1Department of Botany, Mahila Mahavidyalaya, Banaras Hindu University, Varanasi – 221005, Uttar Pradesh, India.
2Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi – 221005, Uttar Pradesh, India.
Corresponding author email: kumaridrnishi@yahoo.co.in
Article Publishing History
Received: 18/07/2020
Accepted After Revision: 10/09/2020
Sterculia alata belongs to family Sterculiaceae. It is a highly medicinal plant and its uses are already in practice in traditional medicines. Its leaves and bark have shown various biological activities. But there is utmost need to identify medicinal potential of other parts of the plant. Extracts of fruits and flowers were prepared in different solvents. Antioxidant activities were assessed by using two most common methods- through β-carotene and Linoleic acid assay and Hydrogen Peroxide (H2O2) scavenging assay. Disc diffusion methods were used to study antibacterial activities of extracts. Both fruit and flower extracts showed antioxidant and antibacterial activities. Aqueous fruit extract showed maximum hydrogen peroxide scavenging activity (80.13±0.29), whereas methanolic extract of flower extract showed comparatively less hydrogen peroxide scavenging activity (78.25±0.12).
Highest antioxidant activity (77.78±0.58) was observed by aqueous extract of fruit through β carotene/ linoleic acid assay. Lower antioxidant activity was shown by flower extract (ethanolic extract: 52.25±0.29). Antibacterial efficacies of fruit and flower extracts were observed against six resistant pathogenic bacteria (Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis ATCC, Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis). Flower extract showed maximum inhibition for E. coli (aqueous extract: 13.00± 0.29) and K. pneumoniae (methanolic extract: 13.33±0.67). Fruit extracts were found effective against S. aureus (aqueous extract: 13.00± 0.58), E. faecalis (methanolic extract: 15.00±0.04), P. mirabilis (methanolic extract: 14.00±0.29) and P. aeruginosa (ethanolic extract: 10.67±0.33). Both fruit and flower extracts can serve as the source of natural antioxidant and antibacterial.
Plant Extract, Antioxidant, Antibacterial, Inhibition Zone
Yadav A, Jaiswal P, Nath G, Kumari N. Antioxidant and Antibacterial Activities of Flower and Fruit of Sterculia alata. Biosc.Biotech.Res.Comm. 2020;13(3).
Yadav A, Jaiswal P, Nath G, Kumari N. Antioxidant and Antibacterial Activities of Flower and Fruit of Sterculia alata. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/32oQEFv
Copyright © Yadav et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Medicinal plants and their parts have been identified as highly useful in treating various diseases since ancient time. Several phytochemicals have shown their efficacies in treating many chronic and deadly diseases such as cancer, diabetes, neurological disorders, AIDS, etc. World Health Organization (WHO) has also witnessed the dependence of about 80% of world population over plant products for medicines. Pharmaceutical activities of any plant is directly associated with the types and concentration of its bioactive compounds (Shihabudeen et al, 2010: Gowri and Vasantha, 2010). Different solvents show different extraction efficiency and therefore, extracts prepared in different solvents show considerable different phytochemical activities (Ernst and Kuppan, 2013: Ngo et al, 2017).
Sedentary life style, poor intake of healthy diet, mental stress and illness are some major reasons for oxidative stress. Oxidative stress causes formation of free radicals. Reactive oxygen and reactive nitrogen species (ROS and RNS) are two major groups of free radicals (Tiwari et al, 2009: Meo et al, 2016). ROS and RNS are being produced due to oxidative stress and cell metabolism. Antioxidants play pivotal role in scavenging of free radicals by inhibiting or delaying process of oxidation and thus they contribute greatly in detoxifying organisms. Nowadays, plant products have been proved as efficient antioxidants and due to their nil or negligible side effects, they are considered superior over synthetic antioxidants. Many plants also show antimicrobial activities due to their phytochemicals. For example, terpenes, phenolics, defensins, essential oil, etc present in plant show potential antimicrobial activities. Such plant products can be good alternative of antibiotics and can deal effectively with multi drug resistant problem (Laws et al, 2019; Pacios et al, 2020).
Sterculia alata Roxb. syn. Pterygota alata popularly known as Buddha Coconut, is a large fast growing moist, deciduous and evergreen bark tree. It belongs to Sterculiaceae family. It is distributed mainly in tropical Asia (Lin et al, 2010). In India, it is found in western ghats, south and central Sahyadris. The plant is an important forest tree and medicinally very important. Due to fast growth, its plantation is being done for afforestation purpose. Presence of several phytochemicals (phenolics, flavonoids, steroids, anthraquinones) have been identified from leaf and stem extracts of Sterculia alata (Jahan et al, 2014; Jahan et al, 2014; El-Sherei et al., 2018). Omran et al (2019) have observed antioxidant and antimicrobial activities of leaves of Sterculia alata. Various studies showed medicinal importance of S. alata. Biosynthesis and accumulation of secondary metabolites in different organs of same plant may be different and it has been observed by their varied distribution patterns at tissue and organ level (Zribi et al, 2014; Azadeh et al, 2020). Thus, extensive study is needed to identify all possible phytochemicals of a plant and to categorize their biological activities. Flowers and fruits of Sterculia alata are still unexplored and identification of their biological activities is highly required. Present study deals with the study of antioxidant and antibacterial properties of flower and fruit extracts of Sterculia alata.
MATERIAL AND METHODS
Collection of Plant Material: Flowers and fruits of S. alata were collected from the campus of Banaras Hindu University, Varanasi during the month of February and March. Flowers and fruits were cleaned under running tap water. Pericarp (separated from fruit) and flowers was shade dried, oven dried at 40-45°C for 2 hour and then grinded in mechanical grinder to make course powder. Extraction was done from 20g of flower and fruit powder in 250 mL of solvent by using a soxhlet apparatus for 24 hours. Ethanol, methanol and double distilled water were used as solvents for extraction. Extracts were then dried at 40°C in rotary evaporator. Extracts were stored at -20°C till use. Percentage yield (w/w) of crude extract was calculated using formula:
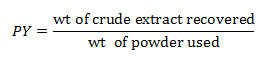
Where PY is percentage yield of extact
Preparation of Sample Extract for Different Assays: To prepare stock samples, about 100 mg extract was dissolved in 50 ml of respective solvent, final concentration 2mg/ml for antioxidant assays. Different volume of samples was used from the stock for various experiments. Stock samples of concentration 100 mg/ml in dimethyl sulphoxide (DMSO) was prepared for antibacterial activity. For the test of susceptibility about 5µl of extracts was taken onto sterile disc.
Antioxidant Activity through β-carotene and Linoleic Acid Assay: Elzaawely et al (2007) method was used with some modifications for β-carotene bleaching assay. For the preparation of stock solution, β- carotene of concentration 2mg/ml was dissolved in chloroform and mixed with 20 µl of linoleic acid followed by addition of 200µl of Tween-20 in a round bottom flask. 50 ml of double distilled water was added in the residue left after complete evaporation of chloroform with vigorous stirring to form an emulsion. Extract (800 µl) was added in the test tube containing 2400µl of emulsion and immediate absorbance was recorded at 470 nm against the blank solution. After that tubes were incubated for 2 h at 50o C then absorbance was recorded. For control, DMSO was added in emulsion in place of plant extract. Percent inhibition was calculated as
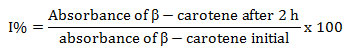
Hydrogen Peroxide (H2O2) Scavenging Assay: Hydrogen peroxide scavenging activity was assessed according to Bokhari et al (2013) with some modifications. For stock solution, H2O2 (4mM) solution was prepared in phosphate buffer (50 mM, pH 7.4). 2 ml (100mg/ml) of plant extract was mixed with 3 ml of H2O2 and absorbance at 230 nm was recorded after 10 min. Phosphate buffer without H2O2was served as blank while H2O2 solution without sample is taken as control. Percent hydrogen peroxide scavenging activity was determined as

Media Preparation and Test Microorganisms for Antibacterial Assay: For bacterial media preparation, Muller Hinton agar (38g/L) and agar (10g/L) were dissolved in double distilled water. Saline (8.5 g/L) was prepared by dissolving in double distilled and autoclaved for 15 min at 1.1kg/cm2 and 121ºC. About 20 mL sterile media was used for plating. Bacterial cells were grown on MHA (Himedia, Mumbai) for 24 h at 37 ºC to form bacterial inoculums. The bacterial suspension turbidity was maintained at 0.5 McFarland turbidity standards (approximately 1×107 CFU/mL). For assessment of antibacterial activity, six bacteria (both gram positive and gram negative) were selected. Test organisms selected were Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis ATCC, Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis.
Bacterial cultures were obtained from Department of Microbiology, Institute of Medical Sciences, BHU, Varanasi, India. Broth cultures of young bacteria were maintained for screening experiments. For antibacterial activity, disc diffusion method (Murray et al., 1995) was used. Bacterial suspension was evenly distributed over the surface of solidified media by using a sterile swab and allowed to dry for 5 min. About 5µL extract was loaded to each sterile disc followed by placing disc on medium surface. DMSO was taken as negative control and plates were incubated in BOD (Remi) incubator for 24 h at 37 ºC. Specific standard drug Streptomycin was used against all Gram positive and Gram negative bacteria. Zones of inhibition were measured in millimeters.
Statistical analysis: All experiments were carried out in triplicates and repeated thrice independently. Data were presented by using SPSS software (version 16 ,Chikago, USA). Data were represented as mean ±SE.
RESULTS AND DISCUSSION
Oxidative stress induced by free radicals is the root cause of several diseases. Intake of antioxidants – both synthetic and natural provides protection from the damage of free radicals. They act as free radical scavengers and prevent or delay the process of oxidation. Plants are most commonly known reservoir of natural antioxidants and they can serve as the source of cost-effective antioxidants. Natural antioxidants generally show almost negligible side effects. Several secondary metabolites such as phenolics, flavonoids, carotenoids, cinnamic acids, benzoic acids, folic acid, ascorbic acid, etc. show antioxidant activities (Hollman, 2001; Sasikumar and Kalaisezhiyen, 2014). Similarly, many phytochemicals such as phenolics, flavonoids, alkaloids, essential oils, etc have shown strong antimicrobial activities (Silva and Fernandes, 2010; Seyidoglu and Aydin, 2020). β- carotene and linoleic acid assay is one of the rapid method to screen antioxidants. Two or more methods are preferred for the assessment of antioxidant activities of any plant specimen to get more reliable result. Therefore, another method to analyze antioxidant activities i.e. H2O2 scavenging activity was also performed. H2O2 scavenging activity of extracts may be due to their antioxidant constituents present in them which donate electrons to H2O2 to neutralize it into H2O (Ebrahimzadeh et al., 2009).
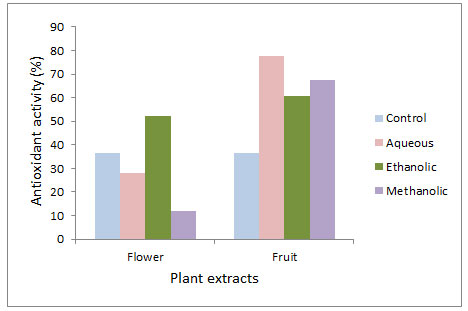
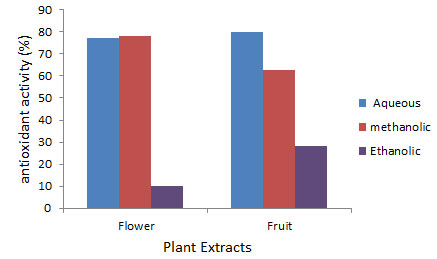
In present study, antioxidant activities of fruit extracts in all solvents were observed better than flower extracts. Antioxidant potential of fruit was observed maximum in aqueous extract (77.78±0.58), whereas activities in methanolic (67.56±0.54) and ethanolic (60.91±0.45) extracts were also significantly high (Fig 1). In flower, maximum antioxidant activity was recorded in ethanolic extract (52.25±0.29), then in aqueous extract (27.84±0.28) and minimum in methanolic extract (11.88±0.32) (Fig 1). Hydrogen peroxide scavenging activity was also observed higher in fruit extract than flower extract. In fruit extract, maximum scavenging activity was reported in aqueous extract (80.13±0.29), significant activity was observed in methanolic extract (62.88±0.08) and minimum in ethanolic extract (28.31±0.69). In flower, methanolic (78.00±0.12) and aqueous extracts (77.14±0.10) showed significant responses (78.00±0.12), but ethanolic extract (10.11±0.31) was not observed effective.
Figure 1: Antioxidant activity through β-carotene/linoleic acid bleaching assay in flower and fruit extract of Sterculia alata.
In present work, six antibiotic resistant test microorganisms were taken to assess antimicrobial effect of plant extracts. All test organisms selected were resistant (Cheesan et al, 2017) and their details are as such- S. aureus (Penicillin resistant; Methicillin resistant S. aureus – MRSA), Enterococcus faecalis (vanomycin resistant), Klebisiella pneumonia (XDR -extensively drug resistant), Proteus mirabilis (chloramphenicol and erythromycin resistant) and E.coli (MDR – multi drug resistance), Pseudomonas aeruginosa (Zerbaxa resistant). Antibacterial activities of plant extracts will pave novel way of treatment of diseases caused by them. The current number of antibacterials is not enough for controlling the evolution of MDR and XDR bacteria. Therefore, many pharmaceutical companies are in search of new antibacterial which can reverse the mechanism of bacterial resistance (Isah, 2019; Pacios et al, 2020).
Figure 2: Hydrogen peroxide scavenging activity in the flower and fruit extracts of Sterculia alata.
Flower and fruit extracts were taken for the assessment of their antibacterial activity. Results of antibacterial activity are shown in Table 1 and 2. Flower and fruit extracts exhibit antibacterial activity against most of the pathogenic bacteria with different potency. Flower extract showed significant inhibition against E. coli (13.00±0.29), E. faecalis (12.33±0.17), K. pneumonia (13.33±0.67) and P. mirabilis (13.83±0.88), but was found least effective against S. aureus (maximum in aqueous extract- 10.33±0.67) and P. aeruginosa (maximum in ethanolic extract- 8.67±0.67). Fruit extract showed significant antibacterial potential against E. faecalis (15.00±0.04), P. mirabilis (14.00±0.29), K.pneumoniae (13.30±0.17), S.aureus (13.00±0.58) and E.coli (11.67±0.44), but inhibition was comparatively low against P.aeruginosa (10.67±0.33).
Table 1. Antibacterial activity of flower extract of Sterculia alata
| Test organisms Inhibition zone diameter (mm) | |||||
|
Aqueous |
Ethanolic |
Methanolic |
Control |
Standard(5µg/ml) |
|
| E.coli
|
13.00±0.29 | 11.83±0.58 | 9.17±0.60 | 0.00±0.00 | 34.17±0.16 |
| S.aureus | 10.33±0.67 | 7.17±0.60 | 10.00±0.58 | 0.00±0.00 | 32.33±0.33 |
| E. faecalis | 10.33±0.33 | 12.33±0.17 | 10.50±0.29 | 0.00±0.00 | 41.50±0.76 |
| K. pneumonia | 7.33±0.89 | 10.00±0.58 | 13.33±0.67 | 0.00±0.00 | 35.50±0.29 |
| P. mirabilis | 8.67±0.33 | 13.83±0.88 | 12.83±0.83 | 0.00±0.00 | 17.33±0.89 |
| P.aeruginosa | 6.00±0.58 | 8.67±0.67 | 7.67±0.17 | 0.00±0.00 | 42.33±0.17 |
Data are means of three replicates (n=3) ± standard error.
Table 2. Antibacterial activity in fruit extracts of Sterculia alata.
| Test organisms Inhibition zone diameter (mm) | |||||
| Aqueous | Ethanolic | Methanolic | Control | Standard(5µg/ml) | |
| E.coli
|
11.67±0.44 | 11.00± 1.15 | 7.83±0.44 | 0.00±0.00 | 39.00±0.50 |
| S.aureus
|
13.00±0.58 | 10.30±0.17 | 12.50±0.29 | 0.00±0.00 | 37.50±0.29 |
| E. faecalis
|
6.17±0.60 | 9.66±0.33 | 15.00±0.04 | 0.00±0.00 | 35.17±0.60 |
| K.pneumoniae
|
0.00±0.00 | 9.50±0.29 | 13.30±0.17 | 0.00±0.00 | 42.33±0.17 |
| P. mirabilis
|
0.00±0.00 | 11.00±0.50 | 14.00±0.29 | 0.00±0.00 | 18.00±0.58 |
| P.aeruginosa | 6.80±0.44 | 10.67±0.33 | 8.50±0.76 | 0.00±0.00 | 45.17±0.13 |
Data are means of three replicates (n=3) ± standard error.
There are many factors, which affects antibacterial potential- type of extract used, its concentration, solvents used in extraction and type of bacteria. Extracts prepared in different solvents show different antioxidant and antimicrobial activities due to different dissolving capacities of metabolites (Altemimi et al., 2017 and Ngo et al., 2017). There are many mechanisms by which phytochemicals act against microbes- by disrupting cell wall of bacteria (Burt 2004, Gill and Holley 2006) or by changing the permeability of bacterial cell membrane and mitochondria (Tiwari et al., 2009). Resistance gene of the bacteria may code for efflux pumps which eject antibiotic from the cells or by inducing enzymes for degradation or inactivation of antibiotic. Many plants possess MDR pump inhibitors to enhance the activity of their own natural antimicrobial compounds. Extracts of such plants can be highly effective if used in combination of ineffective or resistance prone antibiotics (Morel et al, 2003; Abreu et al, 2012). Thus, plants rich in secondary metabolites show significant antioxidant and antimicrobial properties and it is utmost need to explore such plants (Isah, 2019; Pacios et al, 2020).
ACKNOWLEDGEMENTS
The authors are thankful to Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India for providing test organisms for antibacterial studies.
Conflict of Interests: The authors declare that they have no conflict of interest.
REFERENCES
Abreu A.C., McBain A.J. and Simões M. (2012) Plants as sources of new antimicrobials and resistance – modifying agents. Natural Product Reports Vol. 29:1007–21
Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G. and Lightfoot D.A. (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants Vol.6 No.42: 1-23
Azadeh Z., Saeidi K., Lorigooini Z., Kiani M. and Maggi F. (2020) Organ- oriented phytochemical profiling and radical scavenging activity of Alcea spp. (Malvaceae) from Iran. SN Applied Sciences Vol.2: 927
Bokhari J., Khan M.R., Galium A., Umbreen R., Shumaila J. and Zai J.A. (2013) Evaluation of diverse antioxidant activities of Galium. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Vol.102: 24-29
Burt S. (2004) Essential oils: their antibacterial properties and potential application in foods: a review. International Journal of Food Microbiology Vol. 94: 223-253
Cheesan M.J., Ilanko A., Blonk B. and Cock I.E. (2017) Developing new antimicrobial therapies: are synergistic combinations of plant extracts/ compounds with conventional antibiotics the solution? Pharmacognosy Review Vol.11 No.22: 57-72
Ebrahimzadeh M.A., Nabavi S.F. and Nabavi S.M. (2009) Antioxidant activities of methanol extract of Sambucus ebulus L. flower. Pakistan Journal of Biological Science Vol.12: 447-450
El-Sherei M.M., Ragheb A.Y., Mosharrafa S.A., Marzouk M.M., Kassem M.E.S. and Saleh N.A.M. (2018) Pterygota alata (Roxb.) R. Br., Chemical constituents, anti- hyperglycemic effect and anti-oxidative stress in alloxan- induced diabetic rats. Journal of Materials and Environmental Science Vol. 9 No.1: 245- 255
Elzaawely A.A., Xuan T.D., Koyama H. and Tawala S. (2007) Antioxidant activity and contents of essential oil and phenolic compounds in flower and seeds of A. zerumbet (pers) B.L. Burtt and R.M. Sm. Food Chemistry Vol.104: 1648-1653
Ernest D. and Kuppan E. (2013) A review on antimicrobial efficacy of some traditional medicinal plants in Tamil Nadu. Journal of Acute Disease Vol.2 No.2: 99-105
Gill A.O. and Holley R.A. (2006) Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatis. International Journal of Food Microbiology Vol. 108: 1-9
Gowri S.S. and Vasantha K. (2010) Phytochemical screening and antibacterial activity of Syzygium cumini (L.) (Myrtaceae) leaves extracts. International Journal of PharmTech Reasearch. Vol.2: 1569-1573
Hollman P.C.H. (2001) Evidence for health effects of plant phenols: local or systemic effects? Journal of the Science of Food and Agriculture Vol. 81: 842–852
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biological Research Vol. 52:39
Jahan N., Parvin M.S., Das N., Islam M.S. and Islam M.E. (2014a) Studies on the antioxidant activity of ethanol extract and its fractions from Pterygota alata leaves. Journal of Acute Medicine Vol. 4:103-108
Jahan N., Parvin M.S., Khan A., Das N., Islam M.S. and Islam M.E. (2014b) Evaluation of free radical Scavenging and polyphenolic contents of bark of Pterygota alata Roxb. Journal of Scientific Research Vol.6: 543-552
Laws M., Shaaban A. and Rahman K.M. (2019) Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiology Reviews Vol. 43: 490-516
Lin L., Song Z. and Xu H. (2010) A new phenylpropanoid galactoside and other constituents from Pterygota alata (Roxb.) R. Brown. Biochemical Systematics and Ecology Vol. 38: 1238-1241
Meo S.D., Read T.T., Venditti P. and Victor V.M. (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Medicine and Cellular Longevity Vol. 2016: 1245049
Morel C., Stermitz F.R., Tegos G. and Lewis K.(2003) Isoflavones as potentiators of antibacterial activity. Journal of Agricultural Food Chemistry Vol. 51: 5677–9
Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C. and Yolken H.R. (1995) Manual of Clinical Microbiology, 6th Ed. ASM Press, Washington DC: 15-18
Ngo T.T.V., Scarlett C.J., Bowyer M.C., Ngo P.D. and Vuong Q.V. (2017) Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. Journal of Food Quality Vol. 2017: 1-8
Omran M.E., Azza A.S. and Abdel- Rahman S.E. (2019) Chemical constituents, antioxidant and antimicrobial activities of Pterygota alata (Roxb.) leaves extracts grown in Egypt. Novel Research in Microbiolology Vol. J 3 No.3: 366-378
Pacios O., Blasco L., Bleriot I., Fernandez- Garcia L., Bardanca M.G., Ambroa A., Lopez M., Bou G. and Tomas M. (2020) Strategies to combat multidrug- resistant and persistent infectious diseases. Antibiotics, Vol. 9: 65, doi:10.3390/antibiotics9020065
Sasikumar V. and Kalaisezhiyen P. (2014) Evaluation of free radical scavenging activity of various leaf extracts from Kedrostis foetidissima (Jacq.) Cogn. Biochemistry and Anaytical Biochemistry Vol. 3: 150. doi:10.4172/2161-1009.1000150
Seyidoglu N. and Aydin C. (2020) Stress, Natural Antioxidants and Future Perspectives, Chapter, In: The Health Benefits of Foods – Current Knowledge and Further Development. IntechOpen, DOI: http://dx.doi.org/10.5772/intechopen.91167
Shihabudeen M.H., Priscilla D.H. and Thirumurugan K. (2010) Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants. International Journal of Pharmaceutical Sciences and Research Vol. 1: 430-440
Silva N.C.C. and Fernandes J. (2010) Biological properties of medicinal plants: a review of their antimicrobial activity. The Journal of Venomous Animals and Toxins including Tropical disease Vol. 16 No.3:402-413
Tiwari B.K., Valdramidi V.P., O’Donnell C.P., Muthukumarappan K., Bourke P. and Cullen P.J.(2009) Application of natural antimicrobials for food preservation. Journal of Agricultural Food Chemistry Vol. 57: 5987-6000
Zribi I., Omezzine F. and Haouala R. (2014) Variation in phytochemical constituents and allelopathic potential of Nigella sativa with developmental stages. South African Journal of Botany Vol. 94: 255-262