Division of Microbial Technology Post Graduate and Research Department of Zoology Chikkanna Government Arts College, Tirupur-641602, Tamilnadu, India
Corresponding author Email: moniver65@gmail.com
Article Publishing History
Received: 27/07/2017
Accepted After Revision: 21/09/2017
Pseudomonas aeruginosa is frequent pathogen associated with hospital acquired infections exhibiting intrinsic resistance to numerous antibiotics. A total of 80 isolates of Pseudomonas spp. were isolated from blood cancer patient from three tertiary hospitals in and around Tirupur and Erode (Dt). Metallo beta-lactamase and biofilm production is the most worrisome resistant mechanisms observed in P. aeruginosa. Emergence of antimicrobial resistance by pathogenic bacteria is a major health problem in recent years. The biofilm and Metallo beta-lactamase production was tested by combined disc test and tissue culture plate method. Electrophoretic analysis of the plasmid DNA prepared was carried out by agarose gel electrophoresis on 0.7%. Nineteen carcinogenic isolates which was showed more than 50% resistance against tested antibiotics were selected for plasmid isolation. Recurrently, ZrO2 nanoparticles comprises of well-known inhibitory and bactericidal effects. The nanoparticles were obtained and tested against 10 Metallo beta-lactamase and biofilm producing carcinogenic isolates. The nanoparticles showed appreciable activity at all tested concentrations (0.2, 0.4 and 0.6 mg/ml). Thus, it is concluded that the present study designed to determine the efficacy of ZrO2 may serve as a promising antibacterial agent against cancer causing Pseudomonas aeruginosa
Aeruginosa, Pseudomonas, beta-lactamase
Rajasekar. S, Vijayalakshmi .S, Mohankumar A. Antibacterial Activity of Zro2 Against Metallo Beta-Lactamase and Biofilm Producing Carcinogenic Pseudomonas Aeruginosa. Biosc.Biotech.Res.Comm. 2017;10(3).
Introduction
Pseudomonas aeruginosa is one of the most important opportunistic bacteria, causing a wide variety of infections especially in immune compromised hosts such as burn patients, patients suffering from respiratory diseases like cystic fibrosis, and cancer chemotherapy patients (Govan and Deretic, 1996). It is gram negative short rod belong to family Pseudomonacaeae. Infections with P. aeruginosa is occupy very important position as of greatest concern in critically ill and immune compromised patients who have been hospitalized for extended periods of time and have received broad-spectrum antimicrobial therapy or cancer chemotherapy (Pollack, 2000, Ae Mftah et al., 2015).
The pathogenic cancer causing Pseudo bacterial life includes stages where the cells are associated and form a biofilm on a surface (e.g. Costerton et al., 1995). The formation of these surface communities and their inherent resistance to antimicrobial agents are the cause of many persistent and chronic infections (Costerton et al., 1999).
Nowadays the P. aeruginosa presents a serious therapeutic challenge for treatment of both community-acquired and nosocomial infections, and selection of the appropriate antibiotic to initiate therapy is essential to optimizing the clinical outcome (Mavroidi et al., 2005). Unfortunately, selection of the most appropriate antibiotic is complicated by the ability of P. aeruginosa to develop resistance to multiple classes of antibacterial agents, even during the course of treating an infection. Further acquired resistance is also reported by the production of plasmid mediated AmpC beta (â)–lactamase, Extended Spectrum Beta (â)–Lactamase (ESBL) and metallo beta (â)–lactamase (MBL) enzymes.
 |
Plate 1: Isolated of colonies Pseudomonas aeruginosa from Blood Cancer |
Currently, the nanoparticles are increasingly recognized for their utility in biological applications including nano medicine (mariappan et al., 2011). The ongoing worldwide nanotechnology revolution is predicted to impact several areas of biomedical research and other science and engineering applications. Nanoparticles-assisted drug delivery, cell imaging, and cancer therapy are important biomedical applications of nanotechnology (Hua Wang et al., 2011).
Despite their potential biomedical applications, very few studies have reported on the role of zirconia nanoparticles as anticancer materials. As far as the authors know, this is the first report of the in vitro anticancer effect of sulphated zirconia nanoparticles against three cancer cell lines. Specifically, the toxicity of sulphated zirconia nanoparticles against human colon cancer HT29, human breast cancer MCF-7 and human liver cancer HepG2 cell lines was assessed, showing promising results (Ae Mftah et al., 2015).
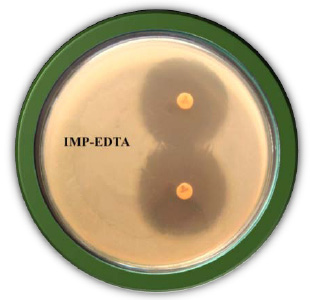 |
Plate 2: Detection of Metallo â-Lactamase by Imipenam EDTA Combined Disc Test |
Moreover, it is reported here that these novel nanoparticles hold promise not just for anticancer applications but also for anti-infection applications. The steady increase in the antimicrobial resistance of microorganisms represents a great public health concern. This requires the search for new unconventional antimicrobial agents. Nanotechnology provides promising materials to fight infectious diseases such as nanoparticles with antimicrobial activities. So hence the present study has made an attempt to point out the antimicrobial properties of the ZrO2 nanoparticles were determined using the agar diffusion method against cancer causing Pseudomonas aeruginosa.
Materials and Methods
A total of 100 clinical cancer blood samples were collected from the jugular vein with a sterile syringe of different age group of blood cancer patients in different clinics and PHC (Primary Health Centre) from in and around Tirupur and Erode District. 80 isolates of Pseudomonas aeruginosa were isolated from the samples. Colony morphology was reported as non-mucoid or mucoid and the isolated colonies were subjected to standard biochemical tests and 16S r-DNA gene sequencing for identification of Pseudomonas aeruginosa. Finally all isolates were kept at – 20oC in media containing 8% dimethyl sulfoxide (DMSO) until use.
The qualitative and quantitative analyzes of the biofilm produced were performed of clinical isolates of P. aeruginosa according to the protocol described by (Xicohtencatl – Cortes et al. 2015). Clinical strains of P. aeruginosa were incubated for growth in trypticase-soy broth (TSB) at 37°C for 24 h. For biofilm formation, 24-well plates containing 1 ml of TSB were inoculated with 50 ml (1.5 x 108 bacteria/ml) of a bacterial suspension of each of the P. aeruginosa strains and incubated at 37°C for 24 h. Biofilms were washed with phosphate buffer solution (PBS) (pH 7.4) and fixed with 2% formalin at 4°C overnight. Subsequently, the fixative solution was removed with PBS and the films were stained with 1 ml of 1% crystal violet for 15 min. Excess crystal violet was removed and 1 ml of methanol at 70% was added for quantification of the biofilm to an optical density of 600 nm.All imipenem resistant isolates were tested for MBL by Imipenem- EDTA combined disc test (CDT).
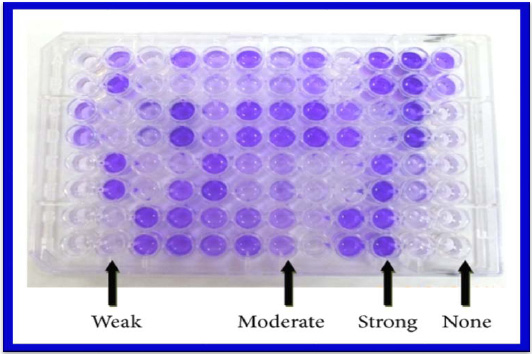 |
Plate 3: Biofilm production by carcinogenic pathogen Pseudomonas aeruginosa |
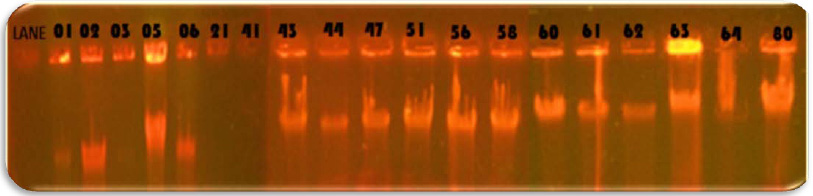 |
Plate 4: Isolation of plasmid from Pseudomonas aeruginosa |
The Imipenem- EDTA combined disc test (CDT) was performed as described by the method of Yong et al. (2002). The test organisms were inoculated on the plates with seeded Mueller Hinton agar as recommended by the CLSI. A 0.5 M EDTA solution was prepared by dissolving 18.61g of EDTA in 100 ml of distilled water and adjusting its pH 8.0. The Mixture was sterilized by autoclaving. After two Imipenem (10µg) discs were placed on the surface of agar plate, add finally 4µl of 0.5 M concentration of EDTA solution. Finally, the inhibition zones of imipenem- EDTA discs were compared after 16 to 18 h of incubation in air at 37°C. In the combined disc test, if the increase in inhibition zone with imipenem- EDTA disc was ≥7 mm it was considered as Metallo Beta Lactamase positive (Lee et al. 2001).
The blood cancer causing P. aeruginosa was treated with various different concentrations (0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL & 100μg/mL) of ZrO2 nanoparticles no significant difference in the growth curve. The growth experiment under the influence of Zirconium nanoparticles thus reveals the non-toxic nature of the Zirconium nanoparticles in the bacterial system. In the present study plasmid DNA was done by boiling preparation method (Holmes & Quigley, 1981; modified by Riggs & McLachlan, 1986).
Antimicrobial activity of protease was carried out by using well diffusion method. The Pseudomonas aeruginosa was inoculated in LB broth and incubated for 24 hrs at 37oC. The turbidity of the broth was adjusted at 0.5 (optical density) using Spectrophotometer. The bacterial culture was inoculated on MHA plates using sterilized cotton swab. Allow it to dry for 2 – 5 min. Well was made using sterile cork borer. Various different concentrations (0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL & 100μg/mL) of different µl of ZrO2 nanoparticles (50µl, 100µl and 150µl) was impregnated in to the well. The plates were swabbed with Pseudomonas aeruginosa. The plates were examined to measure the zone of inhibition around the well.
Results and Discussion
The carcinogenic disease is caused by external factors, such as tobacco, infectious organisms, and an unhealthy diet, and internal factors, such as inherited genetic mutations, hormones, and immune conditions. These factors may act together or in sequence to cause cancer. Currently, emerging and reemerging infectious disease are a major problem in public health and global economics. They are caused by different type of infection such as drug resistant infection, mostly involving bacteria, and many emerging pathogen is increasing significantly over time because they are becoming progressively more resistant to conventional antibiotic compounds. For example, Pseudomonas aeruginosa has been reported as an opportunistic pathogen and one of the most important opportunistic bacteria, causing blood cancer by the intrinsic resistance to many antimicrobial agents. So, the need for the development of new antibiotics to counter drug resistance in bacterial pathogens has been stressed by various researchers worldwide.
Totally 100 carcinogenic blood samples were collected from the jugular vein with a sterile syringe of different age group of blood cancer patients in different clinics and PHC (Primary Health Centre) from in and around Tirupur and Erode District. 80 isolates of Pseudomonas aeruginosa were isolated from the samples. The cancer causing leading pathogen Pseudomonas aeruginosa strains were confirmed by comparing the results with standard biochemical test and 16S-rDNA gene sequence.
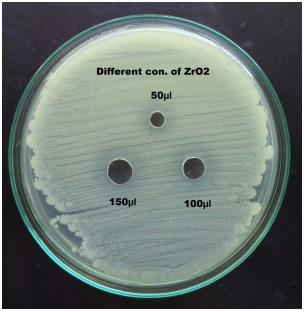 |
Plate 5: Antibacterial activity of ZrO2 against Metallo beta-lactamase and biofilm producing carcinogenic Pseudomonas aeruginosa |
In the study 28.75% of blood cancer causing Pseudomonas aeruginosa isolates was found to be MBL producers. These isolates exhibited a ≥ 7 mm zone enhanced for Imipenem and EDTA combined than the Imipenem disks alone. All the MBL producing isolates were multi drug resistant, most of which showed resistance to more than twelve antibiotics out of the twenty antibiotics tested. Twenty three isolates: PARS01, PARS02 PARS03, PARS05, PARS06, PARS21, PARS41, PARS43, PARS44, PARS47, PARS51, PARS56, PARS58, PARS60, PARS61, PARS62, PARS63, PARS64, PARS74, PARS75, PARS74, PARS75, PARS77, PARS78 and PARS80 which was showed more than above 50% resistance used for Metallo Beta Lactamase production by Imipenem (IMP)-EDTA Combined Disk test of (Yong et al. 2002). The maximum zone of inhibition (mm) produced by the strain No. PARS21 and PARS 77 which showed 40 mm followed by minimum amount of zone of inhibition produced by the strain No. PARS56 and PARS64 which showed 30mm (Plate: 8; Table: 16). Electrophoretic analysis of the plasmid DNA prepared was carried out by agarose gel electrophoresis on 0.7%. Nineteen carcinogenic isolates (PARS01, PARS02, PARS03, PARS05, PARS06, PARS21, PARS41, PARS43, PARS44, PARS47, PARS51, PARS56, PARS58, PARS60, PARS61, PARS63, PARS64 and PARS80) which was showed more than 50% resistance against tested antibiotics were selected for plasmid isolation.
The antibacterial activity of ZrO2 nanoparticles was studied by agar well diffusion method. Three different concentrations (0.2%, 0.4% and 0.6%) were used in this assay against leukemia (blood cancer) causing Pseudomonas aeruginosa. The maximum zone of inhibition 18mm, 19mm and 22mm was observed in strain no. PARS47 followed by the minimum zone of inhibition 15mm, 16mm and 17mm was observed in strain no. PARS64 and PARS56 at 150μl concentration of different µg- 50, 100 and 150 of ZrO2. Mahrukh Khattak et al., (2013) determined the frequency of Pseudomonas aeruginosa in middle and outer ear and to check the antibiotic susceptibility pattern of commonly used antibiotics. The pathogen was highly sensitive to Gentamycin 30 (71.4%), followed by Aztreonam 28(66.6%), Cefixime 22(52.3%), Imipenem 18(42.8%), amikacin 15(35.5%) and Ciprofloxacin 12(28.5%). Females were more susceptible to Pseudomonas infections. Isolates of Pseudomonas aeruginosa from samples showed highest sensitivity (71.4%) to Gentamycin while maximum resistance was showed to Ciprofloxacin (47.6%). In this research, similar results were found in all the Pseudomonas aeruginosa in blood cancer origin were more highly sensitive to Imipenem (97.5%).
Resistance to imipenem has been found to be independent of â-lactamase production and in P. aeruginosa has been attributed to diminished expression of certain outer membrane proteins (Buscher et al., 2000). More than 80% of isolates in this study were sensitive to imipenem (97.5%). Compared with results of a study conducted at the Lagos University Teaching Hospital (LUTH) in which 12.5% were resistant to imipenem, in this study only 5.4% Pseudomonas strains were resistant. Imipenem is a drug that is not readily available in our environment and its cost is also prohibitive.
Plasmid analysis of the multi-resistant strains showed that 18 of the Pseudomonas strains harbored plasmids, eleven of which had similar plasmid band patterns of 1-7 plasmid bands having low to intermediate molecular weights. Plasmid prevalence was higher in the strains from blood cancer. Acquisition of mobile genetic elements is known to be the main mechanism for short term accumulation of resistance determinants in bacterial genomes (Liu et al., 2000).Our finding that 18(78.75%) out of the 46 Pseudomonas isolates contained plasmids with seven different plasmid profiles, coincides with that reported by Tsakris et al., (1992) who found that 10 isolates harbored plasmids out of 35 multi resistant pseudomonas strains, with 6 different plasmid profiles. In another study, Poh et al., (1988) detected 11 different plasmid profiles in Ps. aeruginosa isolates.
Biofilm producing organisms are responsible for many recalcitrant infections and are notoriously difficult to eradicate. They exhibit resistance to antibiotics by various methods like restricted penetration of antibiotic into biofilms, decreased growth rate and expression of resistance genes (Hachem et al., 2007). There are various methods for biofilm detection (Poole, 2004). In the present study the number of isolates showing strong biofilm producers were 41(64%) and moderate biofilm producers were 39(36%) by Tissue culture plate method similar to the study done by Afreenish Hassen et al., (2011). In their study noted that out of 110 isolates from different clinical samples tested for biofilm production, the number of biofilm producers identified by Tissue culture plate method (TCP) was 70(64.7%) and non or weak biofilm producers were 40(36.3%). The recent study has performed the Tissue culture plate method by addition of 1% glucose in trypticase soy broth. Addition of sugar helps in biofilm formation16. This was also reported by studies conducted by Mathur et al., (2006).
Metallo-beta-lactamase enzyme is an emerging threat and cause of concern for physician. The metal ion active sites appear to decrease their susceptibility to beta lactamase inhibitors and enable them to hydrolyze broad spectrum including carbapenems. The Metallobeta-lactamase are plasmid mediated, so the resistance can be spread among hospital pathogen and will cause problems in treating infections (Mehul Chaudhari et al., 2011). In present study, attempt was made to detect Metallobeta- lactamase producing Pseudomonas aeruginosa. Of 80 isolates of Pseudomonas aeruginosa, 23 (2.5%) were resistance to imipenem. All 23 isolates were found to be MBL producers. Of 23 isolates of MBL, 23 (28.75%) were isolated from blood cancer patient. The prevalence of detect Metallo-beta-lactamase producing Pseudomonas aeruginosa in our setup if 2.5%.
Ae Mftah et al., (2015) studied the sulphated zirconia nanoparticles showed high antimicrobial activity against both Gram-positive and Gram-negative bacteria. It was found that the nanoparticles showed the highest activity against Pseudomonas aeruginosa and methicillin-resistant S. aureus, followed by Bacillus subtilis and Salmonella choleraesuis. The first time that the exposure of cancer cells to sulphated zirconia nanoparticles (3.9–1,000 μg/mL for 24 hours) resulted in a dose-dependent inhibition of cell growth, as determined by (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays. Similar promising results were observed for reducing bacteria functions. In this manner, this study demonstrated that sulphated zirconia nanoparticles with bronsted acidic sites should be further studied for a wide range of anticancer and antibacterial applications. Followed by these present study similar results of antimicrobial ability of ZrO2 at different concentration (0.2%, 0.4% and 0.6%) used against blood cancer causing Pseudomonas aeruginosa. Zirconium oxide was used and it shows prominent anti carcinogenic activity against test pathogen.
Conclusion
The study also highlights that MâL incidence is increasing in our region. The metallo beta-lactamase and biofilm production is the most worrisome resistant mechanisms observed in P. aeruginosa. Emergence of antimicrobial resistance by pathogenic bacteria is a major health problem in recent years. The resistance may spread rapidly to various species of Gram-negative bacilli; therefore, to prevent the further spread of MâL producers, it is essential to rapidly detect MâL-positive isolates to aid infection control. Recurrently, tiny nanoparticles, far smaller than the width of a human hair, might help the body’s own immune system fight tumors. Moreover, it is reported here that these novel metallo nanoparticles (ZrO2) comprises of well-known inhibitory and bactericidal effects against cancer causing Pseudomonas aeruginosa.
References
- Ae Mftah, Fatah H Alhassan, Mothanna Sadiq Al-Qubaisi, Mohamed Ezzat El Zowalaty, Thomas J Webster, Mohammed Sh-eldin, Abdullah Rasedee, Yun Hin Taufiq-Yap, and Shah Samiur Rashid(2015). Physicochemical properties, cytotoxicity, and antimicrobial activity of sulphated zirconia nanoparticles. Int J Nanomedicine. 10: 765–774.
- Afreenish Hassan, Javaid Hasman, Fatima and Maria (2011). Evaluation of different methods associated of biofilm formation in the clinical isolates. Braz. J. Infectious disease. 15(4): 305 – 311.
- Buscher, KH., W. Cullman, W. Dick, Wendt. S and Opferkuch. W (2000). Imipenem resistance in aeruginosais due to diminished expression of the outer membrane proteins. J. Infect. Dis. 156: 681- 685.
- Costerton, J.W., P.S. Stewart and Greenberg E.P (1999). Bacterial biofilms: a common cause of persistent infections. Science. 284: 1318–1322.
- Costerton, J.W., Z. Lewandowski, D.E. Caldwell, Korber D.R and Lappin-Scott H.M (1995). Microbial biofilms. Annu Rev Microbiol. 49: 711–745.
- Govan, J.R and Deretic V (1996). Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosaand Burkholderia cepacia. Microbiol. Rev. 60: 539 – 74.
- Holmes, D and Quigley M (1981). A rapid boiling method for the preparation of bacterial plasmids. Anal. Biochem. 114: 193-197.
- Hua Wang, Denise Wingett, Kevin Feris, Madhusudan R Kongara, Alex Punnoose(2011). Fluorescent particles comprising nanoscale ZnO layer and exhibiting cell-specific toxicity. US Patent. US 7,939,560 B2. 1-8.
- Lee, K., Y. Chong, H.B. Shin, Y.A. Kim, Yong D and Yum J.H (2001). Modified Hodge and EDTA-disk synergy tests to screen metallo-lactamase-producing strains of Pseudomonas and Acetobacter Clin Microbiol Infect. 7: 88–91.
- Liu, XZ., I. Zang and Poole K(2000). Interplay between the MexA MexB OprM multidrug Pseudomonas aeruginosa. J. Antimicrobiol. Chemother. 45: 433-436.
- Mahrukh Khattak, Muhammad Saqib Ishaq, Maimoona Gul, M. Medrar Hussain, Ghadir Ali, Amir Mohammad, Khalid Javed and Arshad Parvez (2013). Isolation and identification of Pseudomonas Aeruginosafrom ear samples and its antibiogram analysis. 6(2).
- Mariappan Premanathan, Krishnamoorthy Karthikeyan, Kadarkaraithangam Jeyasubramanian and Govindasamy Manivannan (2011). Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation Nanomedicine: Nanotechnology, Biology, and Medicine. 7: 184–192.
- Mathur, T., S. Singhal, S. Khan, DJ. Upadhyay, Fatma T and Rattan A (2006). Detection of biofilm among clinical isolates of Staphylococci: An evaluation of three different screening methods. Indian J Med Microbiol. 24(1): 25-9.
- Mavroidi, A., A. Tsakris, E. Tzelepi, S. Pournaras, Loukova V and Tzouvelekis L.S (2000). Carbapenem-hydrolyzing VIM-2 metallo-b-lactamase in Pseudomonas aeruginosafrom Greece. J Antimicrob Chemother. 46: 1041–1042.
- Mehul Chaudhari. S., B. Tanuja Javadekar, Govind Ninama and Neelam Pandya1 Jivraj Damor (2011). A study of metallo-beta-lactamase producing Pseudomonas aeruginosain clinical samples of S.S.G. hospital. National journal of medical research. 1(2): 60-63.
- Pollack, M (2000). Pseudomonas aeruginosa. In: Principles and practice of infectious diseases (eds. G.L. Mandell, J. E. Bennett and R. Dolin), Churchill Livingstone, Philadelphia, PA, USA, 5th Ed: 2310-2335.
- Poole, K (2004). Efflux mediated multi resistance in Gram Negative bacteria. Clinical 0Microbiology and Infection. 10 (1): 12–26.
- Riggs, M.G and McLachlan A (1986). Biotechnology Techniques. 4: 310 – 313.
- Tsakris, A., A.C. Vatopoulous, Tzouvelekis L.S and Legakis N.J (1992). Diversity of resistant phenotypes and plasmid analysis in multi resistant 0:12 aeruginosa. Eur.J. Epidemiol. 8: 865-780.
- Xicohtencatl-Cortes, J., V. Monteiro-Neto, Z. Saldana, M. A. Ledesma, Puente J.L, Girón J.A (2009). The type 4 pili of enterohemorrhagic Escherichia coliO157:H7 are multipurpose structures with pathogenic attributes. J Bacteriol. 191:411-421.
- Yong, D., K. Lee, J.H. Yum, H.B. Shin, Rossolini G.M and Chong Y (2002). Imipenem-EDTA disk method for differentiation of Metallo âeta lactamase producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 40: 3798-801.


