Department of Zoology, Banaras Hindu University, Varanasi, UP, India
Corresponding author email: acharya@bhu.ac.in
Article Publishing History
Received: 19/10/2019
Accepted After Revision: 25/12/2019
Carica papaya leaf has widely been used for its medicinal properties; includes anti-cancer, immunomodulatory, and wound healing. However, its molecular mechanisms of enhancing the immune function remain to be unexplored. In the present study, we investigated the effect of aqueous leaf extract of Carica papaya on the production of non-specific effectors molecules and the expression of cell surface receptor by tumor-associated macrophages (TAMs) isolated from Dalton’s lymphoma bearing mice. Nitric oxide production was measured by Griess reagent and release of tumor necrosis factor (TNF-a), IL-6 was determined by ELIZA assay. In addition, FACS analysis used to evaluate the expression of cell surface receptor molecules on TAMs. Here, we found that CPE treatment significantly enhanced the production of effectors molecules nitric oxide (NO), hydrogen peroxide (H2O2), tumor necrosis factor-α (TNF- α) and IL-6 in TAMs. Furthermore, CPE treatments increase the expression of the co-stimulatory molecule (CD80) on tumor-associated macrophages. Thus, our findings suggest that CPE enhanced the anti-tumor response of TAMs by up regulating the production of potent effectors molecules, and modulating the expression of costimulatory cell surface receptor molecule.
Carica papaya, Cell surface molecules, Immunomodulatory, Nitric oxide, Tumor-associated macrophages (TAMs).
Singh S. P, Kumar S, Tomar M. S, Singh R. K, Verma P. K, Kumar A, Kumar S, Acharya A. Aqueous extract of Carica papaya Leaf Elicits the Production of TNF-Α and Modulates the Expression of Cell Surface Receptors in Tumor-Associated Macrophages. Biosc.Biotech.Res.Comm. 2019;12(4).
Singh S. P, Kumar S, Tomar M. S, Singh R. K, Verma P. K, Kumar A, Kumar S, Acharya A. Aqueous extract of Carica papaya Leaf Elicits the Production of TNF-Α and Modulates the Expression of Cell Surface Receptors in Tumor-Associated Macrophages. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2NrykE1
Copyright © Singh et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Cancer is a type of disease that results from uncontrolled growth and division of cells, causes millions of death regardless many developments made in the field of its diagnosis, treatment, and preventive measures (He et al., 2016 and Qin et al., 2017). Currently, treatments available for cancer are surgery, chemotherapy, radiotherapy, immunotherapy, vaccinations, and combination therapy, where chemotherapy is most common and widely used for the treatment of highly metastatic cancer (Greenwell et al., 2015). However, drugs used during chemotherapy shows several side effects and put the life of patient under threat. Therefore, people are forced to look at alternative treatment options against cancer. Since a long time, plant products have been used to treat many diseases, including asthma, ulcers, eczema, jaundice, malaria, diabetes, helminths infections and fever (Nguyen et al., 2013). In modern days, it is one of the primary sources of medicinal drugs in developing countries (Greenwell et al., 2015 Hung et al., 2019).
Earlier, secondary metabolites like alkaloids, phenolics, flavonoids, carotenoids, tannins, saponins, papain and chymopapain (Pandey et al., 2016) isolated from the tropical plant were used as an anti-inflammatory, immunomodulatory, and antiseptic agent with relatively less or no side effects (Recioet al., 2012). Thus, people have shifted their focus to formulate plant product based potential drugs to cure cancer (Sivarajet al., 2014). Carica papaya is a well-known tropical plant containing several bioactive secondary metabolites with immense medicinal value (Khare et al., 2004). Traditionally, people uses different parts of papaya plant, including fruits, seeds, leaves, shoots, and latex to cure various ailments (Nguyen et al., 2013 and Otsukia et al., 2010). The scientific evaluation of papaya plant product show excellent medicinal values, has property to increases antioxidant level, and reduces lipid peroxidation in the blood (Otsukia et al., 2010 and Seigler et al., 2002). It is highly beneficial in wound healing, cardiovascular diseases, dengue fever, cancer, malaria, and hypoglycaemia (Maniyar et al., 2012 and Nunes et al., 2013). Further, strong immunomodulatory, antitumor, anti-inflammatory, antioxidant, and wound healing properties have reported in papaya leaf extract (Imaga, et al., 2013, Gurung et al., 2009 and Anjum et al., 2017).
Recently, immunomodulatory potential of papaya leaf extract reported on several cancer cell lines, but there are very few reports on PBMC (Otsukia et al., 2010), and therefore, required studies in detail. Macrophages are a heterogeneous population of tissue-resident professional phagocytes originated from the terminal differentiation of circulating monocytes (Gordon et al., 2005). They act as a primary line of defence of the host, and mediate innate and adaptive immune response against invading pathogens (Mosser et al., 2008). Stimulation with lipopolysaccharides (LPS), IFN-γ, TNFα, and IL-1β converted resting macrophages into classically activated or M1 phenotype macrophages that shows anti-tumor properties (Edwards et al., 2006). However, continuous exposure of tumor microenvironment and cytokines like IL-4, or IL-13, IL-10 and TGF β secreted by tumor cells changes M1 macrophages into M2 macrophages which are phenotypically and functionally altered population of normal macrophages and support tumor progression (Goswami et al., 2017). The M2 macrophages or frequently called tumor-associated macrophages (TAMs) are the most abundant cells present in the close vicinity of tumor cells, constituted more than 50 % of the total tumor mass (Zheng et al., 2017) provide better opportunity to develop a novel anti-cancer therapy by modulating their altered physiology in a tumor-bearing host.
In the present study, we have studied immunomodulatory potential of C. papaya leaf extract TAMs isolated from Dalton’s lymphoma (DL) bearing mice, and found that aqueous papaya leaf extracts significantly induces anti-tumor activities of TAMs and restore its normal morphology to some extent. DL is a type of transplantable T cell lymphoma of mouse origin that mimics human T cells lymphoma, causes death of the host within a short interval of time, and therefore, our finding justify the purpose of the study.
MATERIALS and METHODS
Preparation of papaya leaf extract
Collected papaya leaf was wash with autoclaved distilled water. Every 20 grams of leaf powder mixed with 400 ml of water was boiled at 60oC until 12.5% ml water was left as a leaf extract. The extracts were filtered by Whatman filter paper followed by 0.22-micron filter (MILLIPORE), and stored at -20oC.
Isolation of tumor-associated macrophage
Tumor-associated macrophages harvested from tumor-bearing mice as described earlier (Gautam et al., 2013). Briefly, a mouse sacrificed by cervical dislocation injected with 2ml chilled PBS in the peritoneal cavity. After intense peritoneal lavaging, peritoneal exudate cells were collected in a Petri plate and incubated at 370C in CO2 incubator for 2 h. Washed the cells three times with incomplete RPMI 1640 to ensure the removal of all non-adherent cells from the plate after incubation and collected only adherent cells.
Characterization of tumor-associated macrophages
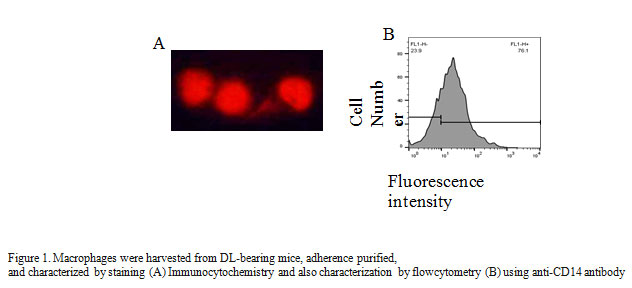
Collected adherent cells were washed in phosphate-buffered saline (PBS) and fixed on Cytospin slide with 4% formaldehyde for 1 h. Block the cells with 0.5% BSA for 1 h at room temperature and permeabilized with 0.2% Triton X-100 for 10 min at room temperature. Washed the cells with PBS, stained with anti-CD14FITC antibody (1:100) and incubated overnight at room temperature. After three washing mounted the cells in DABCO and visualized the cells with Nikon E800 upright fluorescence microscope. Purity of macrophages was further confirmed by flow cytometry analysis using CD14PE antibody describe earlier (Lu-Emerson et al., 2013).
Nitrite assay for estimation of nitric oxide production
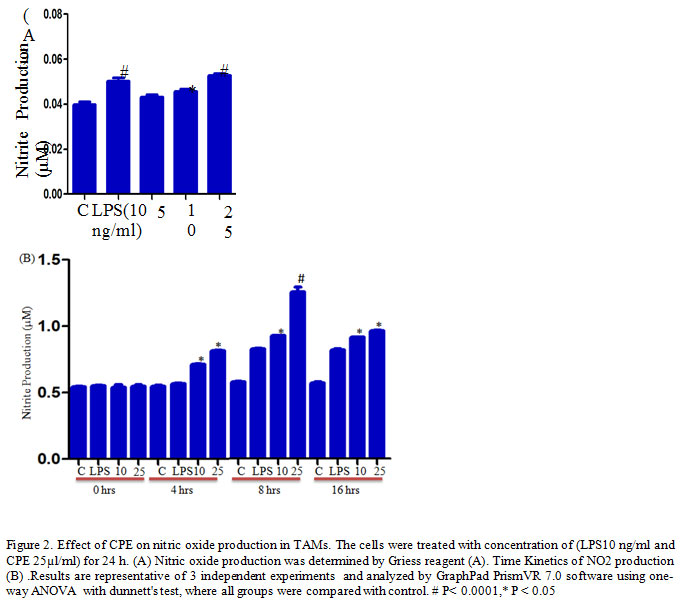
TAMs (1.5x 105 cells/well) culture in 200 μl complete RPMI 1640 in a 96 well ELISA plates treated with different concentrations of Carica papaya leaf extract CPE (5. 10, 25 µl/ml) and LPS (10ng/ml) as a positive control for 24 h. After desired treatments, the supernatant was harvested and mixed with an equal volume of Griess reagent (sulfanilamide, Naphthalene-ethylene-diaminedihydrochloride, and H3PO4) at room temperature for 10 min (Kumar et al., 2006) and the absorbance was measured at 540 nm using a ELISA plate reader.
Assay for reactive oxygen intermediate (ROI) production
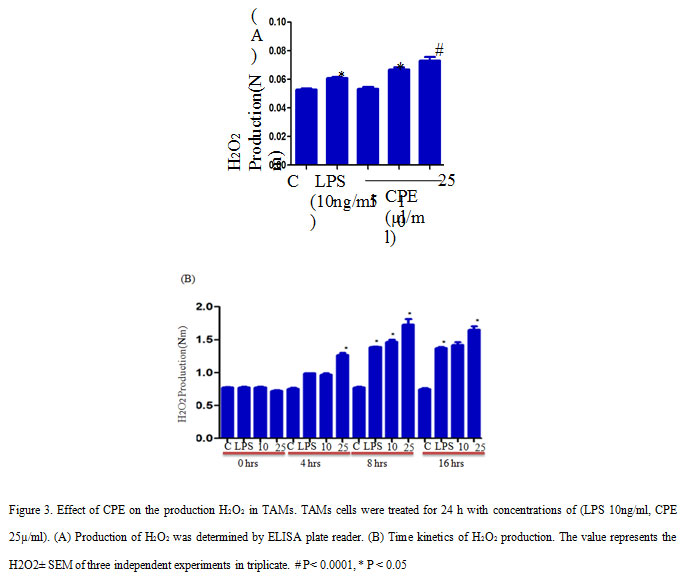
TAMs cells were seeded and treated with CPE similar to the nitrite assay. After treatment, cells were treated with an equal volume of phenol red solution (140mM NaCl, 10mM K2HPO4, 5.5mM dextrose, and 5.5nM horseradish peroxidise), and incubated for 1 hr in CO2 incubator (Kumar et al., 2006). Subsequently, 1M NaOH added in each wells and took the absorbance at 620 nm on ELISA plate reader.
TNF-α and IL-6 production
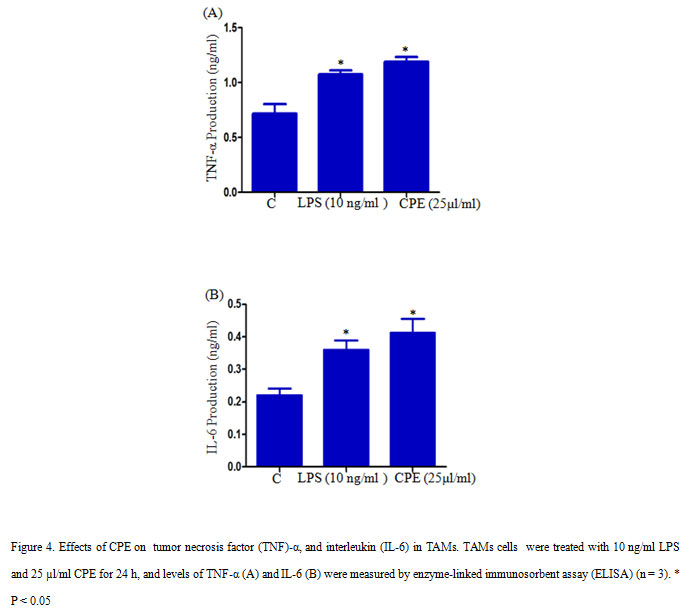
2×105 TAMs per well were seeded in a 12-well culture plate and treated with 10 ng/ml LPS or 25µl/ml CPE and incubated for 24 h in a CO2 incubator. After incubation culture supernatant was collected and measure the level of TNF-α and IL-6 with the help of ELISA assay (Kim et al., 2011), and quantify cytokine concentrations by using standard curves of TNF-α and IL-6.
Flow cytometry analysis for cell surface receptor on TAMs
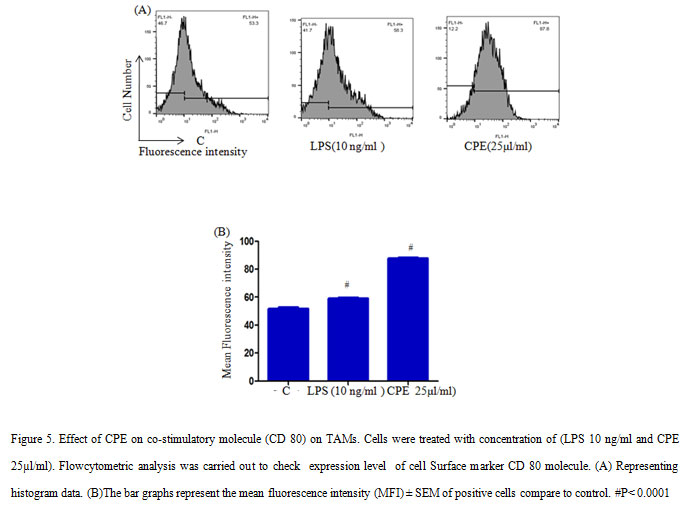
TAMs treated with 10 ng/ml LPS and 25µl/ml CPE incubated in a CO2 incubator for 24 h. At the end of treatment, cells stained with anti-mouse- CD80 conjugated with FITC and anti-mouse CD14 conjugated with PE (Gautam et al., 2015). After washing with PBS, the cells were re suspended in PBS containing 0.1 % NaN3, the fluorescence level was measured with the help of flow cytometer (BD Biosciences, Mountain View, CA, USA) LSRII and the data were analyzed by using Flow Jo software.
RESULTS and DISCUSSION
In the present study, we report that effects of aqueous leaf extract of Carica papaya (CPE) on the production of non-specific effector molecules like, NO, H2O2, and TNF-α, and expression of specific cell surface receptor CD80 on tumor-associated macrophages (TAMs). Macrophages are the part of the innate immune system, and well known to produce non-specific effectors molecules upon stimulation. Here, we isolate and characterized TAMs on the basis of CD 14 expression with the help of flow cytometry (Figure 1A) and found 95 % TAMs after isolation via adherent purification (Figure 1B).CD 14 mainly present on the cell surface on macrophages, and therefore, used as a marker for identifying TAMs (Rőszer et al., 2015).
It has been reported that activation of macrophages by flavonoid or Polyphenols modulates the production of effector molecules (Yahfoufi et al., 2018). Nitric oxide is a lipophilic, toxic gaseous molecule, plays vital role in the inhibition of the tumor growth (Lamattina et al., 2003). Several studies have reported that high concentration of NO induces death in the cells, and secreted by macrophages to kill invading pathogens and tumor cells (Rahat et al., 2013). Herein, we examine the effect of CPE on NO production in TAMs and our result shows that CPE treatment significantly increases NO production in TAMs after for 24 h as compared to the control or LPS stimulated cells (Figure 2A). Furthermore, time kinetics study confirmed that the maximum release of NO observed at 8 h after treatment (Figure 2B) corroborate previous finding of Elmowalid and co-workers who reported that an aqueous extract of Nigella saliva seed enhances NO production in macrophages (Elmowalid et al., 2013).
Further, we investigate the effect of CPE on reactive oxygen intermediate (ROI) production in TAMs and found that CPE treatment increased the H2O2 production in TAMs as compares to control or LPS stimulated cells. Here, we observed that CPE treatment induced more significant production of H2O2 compared to LPS treated TAMs (Figure 3A). Time course study confirmed that maximum secretion of H2O2 from TAMs occur at 8 h of incubation and after that gradually decreased (Figure 3B). Thus, our findings suggest that increased production of RNI, ROI may contribute to reduced tumor growth. Earlier scientists have reported that production of non-specific effector molecules play significant role in enhancing the tumoricidal properties of activated macrophages in vitro and in vivo (Cui et al., 1994).
We further measured the effect of CPE on the production of TNF-α and pro-inflammatory cytokineIL-6 in TAMs. CPE Treatment (CPE 25µl/ml) significantly increased the production of TNF-α and IL-6 in TAMs compares to control (Figure 4 A, B). TNF-α is a multifunctional cytokine, which involves in various cellular processes such as cell survival, apoptosis, and inflammation. Several studies reported that LPS could activate J774.1 cells to produce pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β (Hung et al., 2019). Our result indicated that CPE increased the secretion of pro-inflammatory cytokines TNF-α and IL-6 in TAMs.
In addition, low levels of MHC-II expression reported on macrophages during tumor progression, as a result it failed to stimulate T cells during tumor progression (Guerriero et al., 2018).However, M1-polarization inducers such as anti-CD40 mAb and IFN-γ are able to up-regulate MHC-II expression and other co-stimulating factors (e.g. CD80/86) on macrophages to enhance the adaptive immune response of the host required for tumour rejection. Further, we examined the effect of CPE on the expression of co-stimulatory molecule (CD80) on TAMs and shows that CPE treatment enhanced the expression of CD80 molecules significantly (Figure 5). As a result, macrophages provide costimulatory signal for T cell activation. These results collectively suggested that CPE treatment increases the expression non-specific effector molecules and costimulatory molecules on macrophages that in turn increase anti-tumor properties of TAMs and help to attain the M1 phenotype to some extent.
CONCLUSION
The present study shows that Carica papaya leaf extract (CPE) positively modulated that production of NO, H2O2, TNF-a, and IL-6 production in TAMs. Also, Carica papaya leaf extract upregulated the costimulatory receptor CD80 in TAMs. The results suggested that CPE reversing the M2 phenotype polarization to M1 polarization. However, further studies are essential to explore the molecular mechanism induced by CPE in TAMs, which may improve the treatment of cancer.
ACKNOWLEDGMENTS
This work was financially supported by ICMR, Government of India to Surya Pratap Singh in the form of SRF (No.45/18/2018/BMS/TRM), which is greatly acknowledged. Authors are very thankful to ISLS, BHU for provided FACS machine facility.
CONFLICT of INTEREST
Authors declare no conflict of interest in this scientific observation.
REFERENCES
Anjum, V., Arora, P., Ansari, S.H., Najmi, A.K. and Ahmad, S., (2017) Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of Carica papaya leaves. Pharmaceutical biology, 55(1), pp.2043-2056.
Cui, S., Reichner, J.S., Mateo, R.B. and Albina, J.E., (1994). Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or-independent mechanisms. Cancer Research, 54(9), pp.2462-2467.
C Recio, M., Andujar, I. and L Rios, J., (2012).Anti-inflammatory agents from plants: progress and potential.Current medicinal chemistry, 19(14), pp.2088-2103.
Edwards, J.P., Zhang, X., Frauwirth, K.A. and Mosser, D.M., (2006) .Biochemical and functional characterization of three activated macrophage populations.Journal of leukocyte biology, 80(6), pp.1298-1307.
Elmowalid, G., Amar, A.M. and Ahmad, A.A.M., (2013). Nigella sativa seed extract: 1. Enhancement of sheep macrophage immune functions in vitro. Research in veterinary science, 95(2), pp.437-443.
Gautam, P.K., Kumar, S., Deepak, P. and Acharya, A., (2013). Morphological effects of autologous hsp70 on peritoneal macrophages in a murine T cell lymphoma Tumor Biology, 34(6), pp.3407-3415.
Gautam, P.K. and Acharya, A., (2015). Suppressed expression of cd80 (b7. 1) and cd86 (b7. 2) receptors in tams up-regulated by autologous hsp70–peptide complex in Dalton’s lymphoma bearing balb/c mice. IJRST, 5(III), pp.106-127.
Ginhoux, F. and Guilliams, M., (2016).Tissue-resident macrophage ontogeny and homeostasis. Immunity, 44(3), pp.439-449.
Goswami, K.K., Ghosh, T., Ghosh, S., Sarkar, M., Bose, A. and Baral, R., (2017).Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cellular immunology, 316, pp.1-10.
Greenwell, M. and Rahman, P.K.S.M., (2015) Medicinal plants: their use in anticancer treatment. International Journal of Pharmaceutical Sciences and Research, 6(10), p.4103.
Guerriero, J.L., (2018) Macrophages: the road less traveled, changing anticancer therapy. Trends in molecular medicine, 24(5), pp.472-489.
Gurung, S. and Škalko-Basnet, N., (2009). Wound healing properties of Carica papaya latex: in vivo evaluation in mice burn model. Journal of Ethnopharmacology, 121(2), pp.338-341.
Gordon, S., (2005).Taylor PR. Monocyte and macrophage heterogeneity.Nat Rev Immunol, 5, pp.953-964.
He, L., Gu, J., Lim, L.Y., Yuan, Z.X. and Mo, J., (2016). Nanomedicine-mediated therapies to target breast cancer stem cells. Frontiers in pharmacology, 7, p.313.
Hung, Y.L., Wang, S.C., Suzuki, K., Fang, S.H., Chen, C.S., Cheng, W.C., Su, C.C., Yeh, H.C., Tu, H.P., Liu, P.L. and Huang, M.Y., (2019).Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine, 59, p.152785.
Imaga, N.A., Gbenle, G.O., Okochi, V.I., Adenekan, S., Duro-Emmanuel, T., Oyeniyi, B., Dokai, P.N., Oyenuga, M., Otumara, A. and Ekeh, F.C., (2010). Phytochemical and antioxidant nutrient constituents of Carica papaya and Parquetin anigrescens extracts. Scientific research and essays, 5(16), pp.2201-2205.
Khare, C.P., (2004). Indian herbal remedies: rational Western therapy, Ayurvedic, and other traditional usage, Botany. Springer science & business media.
Kim, S.W., Kim, J.S., Papadopoulos, J., Choi, H.J., He, J., Maya, M., Langley, R.R., Fan, D., Fidler, I.J. and Kim, S.J., (2011). Consistent interactions between tumor cell IL-6 and macrophage TNF-α enhance the growth of human prostate cancer cells in the bone of nude mouse. International immunopharmacology, 11(7), pp.862-872.
Kumar, S., Deepak, P. and Acharya, A., (2006). HSP70 Modulates the Enhanced Production of Reactive Intermediate Metabolites and a Proinflammatory Cytokine TNF-α Expression in a T Cell Lymphoma. European Journal of Inflammation, 4(3), pp.157-169.
Lamattina, L., García-Mata, C., Graziano, M. and Pagnussat, G., (2003).Nitric oxide: the versatility of an extensive signal molecule. Annual Review of Plant Biology, 54(1), pp.109-136.
Lu-Emerson, C., Snuderl, M., Kirkpatrick, N.D., Goveia, J., Davidson, C., Huang, Y., Riedemann, L., Taylor, J., Ivy, P., Duda, D.G. and Ancukiewicz, M., (2013) Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro-oncology, 15(8), pp.1079-1087.
Maniyar, Y. and Bhixavatimath, P., (2012) Antihyperglycemic and hypolipidemic activities of aqueous extract of Carica papaya Linn. leaves in alloxan-induced diabetic rats. Journal of Ayurveda and integrative medicine, 3(2), p.70.
Mosser, D.M. and Edwards, J.P., (2008) Exploring the full spectrum of macrophage activation. Nature reviews immunology, 8(12), p.958.
Murray, P.J. and Wynn, T.A., (2011) Protective and pathogenic functions of macrophage subsets.Nature reviews immunology, 11(11), p.723.
Nguyen, T.T., Shaw, P.N., Parat, M.O. and Hewavitharana, A.K.,( 2013). Anticancer activity of Carica papaya: A review. Molecular nutrition & food research, 57(1), pp.153-164.
Nunes, N.N.D.S., Santana, L.A., Sampaio, M.U., Lemos, F.J. and Oliva, M.L., (2013). The component of Carica papaya seed toxic to A. aegypti and the identification of tegupain, the enzyme that generates it.Chemosphere, 92(4), pp.413-420.
Otsuki, N., Dang, N.H., Kumagai, E., Kondo, A., Iwata, S. and Morimoto, C., (2010) Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. Journal of ethnopharmacology, 127(3), pp.760-767.
Pandey, S., Cabot, P.J., Shaw, P.N. and Hewavitharana, A.K., (2016) Anti-inflammatory and immunomodulatory properties of Carica papaya.Journal of immunotoxicology, 13(4), pp.590-602.
Qin, W., Huang, G., Chen, Z. and Zhang, Y., (2017) Nanomaterials in targeting cancer stem cells for cancer therapy. Frontiers in pharmacology, 8, p.1.
Rahat, M.A. and Hemmerlein, B., (2013). Macrophage-tumor cell interactions regulate the function of nitric oxide. Frontiers in physiology, 4, p.144.
Rőszer, T., (2015).Understanding the mysterious M2 macrophage through activation markers and effector mechanisms.Mediators of inflammation, 2015.
Seigler, D.S., Pauli, G.F., Nahrstedt, A. and Leen, R., (2002). Cyanogenicallosides and glucosides from Passi floraedulis and Carica papaya.Phytochemistry, 60(8), pp.873-882.
Sivaraj, R., Rahman, P.K., Rajiv, P., Narendhran, S. and Venckatesh, R., (2014). Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 129, pp.255-258.
Yahfoufi, N., Alsadi, N., Jambi, M. and Matar, C., (2018). The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients, 10(11), p.1618.
Zheng, X., Turkowski, K., Mora, J., Brüne, B., Seeger, W., Weigert, A. and Savai, R.,( 2017). Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy.Oncotarget, 8(29), p.48436.