MYB33 (Birnbaum et al. 2003). Ostensibly, Arabidopsis infused with MYB33 and mutated mico-RNA results in dramatic pleiotropic developmental defects. A restriction of MYB33 in shoot apices, therefore, is highly recommended for the proper development of Arabidopsis thaliana.
The Regulation of Anther Development in Arabidopsis: In Arabidopsis, anther development involves several steps, particularly, 14 stages that culminate in histogenesis. Neetu (2019) reports that anther development in Arabidopsis is characterized by a complex network of transcription factors that define the 14 stages. Importantly, molecular knowledge of the anther development process is crucial for a clear understanding of the stamen control, which entails the manipulation and study of male fertility. Exposition of the composition and structure of the filament, as well as the anther that produces or contains pollen grains, is tantamount when studying Arabidopsis thaliana as the biological model for the study of genes (Neetu, 2019). Essentially, all Arabidopsis anther genes can be combined into simple pair genes and co-expressed networks to construct a co-expression network. The result is a combination of 254Arabidopsis anther groups. Considering that the combination of co-expressed groups contains a high propensity of functionally related and co-existent genes, it emerges that Arabidopsis thaliana is a uniquely important model that could open up the field of biological research.
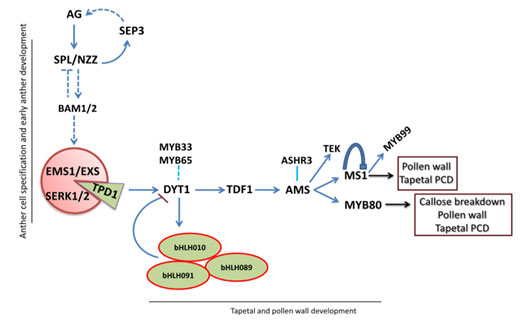
At the transcriptional level, regulation of gene expression helps to control a significant deal of cell physiology in order to manipulate and mediate the development of tissues. Apparently, the development depends on the differential gene expression in all the cells involved in the differentiation and specification of organs (Neetu, 2019). The process is controlled by transcriptional factors that act as regulatory switches. Importantly, the 14 stages that define the anther development stages are categorized into two key phases: microsporogenesis and microgametogenesis. Stages one through to seven make up the microsporogenesis phase and involve the differentiation as well as the meiotic division of microspore cells. The stages from eight to fourteen make up the microgametogenesis phase in which microspores are emitted from tetrads before the mitotic division of the same microspores, the process that results in the production of pollen grains. Degeneration, which is crucial for the maturation of pollen grains and disposal, occurs as the last stage (Neetu, 2019). The following diagram showed the entire process in a nutshell.
Figure 5: Regulatory network of transcription factors of anther development (Juanying et al 2016). the diagram, arrows represent positive regulation whereas the T bar represents negative regulation. Dashed lines represent the putative regulatory function.
In the early stages of anther development, AG acts as the primary trigger where NZZ/SPL acts in a positive loop relative to the AG. The NZZ/SPL up regulates both the BAM1 and BAM2 expression, in a negative loop expression. Notably, EXS/EMS1 forms a receptor complex that includes SERK1, SERK2, and TPD1 that binds to the receptor complex. In the process, tapetal development is activated by TDF expression that triggers DYT through the up regulation of AMS expression (Smalle et al., 1997). In return, AMS up regulates the expression of MS1 and MYB80 that contributes to the development of pollen walls.
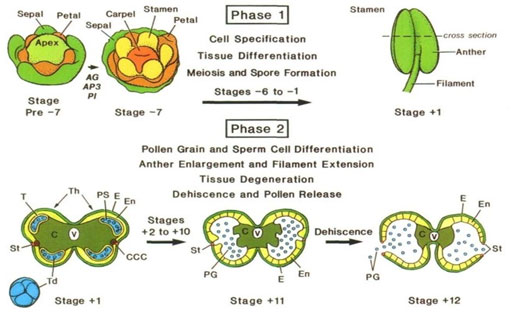
Basic Principles and Applications of Anther Development:The development of anthers occurs in stamen since anther constitutes the male reproductive part of flowers. Also called the sporophytic system, the stamen contains diploid cells that usually undergo meiosis to produce haploid male spores or microspores. These microspores usually divide and differentiate into multicellular gametophytes or pollen grains. As the male part of the flower, the stamen is morphologically distinct and unique component supported by the filament, which is a vascular tissue that serves as the nutrients and water channel (Becker et al., 2003). Broadly speaking, the anther development process occurs in two phases. The anther morphology is established in phase one where the cell and tissue differentiation occurs with the microspore mother cell undergoing meiosis. By the end of the first phase, the anther should contain almost all the specialized cells and tissues. Tetrads of microspores are usually evident in the pollen sacs.
The second phase is characterized by the differentiation of pollen grains. The anther enlarges and advances upwards within the flower through a process called filament extension. Eventually, pollen grains are released (Miransari and Donald, 2016). Through regulation of cellular processes within the flower, differentiation of anther cells occurs with the establishment of tissue patterns. The anther switches from the histo-specification programs of phase one to the cell degeneration and dehiscence program of the second phase (Cecchetti et al., 2008). The developmental events leading to anther formation and the release of pollen grains are exquisitely time (Robert et al., 1993).
Figure 6: An overview of anther development phases and stages (Cecchetti et al., 2008).
Similar to any other developmental process involving cells, anther development is equally subject to defects, particularly, defined as Arabidopsis thaliana male-sterile mutants. Within the stamen, primordia cell-specifications and differentiations appear early and mature faster than other cell types. They generate strange anther morphology from what is expected in ordinary flowering plants. Clearly, defects in anther development result in futile phenotypes and are largely associated with sterility mutant screens (Robert et al., 1993). posits that sterile mutations are a common occurrence in many of the known flowering plants. The mutants range from anther morphology to micro- sporogenesis, pollen dysfunctions, and under-developments.
Protein Phosphorylation for the Regulation of Anther Development in Arabidopsis: The development of stamen, which is the male reproduction part of the flower, is a sophisticated process that involves primordium initiation and early cell divisions. Other crucial process includes differentiation, generation of haploid microspores, and the formation filaments (Smalle et al., 1997). Ordinarily, a mature anther should have four lobes where each lobe contains microspore cells and somatic tissues. The tissues should also contain four sporophytic cell layers independently, epidermis, endothecium, and tapetum. In the first phase defined by the seven stages, archsporial cells undergo differentiation and division to form the building blocks if the stamen. In subsequent stages that define the second phase, anthers are formed and pushed upwards into their position. Additionally, sperm cells are formed as pollen grains mature up. Numerous regulatory factors, however, come into play throughout the two phases (Robert et al., 1993). These factors signify the significance of Arabidopsis thaliana as an important model of study in modern genetic and biomedical research.
Pollen Development and Arabidopsis: The development of heterotrophic pollen grains usually requires energy supplementation with carbon inputs. In the early stages, spores are immersed in a mixture of locular fluid and nutrients from sporophytic petal cells. During the course of pollen maturation, a buildup of carbohydrates with varieties of lipids and starch is paramount (Sangeeta and Maria, 2008). Essentially, nutrient filling throughout all these stages is essential for eminent fertilization. The growth of the plant structure during pollen germination occurs in the early stages although it is manifested a little later in some cases (Robert et al., 1993). Notably, induced male sterility, as is usually attributed to the shortage of carbon or energy sources can be achieved at this stage. As a fact, thorough knowledge of the nutrient regulation process throughout the pollen maturation process is important in agricultural practice.
Regarding Arabidopsis, the maturation of pollens involves the action of lipoid bodies although starch comes as a gift during the initial stages of pollen development. The formation process of lipoid biogenesis bodies, as found in pollens, is analogous or similar to the formation of oil storage bodies in oilseeds. In both cases, the formation process involves two key steps that occur in different organelles. Essentially, the initial step called the Delaware nivo stage involves the synthesis of fatty acids into acyl CoA and carbon. The second step, however, is unique in that it occurs in the specialized endoplasmic reticulum (ER) cells where acyl-CoA mixes with glycerol-3-phosphate (G3P) to produce triglycerides (Sangeeta and Maria, 2008). As an important point to note, carboxylic acids in pollens usually undergo synthesis in non-photosynthetic plastids, a process that depends on the importation of carbon. The carbon is derived from a variety of sugars and glucose-6-phosphate (Glc6P) that is converted into plastids. Unfortunately, existing literature does not document enough information on how the various steps are coordinated to an exceedingly spatiotemporal-specific manner. Besides, it is unclear which step involves rate-limiting for the lipoid body biogenesis process.
Various common proteins are known to exist in the pollen coat of Arabidopsis and maize. Apparently, these proteins have been shown to play a crucial role in pollen-stigma interactions during fertilization in both cases. An example of these proteins is the oleosin-domain supermolecule GRP17 where the enzylem EXL4 genus are from the genus Arabidopsis pollens. Besides being coated, these proteins coordinate the association between pollens and the stigma. Pertaining to maize, however, xylanase is discharged from the pollen coat during the initial stages of fertilization. Importantly, the discharge facilitates the penetration into the silk though the aid of xylan chemical that acts as the catalyst (Sangeeta and Maria, 2008). In both the maize and Arabidopsis cases, molecules usually settle at the surface of pollen grains, which is vital for the interaction of the grains and the stigma during the pollination process.
While acknowledging the mode of interaction between pollens and the sigma, as noted in the case of maize and Arabidopsis, it is important to note that there is only limited literature documenting the role of signals from within pollens during self-pollination process. It was only until recently when the genus Arabidopsis mutant that is associated with nursing came to the limelight among researchers (Harry, 2004). Presumably, the genus is considered to be a possible cause and reason for impaired pollination in vivo where it acts by reducing jasmonic acid levels in pollen grains. Biologists and other researchers postulate that aberrant peroxisome morphology is a possible regulator and controller of peroxisome biogenesis found in pollens. Notably, aberrant peroxisome morphology is expressed in the vegetative cells of pollen grains (Harry, 2004). The interpretation is that the jasmonate acid signals received from within pollen grains usually act as the regulators of the germination process and the interaction with stigma in Arabidopsis.
Tapetal Expression in BnaC.MAGL8.a and its Impact on Male Sterility in Arabidopsis
Monoacylglycerol enzyme (MAGL) is usually used to hydrolyze monoacylglycerol, a process that yields carboxylic acid and alcohol. This protein is known to play a crucial role in the growth and development of vertebrates, its functions in plants remains a relatively unexplored area of study that requires further research (Dennis, 2018). Existing literature shows that MAGL genes often reveals a tapetal expression of BnaC.MAGL8.a, which is a homolog of AtMAGL8. Often, most attempts to combine the two results in male sterility of Arabidopsis thaliana or unintelligent tapetal PCD and defective spore wall in transgenic plants. Besides, tapetal cells can also develop into becoming cavum before degeneration in the last stages of development. Nonetheless, where microspores tend to degenerate with tapetal cells, few pollen grains emerge with an irregularly shaped exinelayer in transgenic plants (Dennis, 2018).
Variations in gene expressions have been explained with the argument that they are potentially a result of the response to stress among other factors that appear to threaten their growth and development.In experiments where microspores are terminated or aborted, the downstream wall biogenesis genes of pollen grains are usually down-graded. Notably, the genes linked to the reactive atomic number eight species exhibit significantly higher stability and equilibrium as their jasmonate signals are upregulated in transgenic plants (Baesso et al., 2018).
Ordinarily, these observations are interpreted as being the result of the expression of BnaC.MAGL8.a in tapetum, which invokes stress response leading to the impairment of pollen development.In the United States, the apparent similarity between atgpat1 mutant and the BnA9:BnaC.MAGL8.a from transgenic plants led to the proposition that monoacylglycerol (MAG) played a crucial role in the development of pollens in genus Arabidopsis (Delker et al., 2006).
The Pathway to Pollen Development Based on the Arabidopsis-Rice Relationship: The development of spores that discharge at the right stage of the pollination and fertilization process is paramount for a given plant species to replicate itself without necessarily compromising its genetic pattern. According to earlier transcriptomic experiments on dilleniid dicot genus, it emerged that staged spores had up to thirteen sets of 977 genes expressed within the male reproductive structure. Besides, up to fifteen genes were noted in the male flora (Delker et al., 2006). A critical analysis of these findings reveals that the scope of organic phenomena throughout the reproductive structure and spore development is replicated in genes.
The ability to control the development of spores is an important breakthrough towards effective selective breeding. Particularly, the process is actualized through the discharge of genetically charged spores; hence, the initiation of hybrid lines in accordance with given expectations (Delker et al., 2006). Oftentimes, hybrids exhibit heterosis and hybrid vigor, which implies that that the new generation of plants could have relatively stronger tissues compared to the parents. Besides, hybrid species tend to mature earlier than usual, or even give higher yields depending on the nature of gene hybridization performed. Although researchers and scientists have a wide scope of generating hybrids, it has been observed that cytoplasmic male sterile (CMS) is commonly used for most crop species such as rice, maize, and cotton (James, 2018).
The CMS approach, depending on the interest or desires of biologists, used in a couple of plant species because it triggers nuclear-mitochondrial interactions. Nonetheless, the process may result from cross-hybridization where it is naturally aided by wind or insect. Based on previous studies, however, researchers have proved that some of the CMS lines have characteristically unique features from those of the parent species. Apparently, arising defects are aberrantly associated with open reading frames (ORFs) in mitochondrial genomes. Nevertheless, some of these mitochondrial defects are recovered through the nuclear encoding of genes, a process that helps to restore the plant fertility in full. Thus, it is possible to manage or reinstate the fertility of species through the selection of the appropriate breeding lines.
Multiple transgenic approaches are available for the conjointly use in the development of hybrid seeds. Scientists have previously demonstrated a great preference for the CMS approach when developing hybrid oilseeds. The technology, as it is used for commercial purposes, is predictable for productive structure needed when specific gene expression is desired. Essentially, available alternative approaches largely rely upon barnase as a combination of two inactive peptides and ulterior reconstitutions. Many of the alternative approaches, however, involve the expression of non-functional barnase and acetolactate synthase. The idea is to achieve male sterility through ulterior interaction and inteine-based splicing of supermolecule fragments. Ostensibly, many of the modern systems are based on suppression and restoration of key genes related to spores development where common examples include MYB103. Unfortunately, these approaches come with the limitation that they are restricted by the inadequacies of existing technologies and limited understanding of their mode of operation.
The Essence of MED30 subunit of mediator complex needed for plant development:The mediator is a giant multi-protein that is considered essential for the transcription of almost all genes transcribed by the polymer enzyme II. Importantly, specific subunits are required to assemble a working, rather, useful intermediator in vitro. Thus, a corresponding loss of function mutants could result in deadly outcomes (Zoe and Da-Bing, 2009). The MED30 subunit is particularly essential in anomal systems although it is absent in yeast. The subunit is reportedly for the survival of all male plants as well as for the development of embryos. In an earlier study, for instance, researchers observed that MED30 spore grains were viable. A few germinated and targeted the ovules much as the embryos aborted after a short period of fertilization. Based on these observations, it was deduced that MED30 is important for paternal management especially during the early stages of embryo development.
DEX1 is required for Exine pattern formation during pollen formation: DEDX1 is a novel plant protein needed in the process of pollen formation in Arabidopsis. To identify the factors and conditions needed for the formation of spore walls, it is necessary to characterize the T-DNA-tagged DEX1 mutations of the Arabidopsis genes that end up having defective spore walls because of irregular formation. This assertion is supported by earlier studies that showed DEX1 mutations encode unique macromolecules that are expected to be membrane-associated but contain numerous calcium-binding domains. In the study, the researches sought to isolate and determine the molecular characteristics of the DEX1 morphological and ultra- structural patterns in plants.
Importantly, it has been noted that the development of the spore wall in DEX1 cells is similar or comparable to many of the existing wild-type plants until the first quaternion stage of development. This observation is consistent with the fact that primexine deposition is ordinarily delayed and significantly reduced in DEX1 plants (Delker, 2006). The natural riffle of the cell membrane in DEX1 and the spacers ascertained in wild-type plants is absent in mutants. Sporopollenin is produced and deposited indiscriminately on the cell membrane in all DEX1 plants. Nonetheless, sporopollenin is not anchored on the spores (Anthony and Frank, 2005). Rather, it forms massive aggregates on the microspores as well as on the bodily cavity walls. As they are supported by the DEX1 structure and the nutrition of the plants, many of the roles of the macromolecules are certainly planned.
Pollen Development in Arabidopsis: The basic qualities of the gametophyte have been studied and documented in detail in previous studies although, in some cases, the qualities used to calibrate the procedures have been challenged for being seemingly obscure. Important concepts of study have been the characteristics of an obscure Arabidopsis and the GTP-Restricting protein-related 1 (GPR1). Apparently, the GPR1is communicated explicitly in ovule, dust, and in the dust tube (Zoe and Da-Bing, 2009). Additionally, upgraded green fluorescent protein-labeled GPR1 restricts both the core and cytoplasm. It also introduces punctate and ring-like structures. GPR1 is known to freak with no deformities in gametogenesis or even seed setting. The dust grains are usually pale shading on a quick glance. On examination under the electron microscope, ordinary designed and slender exines are noted on GPR1 pollen surfaces, which explain the shading pale appearance.
Researchers have also been interested in whether the GPRI transformations have a significant influence on post gametogenesis procedures, such as dust germination, ovule senescence, and dust tube development. Study findings show that GPR1 dust grains tend to sprout fast and their pollen tubes lengthen t a relatively fast pace. Notably, dust grains and ovules from GPR1 freaks show significantly low feasibility compared to the wild type when gauged based on a span of 4-5 days. These observations lead to the inference that the GPR1 capacities, as an inhibiting controller of dust germination, gametophyte senescence, and dust tube development, tend to tweak the preparation process.
Studies dedicated to the exploration of Microsporogenesis have played a key role in the analysis of the wild Arabidopsis thaliana as well as the atomic male-clean mutation BM3, a process that largely involves cytochemical recoloring. The freaks require adenine phosphoribosyl transferase compound made up of purine rescue pathway. Under adenine, the compound changes to AMP. Biologists observe that dust starts to veer off the wild kind soon after meiosis while the quadruplicates of microspores discharge from their callose dividers. The main sign of unusual advancement of dust in the mutant is a dark coloring attributed to the microspore divider. It is actually caused by inadequate intine blend. Under these circumstances, freak microspores do not respond to mitotic divisions. These observations amount to the reassertion that there are evidently conceivable roles of adenine rescue in dust improvements.
REFERENCES
Achard, P., Herr, A., Baulcombe, D.C., Harberd, N.A. (2004). Modulation of floral development by a gibberellin-regulated microRNA, pp. 3357-3365.
Anthony, A. and Frank, G. (2005). The Arabidopsis GAMYB-Like Genes, MYB33, and MYB65, Are MicroRNA-Regulated Genes That Redundantly Facilitate Anther Development. Plant Biology, 12(3), pp. 705–721.
Baesso, B., Chiatante, D., Terzaghi, M., Zenga, D., Nieminen, K., Mahonen, A.P., Siligato, R., Helariutta, Y., Scippa, G.S. and Montagnoli, A. (2018). Transcription factors PRE 3 and WOX 11 are involved in the formation of new lateral roots from secondary growth taproot in A. thaliana. Plant Biology, 20(3), pp.426-432.
Becker, J.D., Boavida, L.C., Carneiro, J., Haury, M. and Feijo, J.A. (2003).Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiology. 133, pp. 713–725.
Birnbaum, K., Shasha,D., Wang J., Jung J. W., Lambert G.M., Galbraith, D.W., Benfey, P. N. (2003). Gene expression map of the Arabidopsis root. Science, 302, 1956–1960.
Boyes, D. C. , Zayed A. M. , Ascenzi, R., McCaskill, A. J. (2001). Hoffman N E., Davis K R., and Görlac J Growth Stage –Based Phenotypic Analysis of Arabidopsis A Model for High Throughput Functional Genomics in Plants. Plant Cell, 13(7): 1499–1510.
Callis, J., Fromm, M.E and Walbot V. (1987). Introns increase gene expression in cultured maize cells. Gene Development, 1, pp. 1183-1200.
Cecchetti, V., Altamura, M., Falasca, G., Costantino, P. and Cardarell, M. (2008). Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. In: the plant cell. Plant Physiology, 7(33), pp. 13–25.
Claudia, H., Jeus, L., Veronica, A., Uta, S., Florian, H., Sang, D., Tang, Z., Eberhard, S., Jorg, K., Klaus, H. (2004). The response regulator 2 mediates ethylene signaling and hormone signal integration in Arabidopsis. The EMBO Journal, 6(10), pp. 3290-3302.
Delker, C., Stenzel, I., Hause, B., Mieresch, O., and Wasternack, C. (2006). Jasmonate Biosynthesis in Arabidopsis thaliana – Enzymes, Products, Regulation. Plant Biology, pp. 297-306.
Dennis, R. (2018). Analysis of anther dehydration: a process required for anther dehiscence and pollen release, University of Nottingham.
Harry, K. (2004). Ethylene signal transduction, Moving beyond Arabidopsis. Available at: http://www.plantphysiol.org/content/135/2/660
Haseneyer, G., Ravel, C., Dardevet, M. et al. (2008). High level of conservation between genes coding for the GAMYB transcription factor in barley (Hordeum vulgare L.) and bread wheat (Triticum aestivum L.) collections. Theor. Appl Genet. 117, 321–331
Hudson, R. R., and Kaplan, N. L. (1985). Statistical properties of the number of recombination events in the history of a sample Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature 402, 761-768.
James, G. (2018). The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Strasbourg France: Ghent University, p.10. Available at: https://www.pnas.org/content/pnas/115/17/E4130.full.pdf
Juanying, Ye, Zhang, Z., You, C, Zhang, X., Lu J., Hong, M. (2016). Abundant protein phosphorylation potentially regulates Arabidopsis anther development. Journal of Experimental Botany, Volume 67, Issue 17, Pages 4993–500.
Miransari, M. and Donald, E. (2016). Gibberellins and Stress. Science Direct, pp. 176.
Neetu V. (2019). Transcriptional regulation of anther development in Arabidopsis. Gene. Volume 689, 20, Pages 202-209
Orgil U, Araki H, Tangchaiburana S, Berkey R and Xiao S. (2007). Intraspecific genetic variations, fitness cost and benefit of rpw 8, a disease resistance locus in Arabidopsis thaliana. Genetics, vol. 176 no. 4 2317-2333
Provart, N. J., J. Alonso, S. M. Assmann, D. Bergmann, S. M. Brady et al., (2016). 50 years of Arabidopsis research: highlights and future directions. New Phytol. 20, 921–944.
Quilichini, T.D., Grienenberger, E., and Douglas, C.J. (2015). The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack. Phytochemistry113, pp. 170–1823.
Robert, B., Thomas, P., and Paul. M. (1993). Anther development: Basic principles and practical applications. The Plant Cell, 5, pp. 1217-1229.
Sangeeta, N. and Maria, I. (2008). Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. The Plant Journal, p.13. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1365-313X.2008.03495.x
Smalle, J., Haegman, M., Kurepa, J., Van Montagu, M. and Straeten, D. (1997). Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proceedings of the National Academy of Sciences, 94(6), pp.2756-2761.
Stefanie.S, Ann. C, Jaco. V and Tony. R. (2015). Gene Networks Involved in Hormonal Control of Root Development in Arabidopsis thaliana: A Framework for Studying Its Disturbance by Metal Stress. International Journal of Molecular Sciences, 30: 22-30
Xiu-Tang, W., Can, Y., Ting-Ting, Y., and Su-Juan, C. (2012). The Arabidopsis LFR Gene Is Required for the Formation of Anther Cell Layers and Normal Expression of Key Regulatory Genes. Molecular plant, 5.Pp. 993-1000.
Yamada, K., Lim J, Dale JM, Chen H, Shinn P, Palm CJ, Southwick AM, Wu HC, Kim C, Nguyen M, Pham P, et al. (2003). Empirical analysis of transcriptional activity in the Arabidopsis genome. Science 302, 842–846.
Yang, X., Zhang, Q. Zhao, K. Luo,Q. Bao, S. Liu, H. Men, S. (2017) . The Arabidopsis GPR1 Gene Negatively Affects Pollen Germination, Pollen Tube Growth, and Gametophyte Senescence. Pubmed magazine, 18(6). 451-453.
Zoe, A. Wilson, S., Benjamin T., and Caiyun Y. (2011). The final split: The regulation of anther dehiscence, Journal of Experimental Botany, 5(8), pp. 167-174.
Zoe, W. and Da-Bing, Z. (2009). From Arabidopsis to rice: pathways in pollen development. Journal of Experimental Botany, 11(3), pp. 317-331.