Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh, Assam, India
Corresponding author email: rsmrpal@gmail.com
Article Publishing History
Received: 13/04/2020
Accepted After Revision: 30/05/2020
Ethnomedicinal survey documents the traditional usefulness of Oxalis debilis Kunth leaves in the management of diabetes mellitus in North-eastern region of India. This study screens the hydro-alcoholic extract (HAE) of leaves of O. debilis for antidiabetic activity in streptozotocin-induced diabetic rats along with antioxidant activity. The antidiabetic activity of HAE was evaluated in streptozotocin-induced diabetic rats at the doses of 250 and 500 mg/kg body weight. Results of antidiabetic activity study revealed that HAE of O. debilis leaves possesses significant hypoglycemic activity (100.52±14.94 and 78.79± 9.67 at 250 and 500 mg/kg, respectively) in diabetic rats (322.64± 11.86) as compared to normal control group (99.88 ± 2.58) after 21st day of treatment. he metformin (5 mg/kg) treated rats also showed significant reduction (76.99 ± 8.63) in plasma glucose level when compared to normal rats. HAE also exhibited promising antioxidant potential in experimental rats. Results indicated possible role of the HAE as herbal antioxidants in the prevention and/or treatment of oxidative stress induced diabetes. The phenolic/flavonoid contents of HAE having antioxidant potential might be responsible for antidiabetic property of O. debilis leaves.
Antidiabetic, Antioxidant, O. debilis, Oxidative stress, Plant flavonoids
Junejo J. A, Zaman K, Hussain N, Rudrapal M, Khan A. Antidiabetic and Antioxidant Activity of Hydro-Alcoholic Extract of Oxalis Debilis Kunth Leave in Experimental Rats. Biosc.Biotech.Res.Comm. 2020;13(2).
Junejo J. A, Zaman K, Hussain N, Rudrapal M, Khan A. Antidiabetic and Antioxidant Activity of Hydro-Alcoholic Extract of Oxalis Debilis Kunth Leave in Experimental Rats. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3g2rL7h
Copyright © Junejo et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Despite the availability of hypoglycemic agents from synthetic sources, diabetes is still life-threatening because of limited therapeutic utility of existing drugs (Riaz et al., 2020). Traditional medicines derived from plants play a significant role in the management of diabetes mellitus (Junejo et al., 2018). WHO recommended the evaluation of traditional plant remedies used in the treatment of diabetes because they are effective with less or no toxicities as compared to synthetic oral hypoglycemic agents (Junejo et al., 2017). Many indigenous Indian medicinal plants have been found to be useful in the treatment of diabetes mellitus (Sekhin-Loodu et al., 2019).
Oxalis debilis (Oxalidaceae) is a tristylous species native to Southern America and is a member of the bulb-forming shrub and distributed widely throughout the world. It is abundantly found in the Brahmaputra valley region of India (Junejo et al., 2006). The traditional uses of herbs and their extracts have been to cure human ailments since ancient times. It has been used traditionally for the treatment of dysentery and diarrhea (Kumar et al., 2012). In modern literature, antioxidant, anticancer, anti-inflammatory, analgesic, antimicrobial, antiamoebic, antifungal, astringent, diuretic and febrifuge activities of this plant species have also been reported (Panda et al., 2016; Rehman et al., 2015).
Ethnobotanical study indicates that leaf decoction of O. debilis have been used by tribal people of North-eastern states of India for the management of diabetes. There are no scientific reports in modern literature on the antidiabetic efficacy of O. debilis leaves. The objective of our present study was to ascertain the scientific basis of using this particular plant species traditionally in the management of diabetes, using streptozotocin-induced diabetic rats. According to WHO guidelines (Junejo et al., 2020c), he extract of O. debilis leaves was prepared using hydro-alcoholic solvent and evaluated for the antidiabetic activity. It has been reported that traditional medicinal plants having antidiabetic activity possess antioxidant potential in experimental animals (Irudayaraj et al., 2012; Debasis et al., 2010). Moreover, since a biochemical relationship exists between diabetic hyperglycemia and cellular oxidative stress, the antidiabetic activity evaluation of the hydro-alcoholic extract (HAE) of O. debilis leaves was carried out along with the study of in vivo antioxidant activity.
MATERIALS AND METHODS
All chemicals and reagents used in the study were of analytical grade and were procured from Rankem, Mumbai and Himedia Laboratories Ltd., Mumbai. Streptozotocin (STZ) was procured from Sigma-Aldrich, Germany. Commercial reagent kits used for determination of biochemical parameters and enzymatic assays were purchased from SPAN Diagnostics Ltd., Surat (India).
Fresh leaves of Oxalis debilis were collected from forest areas of Dibrugarh district, Assam (India) during the month of December 2014. The plant species was identified and authenticated (BSI/ERC/2014/Plant identification/360, dt. 26.08.2014) at the Botanical Survey of India, Eastern Regional Centre, Botanical Survey of India, Eastern Regional Centre, Shillong (India). A voucher specimen (DU/PSC/HRB/B-11/2014) of the identified plant species was deposited in the Herbarium of the Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh.
Preparation of hydro-alcoholic extract (HAE):The air-dried leaves were coarsely powdered (Sieve no. 40) using a cutter mill, and 50 g of powdered leaves was used for the preparation of extract. Powdered leaves were extracted using sufficient quantity (400 ml) of ethanol-water mixture (7:3) by cold maceration for 24 h. The extraction was carried out successively thrice and the combined extract was then concentrated under reduced pressure to dryness in a rotary vacuum evaporator to obtain a thick semisolid-like paste. The crude extract was dried at -40 °C in a lyophilizer and the dried extract (dark brown colour) so obtained was stored in a desiccator until further use. The percentage yield of the dried hydro-alcoholic extract (HAE) was calculated per dry weight of powdered leaves.
Estimation of total phenolic and flavonoid contents (TPC and TFC):The total phenolic content of the HAE was evaluated following the Folin-Ciocalteu colorimetric method and results were expressed as mg of gallic acid equivalent (GE) per g of dry weight of the extract. The total flavonoid content was estimated using the aluminum chloride colorimetric method and results were expressed as mg of quercetin equivalent (QE) per g of dry weight of the extract (Junejo et al; 2020a; Chang et al., 2002). The total phenolic content was calculated from the calibration curve of gallic acid (20, 40, 60, 80, 100 µg/ml, 90% ethanol). The total flavonoid content was calculated from a calibration curve of quercetin (20, 40, 60, 80, 100 µg/ml, 90% ethanol). Results were obtained as mean ± SEM of three replicate studies.
Test animals:Healthy Wistar male albino rats (240–260 g) were maintained under standard environmental conditions (temperature 25±2 °C, relative humidity 50±5 %) with a 12 h light / dark cycle. They were fed on with normal laboratory chow pellet diet and drinking water was given ad libitum. Animals were allowed to acclimatize for 7 days before commencement of the experiment. The animals were used with the approval of the Institutional Animal Ethics Committee (Approval no. IAEC/DU/50 dt. 24.9.13) under guidelines set by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi (India).
Acute oral toxicity study:Over-night fasted rats were randomly divided into six groups of six animals each. Rats of different groups were administered with increasing doses (250, 500, 1000, 2000 and 5000 mg/kg b.w.) of the HAE. One group was maintained as normal control and was given vehicle alone. The acute toxicity study was done as per OECD guideline-423 (Junejo et al., 2020b; Oliveira et al., 2008).
Oral Glucose tolerane (OGT) test :This test was performed in overnight fasted normal rats according to the method reported by Junejo and co-workers (Junejo et al., 2014).
Hypoglycemic activity in STZ-induced diabetic rats:Diabetes was induced (Amira et al., 2016) in overnight fasted animals by a single intraperitoneal (i.p) injection of streptozotocin (STZ, 55 mg/kg b.w. in normal saline). The animals confirmed as diabetic (after 72 h of STZ injection) by the elevated plasma glucose levels (200-300 mg/dl) was used for the experiment. The animals were divided randomly into five groups of six rats in each group. Group I rats served as normal control and were given vehicle (0.5% CMC w/v in normal saline) alone. Group II rats served as diabetic control and were administered with vehicle alone. Group III and IV were treated with HAE at 250 mg/kg b.w. and 500 mg/kg b.w., respectively. Group V rats were received the standard drug, metformin hydrochloride (5 mg/kg b.w.). Treatments were given orally using a canula once daily for a period of 21 days. Blood was collected from the tail vein each time for the determination of glucose levels on 0, 7, 14 and 21 day. Blood glucose levels were measured by the GOD-POD method.
Liver and kidney function tests:The initial and final body weights were measured. Liver tissues were excised, blotted, weighed and stored at -70 oC for assay of glycogen content. Liver glycogen was estimated by the method of Carrol et al. (Carroll et al., 1956). Blood was collected by cardiac puncture in dry test tubes containing a mixture of potassium oxalate and sodium fluoride (1:3) and the serum was separated by centrifugation (2000 rpm, 10 min) for estimation of various biochemical parameters.
Serum insulin levels were measured by the microplate ELISA method using a commercial kit (SPAN Diagnostics Ltd.). Serum lipid profile was also estimated using commercially available kits (SPAN Diagnostics kit). Triglycerides (TG) and Total cholesterol (TC) were estimated by enzymatic methods (HDL (How density lipoprotein) cholesterol by phosphotungstate method and LDL (low density lipoprotein) cholesterol were calculated by Friedewald’s formula (Friedewald et al., 1972).Serum was used to estimate glutamate oxaloacetate transaminase (GOT) glutamate pyruvate transaminase (GPT) and alkaline phosphatase (ALP), total protein (TPR) and creatinine (CRTN). SGOT and SGPT were measured by UV kinetic method and ALKP was estimated by PNPP method. TPR was measured by Bradford Macro method (Bradford, 1976), while CRTN was by picrate method (Saligman et al., 1950).
In vivo antioxidant activity:On 21st day, all the groups of animals were anaesthetized using diethyl ether, liver was dissected out, washed with normal saline and one part was preserved in 10% formalin for histopathological studies. The other part of liver was homogenized by ice chilled Tris-HCl buffer and used for activities/levels of superoxide dismutase catalase, reduced glutathione (GSH), glutathione peroxidase (GPx), and malondialdehyde MDA. The malondialdehyde (MDA) production is a direct indicator of lipid peroxidation (LPO) process that was measured by TBA reaction using an ELISA reader (at 532 nm) (Minami and Yoshikawa, 1979; (Xu et al., 1997).
Histopathological studies: At the end of 21th day of treatment, the animals were fasted for 12 h, anaesthetized using diethyl ether and sacrificed by cervical dislocation. Pancreas was instantly dissected out, excised and rinsed in ice-cold saline solution. Tissue was processed further histopathological observations (Irudayaraj et al., 2012; Kumar et al., 2011; Ozbek et al., 2017).
Statistical analysis:Values are represented as mean ± SEM of three replicate studies. Statistical analysis was performed using the IBM SPSS 19.0 statistical software package, for Windows. Statistical differences at 5% level of probability (p < 0.05) between the groups were analyzed by one-way ANOVA followed by Student’s t-test (Sabu et al., 2002).
RESULTS AND DISCUSSION
TPC and TFC of HAE :The total phenolic content of the HAE, calculated from the calibration curve of gallic acid (R2 = 0.984), was 56.34 ± 2.09 mg gallic acid equivalent (GE) /g of dry wt. of HAE, and the total flavonoid content (R2 = 0.987), calculated from the calibration curve of quercetin was 45.22 ± 2.31 mg quercetin equivalent (QE) /g of dry wt. of HAE.
Acute toxicity study
No sign and symptoms of acute toxicity and mortality up to 2000 mg/kg body weight dose were observed during the whole experimental period. The body weight and food consumption were normal compared to vehicle treated rats. For further studies, the doses were fixed as 250 and 500 mg/kg body weight.
Effect of HAE on OGT test in normal rats:In OGT, HAE (250 & 500 mg/kg) showed significant (p < 0.05) reduction of glucose load (plasma glucose level) as compared to normal control group. The metformin (5 mg/kg) treated group also showed significant (p < 0.05) activity compared to normal control group (Fig. 1).
Figure 1: OGT test. Values are mean ± SEM of three replicate experiments. Activities of HAE and metformin (metformin) are statistically significant at p < 0.05, compared to normal control
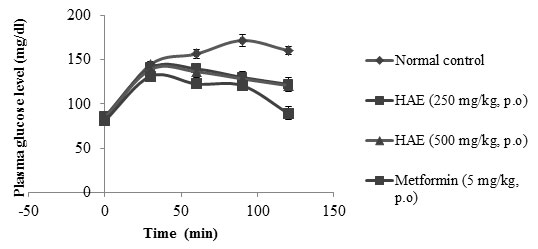
Effect of HAE on blood glucose levels in diabetic rats:STZ-treated diabetic rats exhibited significant increase in the levels of blood glucose in comparison to normal rats. After treatment with HAE the blood glucose levels were significantly (p<0.05) reduced compared to the diabetic control rats at both the doses, viz. 250 & 500 mg/kg. The metformin (5 mg/kg) treated rats also showed significant (p < 0.05) reduction in plasma glucose level when compared to normal rats. Results of the effect of HAE on blood glucose levels in normal and diabetic rats are depicted in Table 1.
Table 1. Effect of HAE on blood glucose levels in diabetic rats
| Group | Days | |||
| 0 day | 7th day | 14th day | 21st day | |
| Normal control | 94.39 ± 2.71 | 102.86 ± 3.51 | 103.49 ± 3.99 | 99.88 ± 2.58 |
| Diabetic control
STZ (55 mg/kg, i.p) |
266.72 ± 8.29 | 289.21 ± 9.57 | 298.12 ± 6.58 | 322.64± 11.86 |
| Diabetic + HAE
(250 mg/kg, p.o) |
252.91± 8.34 | 216.55± 5.21* | 133.72± 7.98* | 100.52±14.94* |
| Diabetic + HAE
(500 mg/kg, p.o) |
255.88 ± 5.61 | 200.61± 9.98* | 103.68± 7.78* | 78.79± 9.67* |
| Diabetic + Metformin
(5 mg/kg, p.o) |
264.56 ± 7.26 | 170.66 ± 8.95 | 116.89 ± 9.04 | 76.99 ± 8.63 |
Values indicate mean ± SEM (n = 6)
*p < 0.05, compared with normal control values
Effect of HAE on body weight, plasma insulin and liver glycogen in diabetic rats
Table 2 depicts the effect of HAE on body weight, levels of plasma insulin and liver glycogen in STZ-induced diabetic rats. In diabetic rats, the body weight, insulin level and glycogen content were significantly decreased. After 21 days of treatment with HAE at 250 & 500 mg/kg, the body weight was significantly (p < 0.05) increased, insulin level and glycogen content were also significantly (p < 0.05) increased as compared to diabetic rats. The activity of HAE was found less than that of metformin (5 mg/kg) treated group.
Table 2. Effect of HAE on body weight, plasma insulin and liver glycogen in diabetic rats
| Group | Body weight in g | Plasma insulin (µU/ml) | Liver glycogen (mg/g tissue) | |
| 0 day | 21st day | |||
| Normal control | 200.50 ± 2.84 | 205.38± 11.49 | 15.31± 3.62 | 72.14 ± 2.23 |
| Diabetic control
STZ (55 mg/kg, i.p) |
205.67 ± 4.88 | 130.38 ± 7.27 | 6.23 ± 6.45 | 32.18 ± 2.25 |
| Diabetic + HAE (250 mg/kg, p.o) | 200.82± 6.81 | 188.30±9.21* | 9.89±5.33* | 59.44±3.76* |
| Diabetic + HAE (500 mg/kg, p.o) | 204.15± 5.29 | 193.96±9.75* | 14.04±3.87* | 67.75±4.38* |
| Diabetic+ Metformin
(5 mg/kg, p.o) |
206.66 ± 2.32 | 192.59 ± 8.30 | 15.65± 6.72 | 72.80 ± 6.67 |
Values indicate mean ± SEM (n = 6)
*p < 0.05, compared with normal control values
Effect of HAE on lipid profile in diabetic rats:The effect of HAE on lipid profile of diabetic rats is displayed in Table 3. In diabetic rats, the levels of triglycerides (TG), total cholesterol (TC), and low density lipoprotein (LDL) were significantly increased and high density lipoprotein (HDL) level was significantly decreased. In HAE (250 & 500 mg/kg) treated groups, the TG, TC and LDL levels activities were significantly (p < 0.05) reduced and the HDL level was significantly (p < 0.05) increased as compared to diabetic control rats, which is in turn comparable to metformin (5 mg/kg) treated group.
Table 3. Effect of HAE on lipid profile in diabetic rats
| Groups | TG (mg/dl) | TC (mg/dl) | HDL (mg/dl) | LDL(mg/dl) |
| Normal control | 86.89 ±7.26 | 152.20 ± 6.56 | 38.29 ± 2.14 | 95.32 ± 4.92 |
| Diabetic control
STZ (55 mg/kg, i.p) |
210.43± 6.84 | 270.83± 14.96 | 30.61 ± 2.60 | 199.33± 15.67 |
| Diabetic + HAE
(250 mg/kg, p.o) |
145.71±5.92* | 162.21 ±5.92* | 40.27 ±5.67* | 117.29±5.46* |
| Diabetic + HAE
(500 /kg, p.o) |
133.21±4.54* | 158.71± 6.91* | 48.29± 5.67* | 100.69± 8.59* |
| Diabetic + Metformin
(5 mg/kg, p.o) |
115.29± 3.79 | 146.59± 11.15 | 54.69 ± 3.28 | 78.89 ± 6.74 |
Values indicate mean ± SEM (n = 6)
*p < 0.05, compared with normal control values
Effect of HAE on SGOT, SGPT, ALKP, TPR and CRTN in diabetic rats
There was a significant increase in activities of SGOT, SGPT and ALKP in diabetic rats. After treatment with HAE (250 and 500 mg/kg) the activities of SGOT, SGPT and ALKP activities were significantly (p<0.05) reduced as compared to diabetic control rats. A significant decrease in serum total protein (TPR) level and a significant increase in creatinine (CRTN) level were observed in diabetic rats. After treatment with HAE at 250 and 500 mg/kg doses for 21 days the TPR level was significantly increased (p < 0.05) and CRTN level was significantly (p < 0.05) decreased compared to diabetic control rats. Metormin (5 mg/kg) treated rats also showed significant effects on blood levels of SGOT, SGPT, ALKP, TPR and CRTN in diabetic rats (Table 4).
Table 4. Effect of HAE on SGOT, SGPT, ALKP, TPR and CRTN in diabetic rats
| Group | SGOT
(U/L) |
SGPT
(U/L) |
ALKP
(U/L) |
TPR
(mg/dl) |
CRTN
(mg/dl) |
| Normal control | 49.78± 6.69 | 46.89± 6.23 | 116.61±4.98 | 8.89± 0.46 | 0.436±0.026 |
| Diabetic control
STZ (55 mg/kg, i.p) |
102.80±8.43 | 88.39 ± 4.59 | 320 ± 8.95 | 4.62± 0.92 | 0.826±0.037 |
| Diabetic + HAE
(250 mg/kg, p.o) |
67.33± 5.35* | 60.21± 4.32* | 146.27±4.89* | 6.95±0.31 | 0.646±0.04* |
| Diabetic + HAE
(500 mg/kg, p.o) |
62.04± 4.32* | 56.73± 5.21* | 138.72±7.29* | 8.73±0.52* | 0.498±0.06* |
| Diabetic + Metformin
(5 mg/kg, p.o) |
58.79 ± 7.61 | 53.91 ± 8.56 | 132 ± 6.52 | 8.98± 2.02 | 0.434±0.062 |
Values indicate mean±SEM (n = 6)
*p<0.05, compared with normal control values
Effect of HAE on liver antioxidant enzymes and MDA:Table 5 displays the activities of SOD, CAT, GSH and GPx in normal and diabetic rats. In STZ-treated diabetic rats, the activities of SOD, CAT, GSH and GPx were significantly increased. There was a significant (p < 0.05) reduction in the activities of these antioxidant enzymes in diabetic rats as compared to normal rats. Metformin (5 mg/kg) also showed significant (p < 0.05) reduction of these enzymes. Increased levels of MDA, an indicator of LPO, in diabetic rats were significantly (p < 0.05) reduced after treatment with HAE (250 & 500 mg/kg) as compared to the normal rats.
Table 5. Effect of HAE on SOD, CAT, GSH, GPx and MDA in normal and diabetic rats
| Treatment | SOD
(U/mg protein) |
CAT
(U/mg protein) |
GSH
(U/mg protein) |
GPx
(U/mg protein) |
MDA
(LPO) (U/mg protein) |
| Normal control | 8.28± 0.06 | 72.16±4.32 | 16.20±0.48 | 23.12 ± 1.89 | 0.52 ± 0.12 |
| Diabetic control
STZ (55 mg/kg, i.p) |
4.23± 0.08 | 42.60±3.69 | 6.20 ± 0.28 | 9.12 ± 0.48 | 0.82 ± 0.03 |
| Diabetic + HAE
(250 mg/kg, p.o) |
6.87± 0.07 | 61.67±3.11* | 11.15±0.54 | 12.97±0.73* | 0.69±0.04 |
| Diabetic + HAE
(500 mg/kg, p.o) |
7.98± 0.08 | 67.56±5.43* | 14.11±0.75* | 19.04±0.43* | 0.58±0.07* |
| Diabetic+ Metformin
(5 mg/kg, p.o) |
8.51± 0.05 | 69.86±3.64 | 15.20±1.20 | 20.34 ± 0.82 | 0.50 ± 0.10 |
Values indicate mean ± SEM (n = 6)
*p < 0.05, compared with normal control values
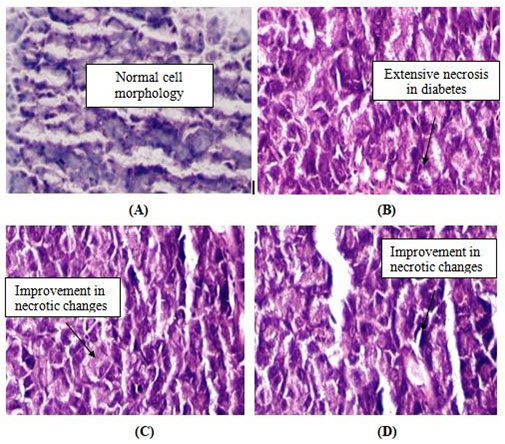
Histopathological observations:Histopathological studies of pancreas (Fig. 2) of STZ-treated diabetic rats exhibited reduction in the dimensions of islets, damaged β-cell population and extensive necrotic changes followed by fibrosis and atrophy (B). HAE (500 mg/kg) and metformin treated rats restored the necrotic and fibrotic changes and also increased the number and increased the size of the islets (C). In normal control group normal acini and normal cellular in the islets of Langerhans the pancreas were observed (A). The changes in pancreas morphology in metrformin treated group (D) are similar to HAE treated rats.
Figure 2: Histology of pancreas of experimental rats after treatment with HAE, 500 mg/kg. (A) Normal control- (B) Diabetic control, (C) Diabetic treated with HAE (500 mg/kg), (D) Diabetic treated with metformin
In STZ-induced diabetic rats (B), the histopathology shows the presence of more shrinkage, increased necrosis and damaged β-cell, whereas, the diabetic treated (HAE) animals (C & D) shows increased number of islets, lesser degree of shrinkage and restoration of necrosis of β-cells of pancreas
Hydro-alcoholic extract (HAE) of O. debilis leaves did not exhibit toxicities up to a dose of 2000 mg/kg b.w. in experimental animals which indicated high margin of safety of bioactive principles present in the extract. HAE treated rats lowered glucose level when compared to normal rats which indicated that the increased glucose tolerance in HAE treated rats was due to insulin secretion from β-cells and increased glucose utilization by the tissues. The HAE treated group exhibited significant reduction of fasting plasma glucose levels as compared to the diabetic control group. The possible mechanism by which HAE brought about its hypoglycemic action might be by improving glycaemic control mechanism and by increasing insulin secretion from regenerated β-cells of pancreas (Jangir and Jain, 2017). It was further supported by histopathological observations which clearly revealed the presence of shrinkage, necrosis and damaged β-cell population in the endocrine region of pancreas in STZ-induced diabetic rats. The diabetic treated (HAE) animals showed increase in the number of islets, lesser degree of shrinkage and restoration of necrosis of β-cells of pancreas. Our finding is consistent with an earlier report by Irudayaraj and co-authors (Irudayaraj et al., 2012). Diabetic rats treated with HAE showed an improvement in body weight in comparison to the diabetic control rats and standard metformin treated rats, signifying the protective effect of HAE in controlling muscle wasting i.e., reversal of gluconeogenesis. Moreover, the ability of HAE to protect body weight loss might be the result of its ability to reduce hyperglycemia (Sudasinghe and Peiris, 2018).
Diabetic rats treated with HAE increased significantly the liver glycogen content as compared to the diabetic control, which could be due to increased insulin secretion. The significant increase in the glycogen levels of the HAE treated diabetic animals might be because of the reactivation of glycogen synthase system.Diabetic rats treated with HAE significantly improved serum TG and TC. The significant control of the levels of serum lipids in the HAE treated diabetic rats might be attributed to improvements in insulin levels. Significant lowering of LDL cholesterol and raise in HDL cholesterol were observed in treated diabetic rats. The HAE extract treated animals showed a weight loss, which probably be due to the lipid lowering activity of the extract or indirectly to the influence on various lipid regulation systems. Lipid lowering activity of the HAE may help in prevention of diabetic complications like atherosclerosis and ischaemic conditions (Junejo et al., 2018)
An increase in the activities of SGOT, SGPT and ALP in plasma of diabetic rats might be mainly due to the leakage of these enzymes from the liver cytosol into the blood stream which was an indicator of the hepatotoxic effect of STZ (Sreedevi et al., 2020). Treating the diabetic rats with HAE reduced the activity of these enzymes compared to the diabetic control group. Reduction in plasma TPR was observed in diabetic rats, which might be due to the negative nitrogen balance, enhanced proteolysis and decreased protein synthesis (Reddy et al., 2017). The plasma protein level was improved in diabetic rats after treatment with HAE. Diabetic rats showed increased level of CRTN, whereas, a significant reduction in the level of CRTN was observed in HAE treated diabetic rats indicated that the HAE prevented the progression of renal damage in diabetic rats (Attanayake et al., 2015).
The activities of enzymatic antioxidant (SOD¸ CAT, GSH and GPx) were increased to normal indicating the efficacy of HAE in attenuating the oxidative stress (OS) and eventual inhibition of LPO in diabetic liver. Decrease in MDA level indicated reduced rate of LPO in HAE treated diabetes. Mechanisms that contribute to increased OS in diabetes include non-enzymatic glycosylation, autooxidative glycosylation and metabolic stress (Jan et al., 2015). A marked increase in the concentration of TBARS and MDA were observed in STZ induced diabetic rats indicating the LPO of tissues under oxidative stress (Banerjee et al., 2017). Since the HAE significantly decreased TBARS levels as well as MDA in liver of diabetic rats indicating strong lipid peroxidation scavenging activity of the HAE as antioxidant agent. Some studies (Kadali et al., 2017) suggest that high molecular weight phenolic compounds including plant flavonoids comprising hydroxyl group and aromatic ring serve as potent free radical scavengers. It is now assumed that the antioxidant activity is responsible for the antidiabetic action of the HAE, and phenolic compounds and flavonoids present in the HAE may be involved in reducing underlying cellular OS and eventual hypoglycemic reactions (Sekhin-Loodu et al., 2019). Plants rich in phenolics, flavonoids and related substances, have antioxidant activity due to their redox properties, and as their free radicals scavenging ability is facilitated by hydroxyl groups (Junejo et al., 2000a). So, the determination of total phenolic and flavonoid contents could be used as a basis of assessing the antioxidant potential of plant extracts. It has been reported that antioxidant properties of plant derived phenolic compounds are brought about mainly via their radical scavenging activities (Junejo et al., 2018).
CONCLUSION
The findings of our present investigation justify the traditional use of O. debilis leaves in ethnomedicine of Northeast India for the treatment of diabetes. The antioxidant activity of O. debilis leaves reported here in signifies the potential of the plant as herbal antioxidant with possible role in the prevention of oxidative stress induced diabetes and associated disease complications. Studies are in progress in our laboratory to isolate bioactive principles from the HAE extract of O. debilis leaves and further exploration of biochemical mechanisms involved in antidiabetic action of isolated compopunds. As this is the first report on the antioxidant activity of O. debilis, thorough phytochemical analyses need be executed in order to identify the possible antioxidant phenolic and flavonoid components having antidiabetic activity.
ACKNOWLEDGEMENT
Authors are thankful to the University Grants Commission (UGC), New Delhi for providing Senior Research Fellowship (Grant number: F1-17.1/MANF-MUS-WES) to first author.
Conflict of Interest
The authors declare that there are no conflicts of interest.
REFERENCES
Attanayake, A.P., Jayatilaka, K.A.P.W., Pathirana, C., Mudduwa, L.K.B. 2015. Acute hypoglycemic and antihyperglycemic effects of ten Sri Lankan medicinal plant extracts in healthy and streptozotocin induced diabetic rats. International Journal of Diabetes in Developing Countries 35:177-183
Amira, R., El, B., Samy, A.H., Abeer, A.A., Yehia, A.H., Tarek, M.M., 2016. Anti-diabetic activity of Holothuria thomasi saponin. Biomedicine and Pharmacotherapy 84, 1472-1487
Banerjee, A., Maji, B., Mukherjee, S., Chaudhuri, K., Seal, T. 2017. In Vitro Antidiabetic and Anti-oxidant Activities of Methanol Extract of Tinospora Sinensis. Journal of Applied Biology Biotechnology 5, 061-067
Carroll, N.V., Longley, R.W., Roe, J.H., 1956. The determination of glycogen in liver and muscle by use of anthrone reagent. Journal of Biological Chemistry 220, 583–593
Chang, C.,Yang, M., Wen, H, J. Chern., 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis 10, 178-182
Debasis, D.E., Chatterjee, K., Ali, M.K., Mandal, S., Barik, B., Ghosh, D., 2010. Antidiabetic and antioxidative effects of hydro-methanolic extract of sepals of Salmalia malabarica in streptozotocin-induced diabetic rats. Journal of Applied Biomedicine 8, 23–33
Friedewald, W.T., Levy, R.T., Frederickson, D.S., 1972. Estimation of VLDL- and LDL cholesterol. Clinical Biochemistry 18, 499–502
Irudayaraj, S.S., Christudas, S., Duraipandiyan, V., Ignacimuthu, S., 2012. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) Lam. leaves in Streptozotocin-induced diabetic rats. Journal of Ethnopharmacology 143, 515–523
Jana, K., Bera, T.K., Ghosh, D. 2015. Antidiabetic effects of Eugenia jambolana in the streptozotocin-induced diabetic male albino rat. Biomarkers and Genomic Medicine 7, 116
Jangir, R.N., Jain, G.C., 2017. Evaluation of Antidiabetic Activity of Hydroalcoholic Extract of Cassia fistula Linn. pod in Streptozotocin-Induced Diabetic Rats. Pharmacognosy Journal 9, 599-606
Junejo, J.A., Gogoi, G., Islam, J., Rudrapal, M., Mondal, P., Hazarika, H., Zaman, K., 2018. Exploration of antioxidant, antidiabetic and hepatoprotective activity of Diplazium esculentum, a wild edible plant from North Eastern region of India. Future Journal of Pharmaceutical Sciences, 4, 93-101
Junejo, J.A., Rudrapal, M., Mohammed, A., Zaman, K., 2020a. New flavonoid with antidiabetic potential from Tetrastigma angustifolia (Roxb.) Deb leaves. Brazilian Journal of Pharmaceutical Sciences; in press
Junejo, J.A., Rudrapal, M., Nainwal, L.M., Zaman, K., 2017. Antidiabetic activity of hydro-alcoholic stem bark extract of Callicarpa arborea Roxb. with antioxidant potential in diabetic rats. Biomedicine and Pharmacotherapy 95, 84-94
Junejo, J.A., Rudrapal, M., Zaman, K., 2020b. Antidiabetic activity of Carallia brachiata Lour. leaves hydro-alcoholic extract (HAE) with antioxidant potential in diabetic rats. Indian Journal of Natural Products and Resources; in press
Junejo, J.A., Zaman. K., Rudrapal, M., Khan, A., Sarwa, K.K., Suryawanshi, V.K., Verma, S., Hussain, N., 2020c. Antidiarrheal and Antipyretic Activity of Ethyl Acetate and Hydro-Alcoholic Extracts of Diplazium esculentum Leaves. Bioscience Biotechnology Research Communication 13, 169-173
Junejo, J. A., Zaman, K., Rudrapal, M., Mondal, P., 2014. Antidiabetic assessment of the hydro-alcoholic leaf extracts of the plant Tetrastigma angustifolia (Roxb.), a traditionally used North-Eastern Indian vegetable. Biomedical and Pharmacology Journal 7, 635-644
Kadali, S.L.D.V.R.M., Das, M.C., Vijayaraghavan, R., Kumar, M.V. 2017. Evaluation of Antidiabetic Activity of Aqueous and Ethanolic Extracts of Leaves of Chloroxylon swietenia in Streptozotocin (Stz) Induced Diabetes in Albino Rats. Biomedcical and Pharmacology Journal 10, 1347-1353
Kumar, S., Kumar, V., Om, P., 2011. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pacific Journal of Tropical Medicine 347-352
Kumar, A., Niketa, D., Rani, S.S., 2012. Sagwal. An absolute review on Oxalis corniculata Linn, International Journal of Research in Pharmaceutical and Biomedical Sciences 3, 1173-1188
Luo, S., Zhangi, D., Renner, S.S., 2006. Oxalis debilis in China: Distribution of Flower Morphs, Sterile Pollen and Polyploidy. Annals of Botany 98, 459–464
Mahdi, A.A., Chandra, A., Singh, K.R., Shukla, S., Mishra L.C., Ahmad, S., 2003. Effect of herbal hypoglycemic agents on oxidative stress and antioxidant status in diabetic rats. Indian Journal of Clinical Biochemistry 18, 8-15
Minami, M., Yoshikawa, H., 1979. A simplified assay method of superoxide dismutase activity for clinical use. Clinica Chimica Acta 92, 337-342
Oliveira, H.C., Santos, D.M.P., Grigulo, R., Lima, L.L., Martins, D.T.O., Lima, J.C.S., Stoppiglia, L.F., Lopes, C.F., Kawashita, N.F., 2008. Antidiabetic activity of Vatairea macrocarpa extract inrats. Journal of Ethnopharmacology 115, 515-519
Ozbek, H., Acikara, O.B., Keskin, I., Kirmizi, N.I., Ozbilgin, S., Oz, B.E., Kurtul, E., Ozrenk, B.C.,Tekin, M., Saltan, G., 2017. Evaluation of hepatoprotective and antidiabetic activity of Alchemilla mollis. Biomedicine and Pharmacotherapy 86,172-176
Panda, E., Pradhan, C., Das, A.B., 2016. Variations in phytoconstituents and antimicrobial activities in ecotypes of Oxalis corniculata L. and Oxalis debilis Kunth. International Journal of Pharmacy and Pharmaceutical Sciences 8, 271-275
Reddy, N.S., Ramanjaneyulu, K., Sabbani, V., Choday, V. 2017. In Vitro and in Vivo Antidiabetic Activity of Rumex Vesicarius Leaves Extract in Streptozotocin Induced Diabetic Albino Wister Rats. Journal of Diabetes and Metabolism 8, 1000745
Rehman, A., Ahmad, I., 2015. Antibacterial, Antifungal, and Insecticidal Potentials of Oxalis corniculata and Its Isolated Compounds. International Journal of Analytical Chemistry 7, 1-5
Riaz, A., Ali, M.N., Qureshi, Z., Mohsin, M., 2020. In Vitro Investigation and Evaluation of Novel Drug Based on Polyherbal Extract against Type 2 Diabetes. Journal of Diabetes Research 2020, 1-9
Saligman, A.M., Chauncey, H.H., Nachlas, M.M., Manheimer, L.M., Ravin, H.A., 1950. The colorimetric determination of phosphatases in human serum. The Journal of Biological Chemistry 7-15
Sekhin-Loodu, S., Rupasinghe, H.P.V., 2019. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Frontiers in Nutrition 6, 53
Sreedevi, P., Ramu Ganesan, A., Moovendhan, M. et al. 2020. Anti-diabetic activity of crude polysaccharide and rhamnose-enriched polysaccharide from G. lithophila on Streptozotocin (STZ)-induced in Wistar rats. Scientific Reports 10, 556
Souza, M.F., Rao, V.S.N., Silveira, E.R., 1997. Inhibition of lipid peroxidation by ternatin, a tetramethoxyflavone from Egletes viscosa L. Phytomedicine 4, 25-29
Sudasinghe, P., Peiris, D.C. 2018. Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflora suberosa L. Peer Journal 6, e4389
Xu, J.B.,Yuan, X.F., Lang, P.Z, 1997. Determination of catalase activity and catalase inhibition by ultraviolet spectrophtometry. Environmental Chemistry 16,73–76


