1,3,4 Department of Biology, College of Science, University
of Jeddah, Jeddah 21589, Saudi Arabia.
2Department of Chemistry of Natural and Microbial Products, Division of Pharmaceutical
and Drug Industries, National Research Centre, Giza, Egypt.
5Plant Biochemistry Department, National Research Centre, 33 El-Behooth
St., Dokki, Giza, P.O. 12622, ID: 60014618, Egypt.
6Chemistry Dept., University College of Al Leith- Umm Al-Qura University, Saudi Arabia.
Corresponding author email: ahmedazazy8@hotmail.com
Article Publishing History
Received: 17/06/2021
Accepted After Revision: 16/09/2021
Moringa oleifera, commonly known as Moringa, is an Antioxidant and Antimicrobial Potential of Moringa oleifera Extract Against Food Pathogens extraordinarily nutritious vegetable tree with a variety of potentially medicinal benefits, also referred to as the Miracle tree due to its multiple uses. The leaves of Moringa oleifera are high in phenolic compounds, which act as antimicrobials and antioxidants. Antioxidant phenolic compounds may stabilize free radicals by compensating for their electron deficiency. Consumption of polyphenol-rich plants as a dietary component provides protection against such cell damage. The present study explores the antimicrobial, antioxidant ability, total phenolic content (TP) and total flavonoid content (TF) of different extracts prepared from leaves of Moringa oleifera grown locally Saudi Arabia. Higher TP, TF and antioxidant activity have been demonstrated by methanol extracts followed by di ethyl ether solvents.
The present study indicates that all extracts may, to some degree; act as radical scavengers due to the existence of polyphenolic compounds. Additionally, Methanol extracts showed significant inhibitory activity against food poisoning bacteria Shigella sonnei 19 ± 1.73, Klebsiella pneumonia17.33 ± 0.57 and Pseudomonas aeruginosa 17.00 ±6.93. The di ethyl ether extracts showed lower activity. Data provided in this study show that Moringa oleifera leaves have great potential for the development of food preservatives and antibiotic drugs. In conclusion the Methanolic solvent could be reasonable choice for antioxidant compounds extraction and the potential uses of M. oleifera as alternative natural preservatives in food products.
Antioxidant, Free Radicals, Foodborne Disease,
Moringa oleifera, Pathogenic Microorganism.
Elazzazy A. M, Almaghrabi O. A, Kadasa N. M. S, Mohamed A. A. Antioxidant and Antimicrobial Potential of Moringa oleifera Extract Against Food Pathogens. Biosc.Biotech.Res.Comm. 2021;14(3).
Elazzazy A. M, Almaghrabi O. A, Kadasa N. M. S, Mohamed A. A. Antioxidant and Antimicrobial Potential of Moringa oleifera Extract Against Food Pathogens. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3yAHeo3“>https://bit.ly/3yAHeo3</a>
Copyright © Elazzazy et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Foodborne diseases considered one of the worldwide health concern especially in developing countries (Sapkota et al., 2012; Kirk et al., 2017), which may occur at any point during the preparation, distribution, and/or consumption of food. Gram-negative and Gram-positive bacteria which have been identified as the causal agents of food spoilage and food borne diseases; Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Bacillus cereus and Staphylococcus aureus (Braga et al., 2005; Pandey and Singh, 2011). While uses of chemical preservatives were thought to be effective against food poisoning outbreaks, their accumulation in the feed and food chain resulted in microbial resistance, which had negative implications for human life (Akinyemi et al., 2006, Bialonska et al., 2010).
As a result, eco-friendly techniques are now needed to not only minimize pathogenic bacteria growth but also to reduce the use of chemical preservatives and to extend the shelf life of food (Clarke et al., 2017). Among these contexts is the use of plant extracts as antimicrobials for food safety, several researchers have demonstrated the antimicrobial activity of plant extracts against food poisoning bacteria as natural sources of antimicrobials and are considered healthy in nutrition and easily consumable (Akinpelu et al., 2015 and Suppakul et al., 2016; Saleem et al., 2020; Ali et al., 2021).
One alternative treatment for bacteria-infected infections is by using natural ingredients, such as Moringa oleifera L. Plant. This plant is called the most important multipurpose and miracle tree in the world, since all parts of the plant are useful for fruit, medicine, cosmetics or purified water (Fahey, 2005). Moringa oleifera (Moringaceae), also known as the “tree of life,” has made a breakthrough in this field. M. oleifera, which is native to India and Africa, appears promising due to its safety for animal and human consumption (Makkar, and Becker, 1996).
Moringa oleifera L. leaf has many active components such as triterpenoids, flavonoids, tannins saponins and alkaloids so pharmacologically has benefits as antimicrobial, antifungal, antihypertensive, antihyper-glycemic, antitumor, anticancer, anti-inflammatory (Mahmood et al., 2010; Sharma et al., 2011). Antimicrobial activity of Moringa oleifera against human pathogens was proved by several investigators (Singh and Tafida, 2014; Morgan et al., 2019; Das et al., 2020; Naseer et al., 2021).
However, the potential of M. oleifera as alternative natural preservatives in food products has not been thoroughly studied. Therefore, the goal of the present study was to evaluate the antioxidant and antibacterial activity of Moringa oleifera leaves extract in vitro against food poisoning diseases caused by E. coli, S. aureus, P. aeruginosa, S. typhi and B. cereus.
MATERIAL AND METHODS
Plants extraction preparation: Fresh leaves of Moringa oleifera were washed, then air dried, powdered. The Moringa leaves powder (5 g) are successively extracted using with methanol and di ethyl ether solvents. the dry extracts of methanol and di ethyl ether completely re-dissolved in methanol.
Total phenolic content: using the spectrophotometric method; a solution was made with 0.5ml of the sample and distilled water, raising the total volume of the sample to 3 ml, then 0.5 ml of Folin-phenol Ciocalteu’s reagent was added, followed by 2 ml of 2 % Na2CO3 solution after 5 minutes, thoroughly mixed. The total phenolic (TP) was calculated using the extrapolation of the calibration curve when the mixture’s absorbance reached 650 nm after 60 minutes in the dark at 30° C. The gallic acid solution was used to establish the curve. The TP was determined as milligrams of gallic acid equivalents (GAE) per gram of dried sample after the phenolic compounds were estimated in triplicate.
Total flavonoids content (Chang, et al., (2002): The total solution was increased to 1 ml by adding methanol to a 0.5 ml sample. The resulting mixture was left unchanged for 5 minutes after adding 4 mL of distilled water and 0.3 mL of a 5% NaNO2 solution. After adding 0.3 ml of ALCL3 solution 10 % the solution could sit for another 6 minutes before being increased to a volume of 10 ml by adding two ml of NaOH solution (1 M) and distilled water. The concentrations of total flavonoid were determined once the absorption read 510 nm after being thoroughly shaken and left for 15 minutes. For the analysis, Quercetin equivalents (QUE mg/g of dry weight) were used.
Antioxidant’s assay: The radical scavenging activity of methanolic extracts was calculated quantitatively. In a nutshell, using 1,1-diphenyl-2-picryl hydrazyl (DPPH) a 0.1 mM DPPH solution was prepared using methanol. At various concentration (100 – 300 g/ml), 1 ml of DPPH stock solution was combined with 3 ml of each methanolic and di ethyl ether extract. As a positive regulation, butyl-4-hydroxyanisole (BHA) was used. After 30 minutes of incubation, discoloration was estimated at 517 nm. At the very least, three measurements were taken. The following equation was used to measure the capacity to scavenge the DPPH• radical: DPPH• scavenging impact (%) = Abs Control/Abs Control 100.
Antimicrobial’s activity of the Moringa extracts: Bacterial strains and growth conditions the reference strains used in this analysis were bacterial isolates selected for their historical relevance to pathological effects on humans and food product degradation. Among the eight food-borne pathogenic bacteria, obtained from the culture collection of Microbiology Dept. King Abdulaziz University, Jeddah, K.S.A, two were gram-positive bacteria, namely, Bacillus cereus DSM 4312, Staphylococcus aureus (ATCC 25923) and six gram-negative; Enterococcus faecalis ATCC (29212), Escherichia coli O157:H7 (ATCC 43889), Klebsiella pneumonia ATCC (700603), Pseudomonas aeruginosa ATCC (27853), Salmonella typhimurium (ATCC 14028), Shigella sonnei, ATCC (25931).
10 mL nutrient agar media was sterilized as part of the preparation. The test organisms were added to this sterilized mixture and shaken vigorously before being transferred to a sterile petri dish via sterile loop and held aseptic. The test species were kept in a nutrient broth after an overnight incubation time at 37°C. They were then standardized at 560 nm to achieve a concentration of 106 colony-forming units per milliliter (CFU/mL).
Agar well diffusion assay: The antimicrobial activity of Moringa oleifera was investigated using the agar-well diffusion method. Fresh overnight cultures of bacteria (100L) were obtained for this purpose. These cultures were uniformly spread on a sterile surface using cotton swabs, and 50 l methanolic plant extracts were mixed in the agar-wells (7 mm). Aseptic conditions were maintained for the latter part of the process. Additionally, an equal volume of methanol (50 l) was added to one of the wells to serve as a negative control. At 37 °C, all the plates were incubated for 24 hours. The average zone of inhibition was calculated using CLSI guidelines, and the experiment was repeated three times.
Minimum bactericidal and minimal inhibitory concentrations (MICs): To evaluate the minimum bactericidal concentration and minimal inhibitory concentrations (MICs) The standard micro-dilution method in 96-well microtiter plates given by the Clinical Laboratory Standards Institute (CLSI) were carried out. The Moringa oleifera active metabolites were serial diluted two folds with Muller-Hinton broth medium then the selected pathogenic bacteria inoculated at 0.5 on the MacFarland scale then, final density be 6 x 106 CFU/well.
The plates were incubated for 24hr/35°C and the bacterial growth was measured using a Bio-Rad Microplate Reader at 600 nm. The lowest concentration that inhibiting the bacterial growth was determined as the MIC values. All experiments were carried in duplicate. The minimal bactericidal concentration (MBC) was performed after the above experiment, after incubation a 5 μL from each well without growth were be inoculated onto Muller-Hinton agar plates. The inoculated plates were incubated overnight at 30°C.
Cytotoxicity Assay: According to the manufacturer’s instructions, the cytotoxic effects of methanolic and Di ethyl ether extract of Moringa oleifera leaves Annexin FITC are used to assess the apoptotic activity of the ovarian cancer cell line (SKOV-3) in relation to tested compounds (BD Biosciences, USA). Cultivated at a density of 3 × 105 cells/well, both treated and untreated SKOV-3 cells were used for the induction of apoptosis for 48 hours. The FACS flow cytometer (BD FACSAria™ II – BD Biosciences) and BD FACSDiva™ Software (BD Biosciences, USA) were used for cell apoptosis analysis.
Statistical Analysis: Variations between the values of selected plant extract samples and controls was performed using a one-way analysis of variance (ANOVA). Statistical significance was described as a P value of less than 0.05.
RESULTS AND DISCUSSION
Total phenolic (TP) and total flavonoid (TF) contents: as shown in Table 1, extraction with methanol was found to provide the highest values of total phenolic and total flavonoid contents (14.70 ±0.28 mg of GAE per g of dried sample and 37.88±0.18 mg of QUE per g of dried sample respectively) in the leaves of Moringa oleifera as compared with extraction by Di ethyl ether (8.95 ±0.59 mg of GAE per g of dried sample and 29.30 ± 1.23mg of QUE per g of dried sample respectively).
Table 1. Total phenolic and flavonoid contents of different extracts of Moringa oleifera leaves
| Solvent extracts | Methanol | Di ethyl ether |
| Total phenolic (mg GAE/g d.w.) | 14.70 ±0.28 | 8.95 ±0.59 |
| Total flavonoid (mg QUE/g d.w.) | 37.88 ±0.18 | 29.30 ± 1.23 |
| Ratio (TP)/(TF) | 0.39 | 0.31 |
Total phenolic and total flavonoid contents are expressed as mean ± S.D (n = 3).
Figure 1: DPPH• scavenging activity (%) of methanolic and di ethyl ether crude extracts of Moringa oleifera. Vertical bars on the columns represent mean ± SD (n = 3).
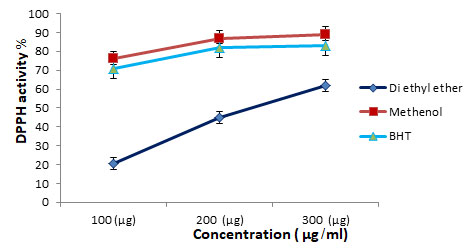
Antioxidant activities: Antioxidants react with DPPH•, reducing a number of DPPH• molecules equal to the number of their available hydroxyl groups. The methanolic extract exhibited the most potent DPPH• scavenging activity (76.9±0.41%) at concentration 100 µg/ml as compared to the Di ethyl ether extract which exhibited (20.7±0.12%) activity at the same concentration.
The same pattern of DPPH• scavenging activity was found in the methanolic and Di ethyl ether extracts at concentration 200 µg/ml and 300 µg/ml. The values were in the ascending order methanolic < BHT extract < Di ethyl ether extract These results indicated that methanolic extract exhibited the highest DPPH radical scavenging activity compared to BHT and the Di ethyl ether extract.
Antimicrobial activity: of the Methanol extracted Moringa leaves and the Di ethyl ether extracted Moringa leaves residue was determined in vitro, using disc diffusion and MIC method against selected eight pathogenic bacteria including two gram positive: Bacillus cereus DSM 4312, Staphylococcus aureus (ATCC 25923) and six gram negative: Enterococcus faecalis ATCC (29212), Escherichia coli O157:H7 (ATCC 43889), Klebsiella pneumonia ATCC (700603), Pseudomonas aeruginosa ATCC (27853), Salmonella typhimurium (ATCC 14028), Shigella sonnei, ATCC (25931). The bacterial growth inhibition of various Moringa leaves extracts was firstly tested and the result was shown in Table 2 &3.
In this study, Moringa leaves extracts were considered active against tested bacterial strains when the zone of inhibition was greater than 6 mm in according to the general rule for the antimicrobial activities of plant extracts (Eilert et al., 1981). The result showed that all Moringa leaves extracts inhibit the growth of Gram-positive bacteria as well as the Gram-negative bacteria. Methanol extracts showed varying degrees of antimicrobial activity on the microorganism tested.
The maximum zone of inhibition was seen in Shigella sonnei 19mg/ml, and the lowest was seen in Escherichia coli O157:H7 10mg/ml. It is thus established that Moringa leaves extracts by methanol contains compound that has antimicrobial property. While Moringa leaves extracted by Di ethyl ether did not show detectable suppression in growth of Escherichia coli O157:H7. The Moringa leaves extracted by Di ethyl ether were less active against all bacterial strains tested.
Table 2. The antimicrobial activities of Moringa leaves extract
| Food-borne pathogens | Zone of inhibition (mm) | |
| Methanol | Di ethyl ether | |
| Bacillus cereus DSM 4312 | 13 ± 6.9 | 10 ± 1.7 |
| Staphylococcus aureus (ATCC 25923) | 16.00±2.00 | 11 ±1.7 |
| Enterococcus faecalis ATCC (29212) | 14 ± 1.53 | 11 ± 0.0 |
| Escherichia coli O157:H7 (ATCC 43889) | 10 ± 1.73 | 00± 00 |
| Klebsiella pneumonia ATCC (700603) | 17.33 ± 0.57 | 14 ±0.0 |
| Pseudomonas aeruginosa ATCC (27853) | 17.00 ±6.93 | 11± 1.73 |
| Salmonella typhimurium (ATCC 14028) | 14 ± 3.0 | 10.33±0.58 |
| Shigella sonnei ATCC (25931) | 19 ± 1.73 | 12.00±1.00 |
Table 3. The minimum Inhibition concentration of Moringa leaves extract.
| Organisms
species |
Methanol | Di ethyl ether | ||
| MIC
(mg/ml) |
MBC
(mg/ml) |
MIC
(mg/ml) |
MBC
(mg/ml) |
|
| Bacillus cereus DSM 4312 | 100 ± 6.9 | 100±1.7 | 100± 3.46 | 200±0.0 |
| Staphylococcus aureus (ATCC 25923) | 100 ± 3.0 | 200±1.7 | 200±0.0 | <200± 1.73 |
| Enterococcus faecalis
ATCC (29212) |
100± 1.53 | 200± 0.0 | 200±0.0 | 200±0.0 |
| Klebsiella pneumonia
ATCC (700603) |
100± 3.46 | 200±0.0 | 100±0.0 | 200±0.0 |
| Pseudomonas aeruginosa ATCC (27853) | 50 ±3.00 | 100±1.73 | 100± 1.73 | <200± 1.73 |
| Salmonella typhimurium (ATCC 14028) | 50 ± 6.93 | 200± 0.0 | 100±0.0 | 200±0.0 |
| Shigella sonnei
ATCC (25931) |
100± 1.73 | 100± 1.7 | 200±0.0 | <200±1.0 |
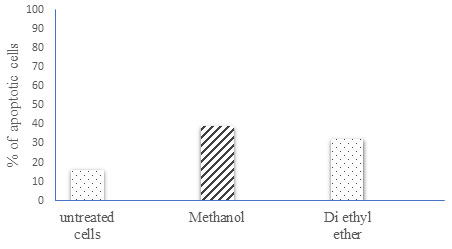
Quantitative analysis of cell apoptosis by flow cytometry: The various Moringa leaves extract used in this study all had varying ability to induce SKOV-3 cell apoptosis. This ability was measured using Annexin FITC staining (Fig 2). The findings indicate that all Moringa leaf extract induces apoptosis in the SKOV-3 ovarian cancer cell line when compared to untreated cells. Moreover, the apoptotic rate is significantly higher after 48 hours when compared to untreated cells. Methanol-treated cells showed the highest percentage of apoptosis, followed by Di ethyl ether extracts (i.e., 39.2 and 34.0 respectively).
Figure 2. Effect of Moringa oleifera on SKOV-3 cell apoptosis. Flow cytometry analysis of apoptosis in SKOV-3 cells either untreated or treated with 10 µg/ml of every compound for 48h. After the treatment period, the cells were stained with Annexin FITC and subsequently analyzed by flow cytometry.
The methanol extracts had higher TPC more than di ethyl ether extract, one possible explanation for this is that the fact that phenolic extraction is greater in more polar solvents, such as methanol, than in non-polar di ethyl extract. This extraction method is the first step in recovering and purifying bioactive compounds from plant materials. The enhanced recovery of antioxidant compounds with methanol is consistent with previous studies (Razali et al., 2012). The polarity of solvents played a crucial role in the extraction process as it would increase the solubility of antioxidant compounds.
While antioxidant activity has been observed in M. oleifera leaf extracts in both in vitro and in vivo conditions as a result of abundant phenolic acids and flavonoids (Verma et al., 2009). However, there are several variables that could influence the composition of M. oleifera tissues linked to their antioxidant function. For example, the season and location of development (Iqbal and Bhanger, 2006) and maturity (Sreelatha and Padma, 2009) have been shown to influence the antioxidant activity. Free radicals (included within lipid peroxidation) play a key role in a variety of chronic diseases, including cancer and cardiovascular disease (Dorman et al., 2003; Saleem et al., 2020; Ali et al., 2021).
At a concentration of 100 g/ml, the methanolic extract had the most potent DPPH, the same pattern of DPPH• scavenging activity was found in the methanolic and Di ethyl ether at concentrations of 200 g/ml and 300 g/ml, the methanolic and Di ethyl ether extracts displayed a similar pattern of DPPH• scavenging activity. Methanolic < BHT extract < Di ethyl ether extract, this ascending order was observed. In comparison to BHT and Di ethyl ether extract, these findings showed that methanolic extract had the highest DPPH radical scavenging activity.
Moringa oleifera methanolic extracts have a higher DPPH• scavenging activity, which may be attributed to their higher total phenolic and total flavonoid contents, as shown in table 1. These hydroxyl phenolic compounds can scavenge DPPH• by donating hydrogen atoms to it. Lu and Foo provided such a clarification (2001). The DPPH scavenging method is now widely used to investigate the antioxidant function of herb extracts (Chatha et al., 2006; Khor et al. 2018; Saleem et al., 2020; Ali et al., 2021).
It is well known that the solvents used for antioxidant extraction have a major effect on the DPPH scavenging capability determination. Indeed, because of their superior structural chemistry, free radical scavenging methods (DPPH) exhibit reduced alcoholic DPPH solutions in the presence of a hydrogen donating antioxidant (Koleva et al., 2002). Phenolic compounds, on the other hand, have been documented and given to be potent hydrogen donors to the DPPH radical (Mohamed et al., 2003).
The bacterial growth inhibition of various Moringa leaves extracts was firstly tested and the result was shown in Table 2. In this study, Moringa leaves extracts were considered active against tested bacterial strains when the zone of inhibition was greater than 6 mm in according to the general rule for the antimicrobial activities of plant extracts (Eilert et al., 1981). The result showed that all Moringa leaves extracts inhibit the growth of Gram-positive bacteria as well as the Gram-negative bacteria. Methanol extracts showed varying degrees of antimicrobial activity on the microorganism tested.
The maximum zone of inhibition was seen in Shigella sonnei 19mg/ml, and the lowest was seen in Escherichia coli O157:H7 10mg/ml. It is thus established that Moringa leaves extracts by methanol contains compound that has antimicrobial property. While Moringa leaves extracted by Di ethyl ether did not show detectable suppression in growth of Escherichia coli O157:H7. The Moringa leaves extracted by Di ethyl ether were less active against all bacterial strains tested. Similarly, Spiliotis et al. (1997) studied the antimicrobial activity of MO oil on various microorganisms and found that the oil was not effective against the microbial activity. However, Lalas et al. (2012), (Adeyinka et al., 2018; Das et al., 2020; Milla et al., 2021) assessed the antimicrobial activity of the oil of Moringa peregrina on various bacterial strains and the extracts proved effective against all microorganisms studied.
The variation in antimicrobial activity of MO extracts reported by different studies could be attributed to the differences in Moringa species and the different extraction method they used. As shown in Table 2, The MIC of the two extracts against test organisms ranged from 50 to 100 mg/mL with methanol having the highest activity at 50 mg/mL against Pseudomonas aeruginosa ATCC (27853) and Salmonella typhimurium (ATCC 14028). which demonstrated a significantly higher activity against the tested bacteria compared to the MO Di ethyl ether extract. For both extracts, the maximum MBC was found at 200 mg/mL. (Table 2).
The extract’s inhibitory activity, MIC, and MBC against the food pathogenic bacteria suggested that Moringa leaves could be used as antimicrobial agent. Adeyinka et al. (2018) reported Staphylococcus aureus, Salmonella typhi or Escherichia coli were sensitive to MO methanol extracts. Similarly, Saadabi and Abu Zaid (2011) also indicated that aqueous extract of MO showed a superior antibacterial activity against gram positive bacteria including Staphylococcus aureus and Bacillus subtilis. It is interesting to note that the presence of types of phytochemicals and their contents in the MO extracts dominates the antimicrobial activity of the extracts (Bukar et al., 2010; Das et al., 2020; Milla et al., 2021).
The type of the solvent used for extraction plays a dominant role on antimicrobial activity of the extract. Seleshe and Kang (2019) reported that MO extract from Methanol and chloroform showed significant antimicrobial activity against Klebsiella pneumoniae and Bacillus cereus, while Water extract showed the lowest inhibition against these microorganisms. In contrary, Ajaiyeoba (2002) and Bukar et al. (2010) indicated that MO extracts from polar solvent (ethanol and water) extraction were more active than the extracts from non- or less polar solvents such as chloroform. In relation to the extracts’ phytochemical content, the presence of saponins, alkaloids, tannins and flavonoids was confirmed to enhance the antimicrobial activity of the plants (Bukar et al., 2010; Sing and Bhat, 2003; Saleem et al., 2020; Ali et al., 2021).
This could possibly be attributed to the difference in the extraction method. This study evaluated the cytotoxic effect of crude extracts (Methanol and Di ethyl ether). The results of the study revealed a higher percentage of apoptosis in the Methanolic MO extract. Second in place was the Di ethyl ether extract. (Fernandes et al. 2016; Khor et al. 2018) confirmed that the cytotoxic effect of MO extracts was found to be selective to cancer cell lines and not to normal cell lines, which were found to be immune. The phytochemicals present in MO flower extract may be responsible for the high cytotoxic activity. Previous study revealed that presence of quinic acid in MO is chemo-preventive in nature (Padmini et al., 2013; Saleem et al., 2020; Ali et al., 2021).
CONCLUSION
Moringa oleifera extracts contain potent antioxidant compounds that have the potential to be applied in the pharmaceutical and food industries. The first step in obtaining these compounds is finding the best extraction solvent for subsequent biological applications. In comparison to Di ethyl ether extract, methanolic extracts of MO have higher antioxidant activity and antimicrobial capacity against foodborne pathogen, Moreover, they show anticancer capacity when tested over ovarian cancer cells. We conclude that the Methanolic solvent could be reasonable choice for antioxidant compounds extraction and the potential uses of M. oleifera as alternative natural preservatives in food products.
ACKNOWLEDGEMENT
This work was funded by the University of Jeddah, Saudi Arabia, under grant No. (UJ-49-18-DR). The authors, therefore, acknowledge with thanks the University technical and financial support.
Conflict of Interest: The authors declared that present study was performed in absence of any conflict of interest.
REFERENCES
Adeyink D., B. Timothy, N. Acharyna (2018). Antimicrobial activity of Moringa oleifera lam. Extract against some Food-Borne Microorganisms and some Human Pathogens. International journal of scientific research Vol 7 (5). DOI : 10.36106/ijsr.
Ali, A., Garg, P., Goyal, R., Kaur, G., Li, X., Negi, P., Valis, M., Kuca, K. and Kulshrestha, S., (2021). A Novel Herbal Hydrogel Formulation of Moringa oleifera for Wound Healing. Plants, Vol 10(1), p.25.
Amaglo N.K., R.N. Bennett, R.B. Lo-Curto (2010). Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem, Vol 122, pp. 1047–1054.
Brand-williams W, M.E. Cuvelier and C. Berset (1995). Use of free radical method to evaluate antioxidant activity. Lebensmittel Wissenschaft and Technologie, Vol 28(1), pp. 25-30.
Chang, C.C., MH. Yang, H.M. Wen, J.C. Chern (2002). Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J. Food Drug Anal, Vol 10, pp. 178-182.
Chatha S.A., F. Anwar, M. Manzoor, J.R. Bajwa (2006). Evaluation of the antioxidant activity of rice bran extracts using different antioxidant assays. Grasas Aceites Sevilla, Vol 57, pp. 328-335.
Clarke, D., A.A. Tyuftin, M.C. Cruz-Romero, D. Bolton, S. Fanning, S.K. Pankaj (2017). Surface attachment of active antimicrobial coatings onto conventional plastic-based laminates and performance assessment of these materials on the storage life of vacuum-packaged beef sub-primals. Food Microbiol, Vol 62 pp. 196–201.
Das, P.E., Abu-Yousef, I.A., Majdalawieh, A.F., Narasimhan, S. and Poltronieri, P., (2020). Green synthesis of encapsulated copper nanoparticles using a hydroalcoholic extract of Moringa oleifera leaves and assessment of their antioxidant and antimicrobial activities. Molecules, Vol 25(3), p.555.
Ekor, M. (2014).The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety, Frontiers in Pharmacology, Vol 4, pp. 1–10,
Fahey, J.W. (2005). Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Trees Life J. pp. 1-5.
Fernandes, E.E., A.V. Pulwale, G.A. Patil, A.S. Moghe (2016). Probing regenerative potential of Moringa oleifera aqueous extracts using in vitro cellular assays. Pharmacognosy Res. Vol 8(4), pp. 231–237.
Iqbal, S. and M.I. Bhanger (2006). Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Comp. Anal.Vol 19, pp. 544–55
Khor, K.Z., V. Lim, E.J. Moses, N. Abdul Samad (2018). The In vitro and in vivo anticancer properties of Moringa oleifera. Evid Based Complement Alternat Med. Vol 10(7), pp. 1243-1252.
Koleva, I.I., T.A.Van, J.P. Linssen, A. De Groot, L.N. Evstatieva (2002). Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal., Vol 13, pp. 8–17.
Mahmood, K.T., T. Mugal, I.U. Haq (2010). Moringa oleifera: A natural gift—A review. J. Pharm. Sci. Res. Vol 2, pp. 775–781.
Makkar, H.P. and S.K. Becker (1996). Nutritional value and antinutritional components of whole and ethanol extracted of Moringa oleifera leaves. Anim. Feed Sci. Technol., Vol 63, pp. 211–228.
Milla, P.G., Peñalver, R. and Nieto, G., (2021). Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants, Vol 10(2), p.318.
Mohamed, A.A., S.I. Ali and F.K. El-Baz (2013). Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PloS one Vol 8(4), e60269
Morgan, C.R., C. Opio, S. Migabo (2019). Chemical composition of Moringa (Moringa oleifera) root powder solution and effects of Moringa root powder on E. coli growth in contaminated water. S Afr J Bot.
Naseer, B., Iqbal, S., Wahid, N., Jamshaid Qazi, H., Nadeem, M. and Nawaz, M., (2021). Evaluation of antioxidant and antimicrobial potential of rutin in combination with butylated hydroxytoluene in cheddar cheese. Journal of Food Processing and Preservation, Vol 45(1), p.e15046.
Nikkon, F., Z.A. Saud, M.H. Rahman, M.E. Haque (2003). In vitro antimicrobial activity of the compound isolated from chloroform extract of Moringa oleifera Lam. Pakistan J. Biol. Sci. Vol 6, pp. 1888–1890.
Padmini, E. and I. Lakshmipathy (2013). Quinic acid as a potent drug candidate for prostate cancer – A comparative pharmacokinetic approach. Asian J Pharm Clin Res, Vol 6, pp. 211-222.
Ru, P., R. Steele, P.V. Nerurkar, N. Phillips, R.B. Ray (2011). Bitter melon extract impairs prostate cancer cell-cycle progression and delays prostatic intraepithelial neoplasia in TRAMP model. Cancer Prev Res (Phila), Vol 4, pp. 2122-2130.
Razali, N., S. Mat-Junit, A.F. Abdul-Muthalib, S. Subramaniam, A. Abdul-Aziz (2012). Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L., Food Chemistry Vol 131 (2), pp. 441-448.
Sapkota, R., R. Dasgupta, D.S. Rawat (2012) Antibacterial effects of plants extracts on human microbial pathogens & microbial limit tests. Int. J. Res Pharm. Chem, Vol 2(4), pp. 926–936.
Saleem, A., Saleem, M. and Akhtar, M.F., (2020). Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. South African Journal of Botany, Vol 128, pp. 246-256.
Seleshe, S., S.N. Kang (2019). In Vitro Antimicrobial Activity of Different Solvent Extracts from Moringa stenopetala Leaves. Prev Nutr Food Sci. Vol 24(1), pp. 70-74. doi:10.3746/pnf.2019.24.1.70
Sharma, V., R. Paliwal, P. Sharma and S. Sharma (2011). Phytochemical analysis and evaluation of antioxidant activities of hydro-ethanolic extract of Moringa oleifera Lam. pods. J. Pharm. Res, Vol 4, pp. 554–557.
Singh, K. and G.M. Tafida (2014). Antibacterial activity of Moringa oleifera (Lam) leaves extracts against some selected bacteria. Int J Pharm Pharm. Sci Vol 6, pp. 52–54
Singleton, V.L., R. Orthofer, R.M. Lamuela-Raventos (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, Vol 299, pp. 152–178.
Sreelatha, S. and P.R. Padma (2009). Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. Vol 64, pp. 303–31
Verma, A.R., M. Vijayakumar, C.S. Mathela, C.V. Rao (2009). In vitro and In vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem.Toxicol, Vol 47, pp. 2196–2201.


