Department of Zoology and Fisheries, Yashavantrao Chavan Institute of Science, Satara-415001, India
Corresponding author Email: patilneha1227@yahoo.in
Article Publishing History
Received: 07/07/2019
Accepted After Revision: 09/09/2019
Antiangiogenesis is the complex mechanism used for the inhibition of growth of blood vessels from the pre-existing vasculature. Blood vessel is known to perform a crucial role in development of tumor. Blocking of angiogenesis through the control over the action of the cytokine VEGF could be possible mechanism in cancer therapy. This is very important mechanism used to prevent the proliferation of blood vessels towards growth of tumors. It has proved to be potentially attractive approach in case of controlling dreadful disease like Cancer .Knowing the importance of this process, for the first time,the study was aimed to investigate the antiangiogenic potential of endophytic fungi from the medicinal plant Lawsonia inermis linn. We successfully isolated Alternaria alternate from the leaves of Lawsonia inermis Linn and identified microscopically as well as by 18srRNA and ITS sequence analysis. The GC-MS analysis revealed the presence of wide range of bioactive secondary metabolites showing signifi cant antimicrobial property against four human bacterial pathogens .The ethyl acetate extract of Alternaria alternata showed maximum zone of inhibiton of 21.8±0.8mm against Pseudomonas aeroginosa. After these analysis, antiangiogeneic property of Alternaria alternata ethyl acetate extract was studied by Chick Chorioallantoic membrane assay (CAM) which showed the treated CAM has (84±2.60) less no. of tertiary blood vessels as compared to the control (124± 2.64). Therefore the results suggest that Alternaria alternata can be considered as a potential source of antiangiogenic agent. Further investigation of characterization and structure
elucidation of active compounds from this extract is needed to know the exact antiangiogenic component.
Angiogenesis, Angiogenesis, Vegf, Endophytic Fungi,Secondary Metabolites Chorioallantoic Membrane (Cam) Assay, Lawsonia
Bendre N. N, Gonjari G. R. Antiangiogenic Potential of Endophytic Fungi Alternaria Alternata Isolated from Lawsonia inermis Linn. Biosc.Biotech.Res.Comm. 2019;12(3).
Bendre N. N, Gonjari G. R. Antiangiogenic Potential of Endophytic Fungi Alternaria Alternata Isolated from Lawsonia inermis Linn. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/2VMjBpO
Copyright © Bendre and Gonjari, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Angiogenesis is the dynamically regulated process where new blood vessels are escalated from the previously formed blood vasculature. It is known to be essential for normal physiological process like embryonic devel- opment, organ formation, reproductive system, cyclical ovarian function, tissue renewal, and wound healing. Also it also play a vital role in pathological conditions like rheumatoid arthritis, arteriosclerosis, myocardial infarction, ischaemic diseases, diabetic retinopathy and cancer. The later pathological disease called Can- cer has drawn more attention of researchers some few year back. The reason behind the cancer being the most dreadful disease is the unending requirement of oxygen and nutrients supply from the growing blood vessels which later on make it critical, metastatic and leads to death of the person.
According to Folkman (1971) tumor growth is known to depend on angiogenesis where it is triggered by chem- ical signals from tumor cells in a phase of rapid growth. Tumors do not grow progressively unless they induce a blood supply from the surrounding stroma. Cancers that lack angiogenesis remain dormant. Therefore in order to get control over the tumor cells blood vessels sup- ply to cancer cells should be inhibited through process called antiangiogenesis.,Since in the absence of vascu- lar support tumors become necrotic, or even apoptotic, (Holmgren et al 1995). As per Judah Folkman, the main reason behind the excessive formation of blood vessels around the tumor is due to the Vascular Endothelial Growth Factor-A (VEGF-A) is supposed to be a potent angiogenic mitogen inducing migration and prolifera- tion of endothelial cells. Blockade of VEGF has proven to be effective way of inhibiting tumor angiogenesis. Therefore Folkman and his colleagues were the first to propose using inhibition of tumor vasculature for- mation as anticancer therapy (Folkman 2003). Of late researchers are now actively involved in the develop- ment of novel anti cancerous drugs throughout the world .According to some reports, many of the available anticancer drugs are exhibiting toxicity to proliferating normal cells along with the cancer cells also the repeti- tive dosage is enhancing its resistance which results in increasing the the need for bioactive components from natural products, (Remesh 2017).
Natural products are naturally derived metabolites and by products obtained from microorganisms, fungi, plants or animals, (Baker et al 2000). According to Schulz (2001), novel secondary metabolites are derived from organisms that occupy unique biotopes with unu- sual environment are known to be prolific producers of bioactive metabolites. Endophytes are one of those microbes that have the ability to thrive in the specific biotopes like higher plants which are traditionally been exploited for medical, industrial and agricultural use. Endophytic fungi are the microbe that colonize the internal tissues of plant without causing any infection or harm to them, (Bacon and White 2000, Remesh 2017).
In recent advances of research, endophytes are viewed as a excellent source of bioactive natural product as they are known to possess therapeutic value for the preven- tion and treatment of various types and stages of cancer. As per the estimation Dreyfuss and Chapela Rossman, there are as many as 1 million species of endophytic fungi unexplored and undescribed. There are very few research has been reported on the study of angiogen- esis and antiangiogenesis property of endophytic fungi using Chick chorioallantoic membrane assay. it was found that Hulikere et al, (2016) studied the antiangi- ogenic and antioxidant activity of endophytic fungus Penicillium citrinum and Cladosporium Cladosporoides from seaweed (Sargassum wightii). This research work has created impetus in us to investigate the antiangio- genic potential of bioactive secondary metabolites of endophytic fungi isolated from medicinal plant Law- sonia inermis Linn (Mehndi) located in Satara District, (Maharashtra), India, Using chick Chorioallantoic mem- brane assay (CAM).
Chick embryo chorioallantoic membrane in vivo assay is one of the best established and most popularly used model for angiogenesis and cancer studies. As this method is Cost effective, less time consuming, easy to perform, reproducible and has visible vasculature which make this useful for both intravascular and topi- cal administration of study agent, relatively rapid assay method and can be adopted very easily to study tumor growth, (Ribatti et al, 2001). Therefore in order to dis- cover alternative treatment for cancer chemoprevention, the chorioallantoic membrane assay is used to analyse various natural compounds that could reduce or inhibit several pathways involving malignancies and excessive angiogenesis related diseases.
Material And Methods
Collection of plant material
In order to obtain endo- phytes, L. inermis has been selected on the basis of their unique biology, age, endemism, ethnobotanical his- tory, or environmental setting. It seems that endemic plants growing in moist, warm climates or in areas of great biodiversity are among the first choices for study, so accordingly L. inermis is collected from Satara dis- trict in Northern Western Ghats. The properly identified and authenticated plant was used in the current study. Healthy and mature leaves were collected from the field grown plants were brought to laboratory and processed within 24 hrs of collection or stored in icebox.
Isolation of endophytic fungi from
Lawsonia inermis: To isolate endophytic fungi from leaves of Lawsonia inermis Linn, Collected leaves were washed thoroughly with water to expel the unwanted material from the surface. Each leaf was surface sterilized by using the method described by Arnold et al (2007) with minor changes. For sterilization of surface of leaves, leave were immersed in 70 % ethanol for 1 min, further dipped in 0.1% HgCl2 for 1 min and later washed in autoclaved distilled water, (Bisht et al 2016) and blot dried on filter paper. Finally the leaves were cut aseptically into 1 cm long segments and placed on Potato dextrose agar plate supplied with 50ug for /ml of streptomycin to avoid any bacterial contamination .later on the plates were sealed with parafilm and incubated at 28±20c for 8-10 days in incubator. After the respective days, the hyphal tips of fungi emerging from leaves were transferred aseptically to fresh PDA slants to get pure culture for fungi.
Identification of Isolated fungi
The fungal isolates mounted on the sterile slides then it was stained with lactophenol cotton blue and then examined in 100x light microscopy. Morphological identification of the isolates was carried out on the basis of surface tex- ture, pigmentation, and spores at the hyphal tips which helped to identify the endophytic fungi at the species level. The authorized identification of endophytic fungi was done from National Fungal Culture Collection of India (NFCCI), Agharkar Research Institute, and Pune, India. The identified fungal strains were isolated and then sub-cultured in a Petri dish which contains ster- ile PDA media. To preserve as a pure culture, the endo- phytic fungi were inoculated in PDA slant.
Molecular identification: Fungal
Genomic DNA was iso- lated using Qiagen DNeasy kit. Fungal ITS region gene was amplified using standard PCR reaction. The primer pair ITS1 and ITS4 was used in a PCR reaction with an annealing temperature of 54°C. After amplification, products were purified by using Invitrogen PCR prod- uct purification kit (Life technologies, USA) and were directly sequenced using an ABI PRISM BigDye Termi- nator V3.1 kit (Applied Biosystems, USA). The sequences were analyzed using Sequencing Analysis 5.2 software. BLAST analysis was performed at Blast N site at NCBI server (http://www.ncbi.nlm.nih.gov/BLAST).
Fermentation and extraction of secondary metabolites
Erlenmeyer flasks (250 ml) containing 100 mL potato dex- trose broth were used for cultivation of fungal isolates. The broth was inoculated with two loops of fungal isolates and incubated at room temperature for 21 days under sta- tionary conditions with intermittent shaking. The extrac- tion was carried out according to the method described by Radji et al (2011). The broth culture was filtered by double-layered muslin cloth to separate the mycelia and filtrate. To the filtrate equal volume of ethyl acetate (1:1) was added, mixed well for 10 minutes and kept for 5 minutes till the two clear immiscible layers formed. Then the upper layer of ethyl acetate was collected using sepa- rating funnel as it contained the extracted compounds. The mycelium was ground properly in a pestle and mor- tar using ethyl acetate as a solvent and then it was fil- tered using cheesecloth. Both mycelia and culture filtrate extracts were pooled together and evaporated to dryness in the hot air oven. The extract residue was dissolved in Dimethyl Sulfoxide (DMSO) and stored at 4°C to be used as a stock solution for spectroscopic characterization and antimicrobial assay.
Antimicrobial screening by Agar Well diffusion assay
Agar well diffusion assay method described by Magaldi et al with little modification has been used for the evalu- ation of antimicrobial property. The crude extracts of fungal endophytes were used and dissolved in DMSO. The test organisms viz. Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus were obtained from the Department of Microbiology, Yashavantrao Chavan Institute of Science, Satara, India. The wells of 1cm in diameter were bored in the media with the help of sterile cork borer. The well was filled with 40 μl of crude extracts of different fungi. For nega- tive control, one well was filled with only 40 μl DMSO. These plates were then refrigerated at 4 oC for 4-6 hrs for antimicrobial diffusion and then incubated at 37oc for 24 hrs. The clear zones of inhibition formed around the wells were measured by counting the diameter of a circle in millimeters. The test was performed in triplicate with each bacterial strain and mean zone of inhibition was recorded.
Gas Chromatographic Mass Spectroscopic profile
To identify the compounds from the fungal extract, GC-MS analysis was carried out using Shimadzu model QP-2010 with a nonpolar 60 M RTX 5MS column. The carrier gas used was Helium and the temperature programme was adjusted to initial oven temperature at 40 °C for 3 min and the final temperature of oven was 480 °C with rate at 10 °C. One microliters (1 μl) sample was injected using a split less mode. Mass spectra was recorded over 35–650 amu range with electron impact ionization energy 70 eV. The total time required to run the sample was 45 min. Relative quantitative determinations were made by relating respective peak areas to total ion chro- matogram (TIC). Unknown components compared with known mass spectra of National Institute of Standards (NIST) for molecular identification of compounds. Name, molecular weight, retention time and peak area percent- age of the test material was tentatively determined.
Chick Chorioallantoic membrane (CAM) In vivo assay:
The antiangiogenic potential was detected by using CAM assay model as described by Domenico R. et al (1996). Fertilized eggs of Gallus gallus were purchased from Satara Poultry hatchery, then were cleaned and disin- fected with 70% alcohol and divided in three groups for 48 hrs, 72 hrs and 96 hrs. The eggs were incubated in an aseptic incubator in vertical position such as blunt end of egg faced upward and was maintained at 370C temperature and humidity at 70%.
Dose administration
For the dose administration Hanks Balanced Salt Solution (HBBS) was used as saline. Endophytic fungal extract was prepared in ethyl acetate, of which 20 ug/ml of extract was dissolved in 1ml DMSO. All treatments were given in final volume of HBSS (mg/ml). At different developmental stages (48 hrs 72 hrs 96 hrs) dose was initiated by making a small window at the blunt end of egg. With the help of insulin syringe of 0.2 ml of dose was injected on to the chorioallantoic membrane and then sealed with surgi- cal adhesive tape. The development was continued up to 144 hrs. (Korn & Cramer, 2009). After 144 hrs The eggs were then opened for morphometric evaluation. Later the CAM samples were fixed in CAF (Calcium Acetate Formalin) fixative to carry out further histopathological process and finally stained with Haematoxylin and eosin for microscopic evaluation.
Results And Discussion
From the present study, after following the standard iso- lation procedure of endophytic fungi from the leaves of medicinal plant Lawsonia inermis Linn, Alternaria spp. was isolated successfully. Pure culture was maintained by sub culturing it at least three times.
From the preliminary identification of fungi it was observed that the fungal colony had olivaceous black or dark gray color with white margin with woolly texture (Fig:1) while features like elongated chains of septate brownish conidia with short beak and smooth surface were observed in the microscopic examinations which were confirmed from National Fungal Culture Collec- tion of India (NFCCI), Agharkar Research Institute (ARI) Pune. Therefore as per the morphological and micro- scopic observations the isolate was identified as Alter- naria spp, further confirmation up to species level was confirmed by molecular analysis. Molecular identifica- tion was done with the help of 18s rRNA gene and Inter- nal transcribed Spacer (ITS) region which identified the Alternaria spp as Alternaria alternata based on BLAST results showing 100 percent identity, 100 % query cover- age with nucleotide homology and phylogenetics analy- sis The information regarding close homologs is repre- sented in the alignment view (fig: 2).
 |
Figure 1: A) Collected plant sample of Lawsonia inermis Linn, B) emergence of mycelia from cut leaf sections, C) colony morphology of Alternaria alternata, D) microscopic view |
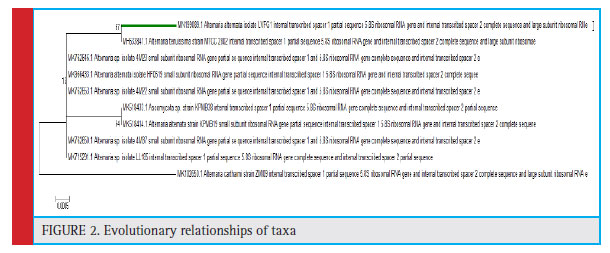 |
Figure 2: Evolutionary relationships of taxa |
The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei (1987). The optimal tree with the sum of branch length = 0.01238757 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches, (Felsen- stein 1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evo- lutionary distances were computed using the Kimura 2-parameter method (Kimura M. 1980) and are in the units of the number of base substitutions per site. The analysis involved 10 nucleotide sequences. Codon posi- tions included were 1st+2nd+3rd+Noncoding. All posi- tions containing gaps and missing data were eliminated. There were a total of 486 positions in the final data- set. Evolutionary analyses were conducted in MEGA6 (Tamura et al 2013).
The Agar well diffusion assay was performed to eval- uate the antimicrobial property of the Alternaria alter- nata ethyl acetate extract. In this, it showed promising inhibition of four human bacterial pathogens (table 1). In this assay 10 μl of extract was dissolved in DMSO which also was used as a negative control to state whether the inhibition was not occurred due to solvent. The evalu- ation was carried out on the basis of classification as (0-6mm)- no activity, (7-9mm )-not significant, (10- 12mm )- poor activity (13-15mm )- low activity (16-18 mm)- good activity, and above 18 mm significant activ- ity. From this it was observed that the extract showed significant activity with maximum zone of inhibition against Pseudomonas aeruginosa (21.8mm), while Bacil- lus subtilis showed good activity with (17mm) inhibi- tion while S.aureus showed poor activity with (13.2mm) inhibition and E.coli showed low activity with 9.4 mm inhibition (table no.1). Thus the above results indicates that the endophytic fungi Alternaria alternata possess significant antimicrobial property which was then fur- ther characterized for secondary metabolite profile and investigation of antiangiogenic potential.
| Table 1: Zone of inhibition (in mm) of fungal endophytes crude extracts by Agar well diffusion method | |
| Name of organism | Diameter of zone of inhibition (mm) |
| P. aerogenusa | 21.8±0.8 |
| B . subtilis | 17±0.6 |
| E.coli | 9.4±0.2 |
| S.aureus | 13.2±1.3 |
| DMSO | — |
| (–) –No inhibition, DMSO – Dimethyl Sulfoxide | |
Antiangiogenic activity was studied by Chick chorio- allantoic Membrane assay using Window method. After 144 hrs, the eggs were opened for morphometric and histological analysis. For morphometric study, firstly the CAM area was measured as described by Melkonian et al (2002), from the (fig :3) and (table no. 2), it can observed that there has been 24.6 % decrease in the total CAM area of eggs treated with A. alternata extract than HBSS control eggs. Also the tertiary blood vessels on CAM area were counted on manually on Computer by con- sidering the sprouting points, it was observed that there was significant decrease in the no. of tertiary blood ves- sels showing 32.7% inhibition while normal and HBSS (Hanks balanced Salt Solution) control showed increase in the number of blood vessel and branches.These sup- pression of blood vessels were confirmed by histologi- cal section stained with Haematoxylin and eosin show- ing inhibition of proliferative vessel and reduction in capillary plexus of treated vessels as compared to nor- mal. (Fig:4) For morphometric analysis- CAM area was measured according to method described by Melkonian et al (2002) as, CAM area = (1/2A) x(1/2B)x π, where, A=Longest length,B=Longest width, and π=3.14. For Histological analysis- Normal, controlled and treated CAMs were fixed in CAF Fixative, a section of tertiary blood vessels was cut and processed in alcohol grades for dehydration and then embedded in paraffin. The par- affin blocks were then sectioned of 6μ size using rotary microtome. these vertical sections were stained with H-E technique. Finally the slides were observed under light microscope for histological changes in structure of blood vessels.
| Table 2: Influence of endophytic fungi Alternaria alternata methanolic extract on number of blood vessels. | ||
| Group | Total Number Tertiary blood vessels | Total CAM area |
| Normal | 115 ±2.9 | 26.8±0.2 |
| Sham | 111±5.5 | 26.8±0.1 |
| HBSS | 124±2.6 | 27.6±0.1 |
| Al.al extract | 84±2.6 | 22.6±0.4 |
| (HBSS-Hanks Balanced salt Solution, Al. al –Alternaria alternata) Results expressed in mean±S.E,P-value <0.05 considererd most significant value | ||
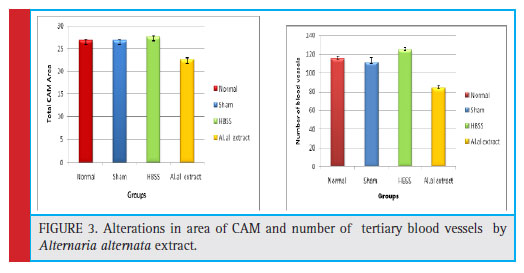 |
Figure 3: Alterations in area of CAM and number of tertiary blood vessels by Alternaria alternata extract. |
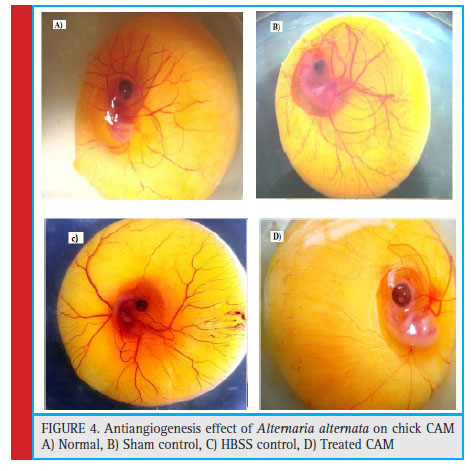 |
Figure 4: Antiangiogenesis effect of Alternaria alternata on chick CAM A) Normal, B) Sham control, C) HBSS control, D) Treated CAM |
Gas Chromatographic –Mass Spectroscopic analysis
The GC-MS analysis was carried out for fungal extract that showed the potential antimicrobial property. There- fore the ethyl acetate fungal crude extract of Alter- naria alternata showed total twelve peaks indicating the presence of twelve compounds (Fig. 6). Among them Hexadecanoic acid, methyl ester (35.73) showed high- est percentage of area followed by 9-Octadecenoic acid, methyl ester(E)- (24.55), 2-Fluorobenzoic acid, Hepta- decyl ester (12.79), 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (3.66), Methyl tetradecanohate (2.19), 2-Ethyl-1hexanol (1.94), Methyl hexadec-9-enoate (1.85), 1,2 Benzenedicarboxylic acid (0.93), Nonanedioic acid, dimethyl ester (0.92), 1-Nonadecene (0.91), and Heneicosane (0.82) (Table 3).
Compounds detected were predominantly derived from the ethyl extract especially hexadecanoic acid methyl ester which is an aliphatic acid ester reported to cause growth inhibition and apoptosis induction in human gastric cancer cells (Yu et al 2005). Number of com- pounds detected including 9-Octadecenoic acid, methyl ester (E)- (24.55) are to possess anti-inflammatory and cancer preventive properties.
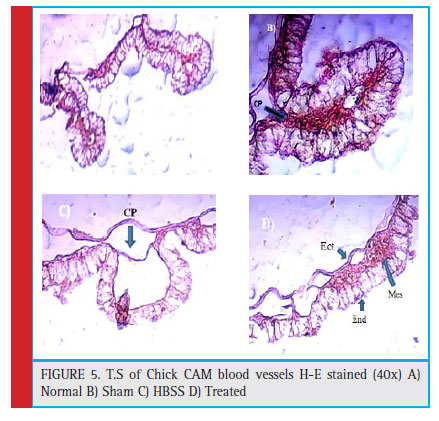 |
Figure 5: T.S of Chick CAM blood vessels H-E stained (40x) A) Normal B) Sham C) HBSS D) Treated |
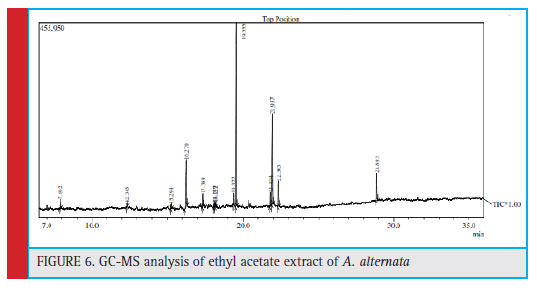 |
Figure 6: GC-MS analysis of ethyl acetate extract of A. alternata |
| Table 3: Phytoconstituents in Alternaria alternata ethyl acetate extract | ||||||||
| S. No | Name | Peak | R.T | I.T | F.T | Area | Area (%) | |
| 1. | 2-Ethyl-1-hexanol | 1 | 7.892 | 7.845 | 7.955 | 41804 | 1.94 | |
| 2. | 1,2-Benzenedicarboxylic acid | 2 | 12.345 | 12.300 | 12.395 | 20046 | 0.93 | |
| 3. | Nonanedioic acid, dimethyl ester | 3 | 15.294 | 15.260 | 15.325 | 19804 | 0.92 | |
| 4. | 2-Fluorobenzoicacid, heptadecyl ester | 4 | 16.270 | 16.160 | 16.335 | 275250 | 12.79 | |
| 5. | Methyl tetradecanoate | 5 | 17.389 | 17.345 | 17.420 | 47222 | 2.19 | |
| 6. | 1-Nonadecene | 6 | 18.149 | 18.100 | 18.180 | 19606 | 0.91 | |
| 7. | Heneicosane | 7 | 18.222 | 18.180 | 18.255 | 17585 | 0.82 | |
| 8. | Methyl hexadec-9-enoate | 8 | 19.322 | 19.280 | 19.360 | 39844 | 1.85 | |
| 9. | Hexadecanoic acid, methyl ester | 9 | 19.555 | 19.495 | 19.610 | 769183 | 35.73 | |
| 10. | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 10 | 21.824 | 21.765 | 21.870 | 78770 | 3.66 | |
| 11. | 9-Octadecenoic acid, methyl ester, (E) | 11 | 21.937 | 21.885 | 21.980 | 528586 | 24.55 | |
| 12. | Methyl stearate | 12 | 22.365 | 22.305 | 22.415 | 148763 | 6.91 | |
Conclusion
Many reports suggested the various medicinal properties of endophytic fungi Alternaria alternata as antimicrobial, antifungal, antidiabetic, anticancerous but this study has first time reported the anitangiogenic potential of terres- trial endophytic fungi Alternaria alternata isolated from leaves of medicinal plant Lawsonia inermis. The GC-MS analysis revealed the presence of 12 bioactive compounds which showed significant antimicrobial property against four bacterial pathogens showing significant zone of inhi- bition (21.8±0.8mm) against Ps.aeruginosa. Due to pres- ence of this bioactive metabolites, we were able to evalu- ate the antiangiogenic potential of Alternaria alternata ethyl acetate extract by observing the maximum inhibi- tion of blood vessels in treated CAM than the control one. This research work defines the very important biological potential which can be considered as potential candidate for antiangiogeneic treatment.
Acknowledgement
The authors are thankful to the Head, Department of Zoology and Fisheries, Department of Biotechnology and Principal, Yashavantrao Chavan Institute of Science, Satara for providing necessary laboratory facilities. We are also thankful to Research Advisory Committee of the institute for financial support. Authors extend their gratitude towards the authorities of Agharkar Research Institute, Pune for the identification of fungal endo- phytes and Dr. Jaykumar J. Chavan for technical help with chromatographic analysis.
References
Arnold A. E., Herre E .A., Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pat- tern and process in Theobroma cacao (Malvaceae). Mycologia, 2003, 95(3):388-398.
Bacon, C. W., and J. F. White. 2000. Microbial endophytes. Marcel Dekker Inc., New York, N.Y.
Bisht R, Sharma D, Agrawal PK (2016). Antagonistic and anti- bacterial activity of endophytic fungi isolated from needle of Cupressus torulosa D.Don.Asian J Pharm clin Res 9(3):282- 288)
Borgstorm P, Bourdon MA, Hillan KJ, Sriramarrao P, Ferrara N (1998) Neutralizing anti-vascular endothelial growth fac- tor antibody completely inhibits angiogenesis and growth of human prostrate carcinoma micro tumors in vivo. Prostrate 35:1-10.
Dreyfuss MM, Chapela IH (1994) Potential of fungi in the dis- covery of novel, low molecular weight pharmaceuticals. In: Gullo VP a. Endophytes and microbiomes. Annu Rev Phyto- pathol 49:291–315
Felsenstein J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39:783-791.
Folkman J. Angiogenesis inhibitors: a new class of drugs. Can- cer Biol Ther. 2003;2(suppl 1):S127–33. [PubMed]
Folkman J., 1971. Tumor angiogenesis: Therapeutic implicatio ns.N.Engl.J.Med.,285;1182-1186.
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29(6, Supplement 16):15–18.].
Holmgren L, O’Reilly MS,Folkman J (1995) Dormancy of micrometastasis: balance proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med.,1:149-153,).
Kimura M. (1980). A simple method for estimating evolution- ary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16:111- 120.
Korn, M.J. and Cramer K.S. (2009), Windowing Chicken eggs for developmental studies, J.Vis. EVP .8:306
Manjunath M Hullikere, Chandrashekhar G. Joshi, D. Ananda, Jagadeesh Poyya and T. Nivya, (2016) Antiangiogenic, wound healing and antioxidant activity of Cladosporium cladospori- oides (Endophytic Fungus) isolated from seaweed (Sargassum wightii), 211http://dx.doi.org/10.1080/21501203.2016.1263688
Manjunath M, Hullikere Chandrashekhar G. Joshi, T. Nivya, D. Ananda and N.G Raju (2016) Antiangiogenic and antioxidant activity of endophytic fungus isolated from seaweed (Sargas- sum wightii) Asian J.Biochem.,11:168-176.
Magaldi S, Mata-Essayag C, de Capriles, H. (2004) Well diffusion for antifungal susceptibility testing, Int. J. Infect. Dis.8:39-45.
Melkonian G., Chung, L., Marr, R. Tong, C and Talbot, P (2002). Main stream and Side stream cigarette smoke inhibit growth and angiogenesis in the day 5 chick chorioallantoic membrane, Toxicological Sci. 68:237-248
Radji M, Sumiati A, Rachmayani R, Elya B.(2011) Isolation of fungal endophytes from Garcinia mangostana and their antibac- terial activity. African Journal of Biotechnology, 10 (1):103-07.
Remesh A. (2017) Toxicities of anticancer drug and its manage- ment. Int.J.Basic Clin.Pharmacol.1-2,doi:10.5455/2319-2003, ijbcp000812
Ribatti, D., B Nico, A.Vacca, L. Roncali, P.H. Burri and V. Djonov (2001). Chorioallantoic membrane capillary bed: A useful target for studying angiogenesis and anti-angiogenesis in vivo. Anatom Rec 264:317-324.
Domenico, R., Angelo, V., Luisa, R., & Franco, D.(1996),The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. International Journal of Develop- mental Biology, , 40, 1189-1197.
Saitou N. and Nei M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4:406-425.
Schulz B, Boyle C, Draeger S, Rommmert AK, Krohn K (2002) -Endophytic fungi :a source of novel biologically active sec- ondary metabolites .Mycol Res. 106;996-1004.
Tamura K., Stecher G., Peterson D., Filipski A. and Kumar (2013). MEGA6: Molecular Evolutionary Genetics Analy- sis version 6.0. Molecular Biology and Evolution30: 2725- 2729.
Yu F, Lian X, Guo H, McGuire P, Li R, Wang R, Yu F. Iso- lation and characterzation of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells J. Pharm. Pharmaceut Sci. 2005,8(3):528-535.


