Department of Bioinformatics, Sathyabama Institute of Science and Technology
Corresponding author email: jemmychristy.bioinfo@sathyabama.ac.in
Article Publishing History
Received: 02/04/2020
Accepted After Revision: 24/05/2020
A stroke happens when the blood supply to the brain regions is interrupted or diminished leading to loss of oxygen, supplements, thereby brain death. The limited treatment options have given a way for natural compounds. Xyloketal B and CEP 1347 are isolated from Xylaria sp and another marine bacteria Nocardiopsis sp, widely found in the South China Sea, having potent anti-inflammatory, anti-apoptotic and neuroprotective properties. In this study, the marine compounds Xyloketal B and CEP 1347 are screened for its pharmacokinetic profile of a drug molecule to define the pharmacodynamic mechanisms using ADME (absorption, distribution, metabolism, and excretion) properties. Whereas, the potential anti-inflammatory targets such as PGR, STAT3, VEGFA, MMP9, SRC, CDK2, ErbB4 were obtained from the previous study on the functional associations between the inflammatory responses and ischemia (which includes ischemic stroke, cardio embolic stroke and hemorrhagic stroke) via gene regulatory network and pathway analysis approaches. Then, the marine compounds (Xyloketal B and CEP- 1347) are docked with anti-inflammatory target such as PGR, STAT3, VEGF, MMP9, SRC, CDK2, and ErbB4. Based on the strong inter molecular interactions using docking approach (having Six or more hydrogen bonds) the highest dock score, we conclude that inhibiting the anti-inflammatory targets, its signals and corresponding pathways can improve the stroke recovery by reducing the loss of nerve function, apoptosis of neuronal cells to promote morphogenesis, to promote immuno modulation which provides a new target for the clinical treatment of ischemia conditions. Studies have shown that Xyloketal B and CEP-1347 are novel inhibitors of many vital pathways, regulates of apoptotic neuronal events, reduces cell and tissue damage, reduces the level of inflammatory cytokines during ischemia and can be represented as anti-stroke therapy for superior stroke retrieval in the future.
Ischemia, Anti-inflammatory targets, Xyloketal B, CEP-1347, ADME descriptors, Receptor- ligand interactions.
Christy J, Shankari H. S. Anti-Inflammatory Potential of Marine Derived Compounds Xyloketal B and CEP 1347 for the Treatment of Ischemic Stroke. Biosc.Biotech.Res.Comm. 2020;13(2).
Christy J, Shankari H. S. Anti-Inflammatory Potential of Marine Derived Compounds Xyloketal B and CEP 1347 for the Treatment of Ischemic Stroke. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3c7iZTm
Copyright © Christy and Shankari This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
A stroke is a cerebrum assault, which happens when the blood stream to the regions of the brain is confined prompting limited oxygen supply that is required for cellular metabolism to keep the tissues alive. When the tissues become ischemic, except if the blood stream is quickly reestablished it leads to brain death. Ischemic stroke is brought about by the blockage of artery in the brain regions, representing around 87% of stroke cases, which adds to the major death cases and post-stroke inability in patients. Whereas, hemorrhagic strokes are caused when blood vessels blast inside (which is known as intracerebral drain) or on the surface of the brain (which is known as subarachnoid discharge). Hemorrhagic strokes are the most serious form and are related to higher danger within months. In cardioembolic stroke cases, the heart pumps unwanted materials into the brain circulating regions, bringing about the occlusion of the brain vessel and damage to the brain tissues (Arboix and Alió, 2010, Simats, et al., 2016; Chandra, et al.,2017, Agrawal et al.,2018).
According to the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC), 15 million people suffer stroke worldwide each year. In affected population, five million people die and a further five million have been permanently disabled. Stroke costs the United States an estimated $34 billion each year and £26 billion a year in the United Kingdom. By 2035, the prevalence of stroke will increase by more than 20% over 2016 and the direct medical costs are projected to reach $184.1 billion, which is a great challenge to human society and healthcare system. There are very limited number of FDA approved medications available for the treatment of stroke. A combination of medications is generally used to treat the condition and to prevent it happening again of such as alteplase (intravenous tissue plasminogen activator) that can dissolve the clots and restore the blood flow to brain is given via injection, antiplatelet, anticoagulants help to reduce the chances of another clot formation (such as aspirin, warfarin) is prescribed. Similarly, statins are prescribed to reduce the level of cholesterol in blood (French et al., 2016).
This gives wide opportunity for the natural compounds to act as protective agents, and which many experimental and clinical trials being performed. Marine compounds have diverse chemical structures, functions and therapeutic properties due to the physical and chemical marine environmental conditions ( Agrawal et al.,2018) which have led to the successful clinical studies of Xyloketal B and CEP 1347 for the development of marine derived anti-inflammatory drugs targeting ischemia conditions. Xyloketal B is a natural compound isolated from the mangrove fungus, Xylaria sp and CEP- 1345, isolated from Nocardiopsis sp widely found in the South China Sea and the compounds Xyloketal B is been widely studied for free radical damage, atherosclerosis, and CEP- 1347 for Parkinson disease (Maroney et al., 2001 Gong et al., 2018 Huang et al., 2019 ).
MATERIALS AND METHODS
Identification of the potential anti-inflammatory targets: The differentially expressed genes were obtained for all the 3 ischemia datasets such as ischemic stroke (GSE37587), cardio embolic stroke (GSE58294) and hemorrhagic stroke (GSE13353) from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo)and analyzed using R programming, GEO2r, is a web tool based on t-test (ANOVA) or analysis of variance and is useful to compare two or more groups of samples in a GEO series, to identify differentially expressed genes. The DEG’s are then screened out through the cut off criteria of adjusted Log2 (FC)| > 1 (Fan et al., 2017).
Similarly, the inflammatory properties were also studied using inflammatory genes obtained from Gene cards and Harmonizome database. Then, the 2,422 overlapping genes were obtained using Venn diagram, for which protein- protein interaction reactome network has been constructed having 7,411 nodes and 61,158 edges. Subsequently, using graph theory approach 63 clusters were identified using MCODE analysis to detect the dense and closely related group of proteins that share similar functionality with Degree Cut-off as2, K-Core as 2(Christy and Priyadharshini,2018). Out of which, 10 clusters have been selected having nodes <= 10, which are classified as group of proteins that share a similar functionality (Myers et al.,2015). Now, pathway analysis and enrichment analysis are made to study the functional associations of the genes and the conditions. This has led to the identification of the potential anti-inflammatory targets based on further refinement and target proteins were listed in Table 1.
Table 1: Anti-inflammatory targets
| Genes | Names | FDA Approval | PDB ID | Favourable Region |
| PGR | Progesterone receptor | Yes | 2OVH | 95.6% |
| STAT3 | Signal transducer and activator of transcription 3 | Yes | 1BG1 | 80.6% |
| MMP9 | Matrix metalloproteinase-9 | Yes | IGKC | 90.4% |
| VEGFA | Vascular endothelial growth factor A | Yes | 1FLT | 92.6% |
| ErbB4 | Receptor tyrosine-protein kinase erbB-4 | Yes | 2R4B | 81.9% |
| CDK2 | Cyclin-dependent kinase 2 | Yes | 1URW | 89.3% |
| SRC | Proto-oncogene tyrosine-protein kinase Src | Yes | 2SRC | 89.4% |
Target Protein retrieval and pre pre-processing : The structure of the anti-inflammatory targets such as PGR, STAT3, MMP9, VEGFA, CDK2, ErbB4, SRC were downloaded from PDB in PDB format. The hetero atoms and alternative conformers are then deleted to provide enrichment to the receptor- ligand interaction study. To make the protein stable, we have minimized the energy by applying force field CHARMm (Brooks et al.,2009) (Chemistry at HARvard Macromolecular Mechanics) and energy minimization algorithm conjugate gradient with the help of DS 2.1. The binding site of the proteins has also been cross validated with Cavity plus web tool, which provides potential binding sites on the surface of a given protein and rank them with ligandability and druggability scores based on structural geometry and CAVITY algorithm. The ligandability value represents the possibility of designing small ligands with high binding affinities and the druggability predicts the possibilities of the cavity being a good target. It predicts maximum pKd, DrugScore and Druggability of all the detected cavities and this cavity score is influenced by cavity volume, pocket lip size, hydrophobic volume, cavity surface area, and hydrogen-bond-forming surface area (Xu et al.,2018)
Therapeutic ligand PK/PD property screening: The 3D structure of the Xyloketal B and CEP 1347 is obtained from PubChem in SDF format. The obtained structure is then screened for its drug likeliness property using Discovery studio 2.1. The properties such as absorption, distribution, metabolism, excretion and toxicity (ADMET), which are the important parameters to determine a compound for therapeutic use. These properties are co-related with the descriptors such as BBB, which determines the distribution of compounds in human body, low molecular weight for absorption. The output ellipses describe the regions where well absorbed compounds are expected to be found, and 95%, 99% confidence limit ellipse corresponding to BBB and intestinal absorption models are indicated (Ponnan et al.,2012)
Intermolecular Docking assessment: The targets are downloaded from Protein data bank in PDB format and the potential binding sites are identified using DS 2.1. A total of 7 proteins were docked with Xyloketal B and CEP-1347 using Ligand Fit protocol and CHARMm force field in DS which provides shape-based docking for accurately docking the ligand into the protein active sites. The active sites are predicted using grid search and ERASER algorithm. This method then combines monte carlo conformational search along with shape comparison filter for generating ligand poses consistent with active site shape. Then the docked poses are analyzed using potential of dock score and non-bonded interactions (Meng et al.,2011). The scoring functions are used to identify correct poses from incorrect poses, which is the sum of all non-bonded interactions such as hydrogen bond, hydrophobic effect, ionic interactions and binding entropy expressed in terms of like LigScore ,PLP, JAIN, PMF, and dock score (Ferreira et al.,2015).
RESULTS AND DISCUSSION
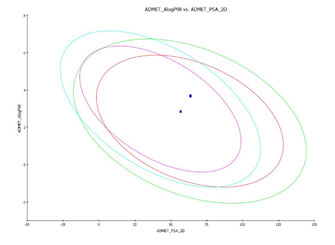
ADMET Screening of ligands : In silico ADME analysis is performed using ADME Descriptors using Accelrys Discovery studio 2.1, in which various pharmacokinetics parameters such as Blood brain barrier (BBB), Hepatotoxicity, Plasma protein binding (PPB), cytochrome p450 inhibition were predicted for Cep-1347 and Xyloketal B. These levels influence the drug intensity and drug exposure to the tissues and thereby influencing the pharmacological activity of the compounds (Li ,2001). The results of the pharmacokinetic screening revealed that xyloketal B and CEP- 1347 followed Lipinki’s rule of five for bioavailability. However compound CEP 1347 shows high lipophilic nature due to high log p value, whereas, xyloketal B has shown 99% and 95% confidence level for intestinal absorption and BBB and falls inside the ellipse model. Figure1 ADMET result for Xyloketal B showing 95% and 99% confidence limit ellipse corresponding to Blood brain barrier and intestinal absorption models. Red and green color indicates absorption and blue and pink indicates BBB. ADMET result for CEP 1347 showing 95% and 99% confidence limit ellipse corresponding to Blood brain barrier and intestinal absorption models. Red and green colour indicates absorption and blue and pink indicates BBB and the Figure 2 depicted the details. Table 2 ADMET descriptors for Xyloketal B and CEP 1347
Table 2. ADMET descriptors for Xyloketal B and CEP 1347
| Parameters | Xyloketal B | CEP 1347 |
| BBB | 2 | 4 |
| PPB | 0 | 2 |
| CYP450 | 0.30 | 0.33 |
| Hepatotoxicity | 0.6 | 0.6 |
| Intestinal absorption | 0 | 1 |
| Aq. Solubility and drug likeliness | 0 | 0 |
Figure1: ADMET result for Xyloketal B and CEP 1347 showing 95% and 99% confidence limit ellipse corresponding to Blood brain barrier and intestinal absorption models. Red and green color indicates absorption and blue and pink indicates BBB
Figure 2
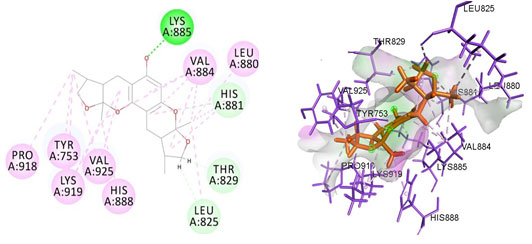
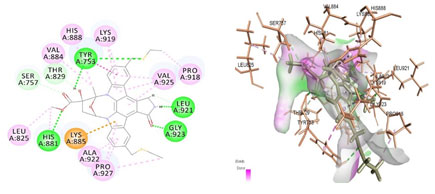
Figure 3: Docked poses of PGR druggable cavity with xyloketal B. Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Lys 885) mediates the hydrogen bond,then His 881,Thr 829,Leu 825 formed the carbon hydrogen bond and residues Leu 880, Val 884, Val 925, His 888, Lys 919, Tyr 753, Pro 918 involves in pi-Alkyl interaction with the receptor PGR.
Study of interactions between Xyloketal B, CEP 1347 and anti-inflammatory targets
Possible binding modes of Xyloketal B with the anti-inflammatory target receptors PGR, STAT3, VEGFA, SRC, CDK2, ErbB4, MMP9 were studied using Ligand Fit module in DS 2.1. The best docking pose and their dock score (Ligand/ Receptor interaction study + Ligand internal energy) as well as the non-bonded interactions (strong hydrogen bonds such as carbon and conventional hydrogen bonds) are noted to assess the affinity of the bonding. Higher the number of hydrogen bonds, more the solubility and more permeability for passive diffusion of the lead compounds. For cross validation of receptor ligand docking, cavity plus results has been used to show the interactions more favourable for validation.
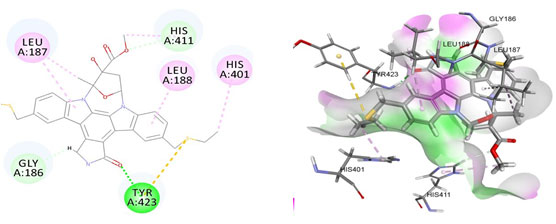
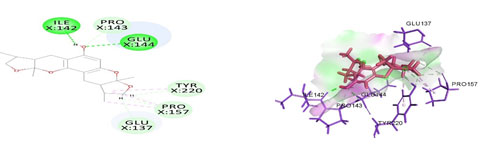
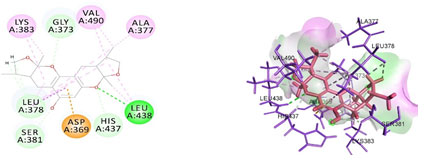
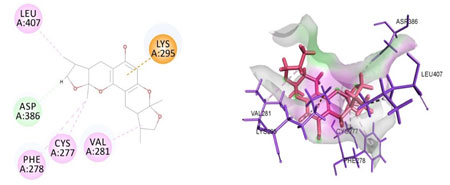
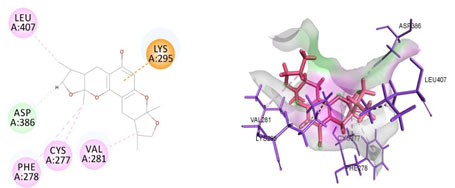
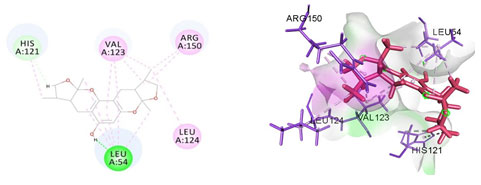
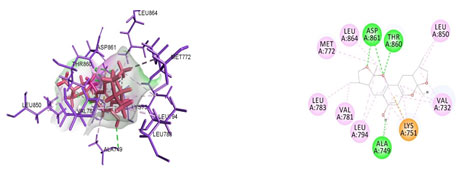
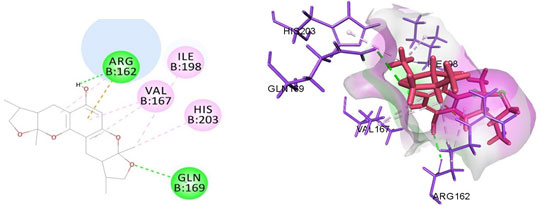
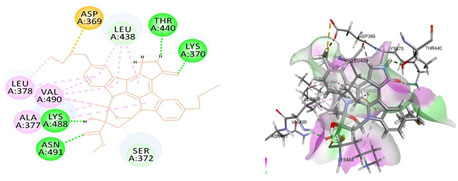
Cavity module screening of anti-inflammatory targets revealed their draggability as well as ligandability nature. Xyloketal B intermolecular interactions with PGR listed in Table 4 and depicted in the Figure 3. Lys885, His881, Thr 829, Leu825 were the residues from druggable cavity mediated the hydrogen bond interaction within 2.5Ao distance. Within the same cavity pi-alkyl bonds interactions mediated pocket residues stabilize the intermolecular interactions. In general Pi-sigma interactions like Pi-alkyl and Pi-Sulphur which mainly largely comprise charge transfer aids in intercalating the drug within the druggable cavity of the receptor. Similarly, with STAT3 and other anti-inflammatory targets also mediated Hydrogen bond in addition to pi cation and pi alkyl bonds and interacting residues were listed in table 4 and depicted in figure 4. Residues Leu 438, His 437, Ser 381, Leu 378, Gly 373, Lys 383, Val 490, Ala377, Asp369 favoured the maximum number of interactions in the proposed target Table 5 and Figure 4. Glu 144, Ile 142 mediates the hydrogen bond, then Pro 143, Tyr 220, Pro 157, Glu 137 formed the carbon hydrogen bond interaction with the receptor VEGFA depicted in figure 5 and Table 6. Xyloketal B affinity to SRC analysed based on molecular docking and the interactions depicted in Figure 6 and residues involved in interactions are listed in Table 7.CDK2 intermolecular interactions with Xyloketal B mediated by hydrogen bonds and listed in Table 8, interacting residues and bonding distance were depicted figure 7.Similarly ErbB4 and MMP9 mediated interaction with Xyloketal B were depicted in Figure 8 and Table9 , Figure9 and Table 10 respectively.
Table 3. Output of cavity module for anti-inflammatory targets
| Proteins | Druggability |
| PGR | Druggable |
| STAT3 | Druggable |
| MMP9 | Druggable |
| VEGFA | Druggable |
| ErbB4 | Druggable |
| CDK2 | Druggable |
| SRC | Druggable |
Table 3. Non-bonded interactions between Xyloketal B and PGR
| Ligand | Dock score | Amino acid
(PGR) |
Atom | Distance | |
| RECEPTOR | LIGAND | ||||
| Xyloketal B | 66.019 | LYS 885
LYS885 HIS 881 THR 829 LEU 825 |
HZ1
HE2 HA OG1 O |
O5
O5 O4 H36 H37 |
2.96989
2.28316 2.09333 2.25979 2.76227 |
Table 4. Non- bonded interactions between Xyloketal B and STAT3
| Ligand | Dock score | Amino acid
(STAT3) |
Atom | Distance | |
| RECEPTOR | LIGAND | ||||
| Xyloketal B | 52.471 | LEU 438
LEU 378 LEU 378 GLY373 SER 381 HIS 437 |
HN
O 0 HA2 HB2 HA |
O4
H34 H35 O1 O5 O4 |
2.5787
2.58422 2.67216 2.35742 2.23962 2.43909 |
Table 5. Non- bonded interactions between Xyloketal B and VEGFA
| Ligand | Dock score | Amino acid
(VEGFA) |
Atom | Distance | |
| RECEPTOR | LIGAND | ||||
| Xyloketal B | 28.394 | GLU 144
ILE 142 PRO 143 PRO 157 TYR 220 GLU 137 PRO 157 |
HN
O HA O OH OE1 O |
O5
H51 O5 H36 H36 H37 H37 |
2.09583
1.68155 2.36209 2.44219 2.25501 2.59563 2.9771 |
Table 6. Non- bonded interactions between Xyloketal B and SRC
| Ligand | Dock score | Amino acid
(SRC) |
Atom | Distance | |
| Ligand | Receptor | ||||
| Xyloketal B | 69. 839 | ASP 386 | H34 | OD2 | 2.94002 |
Figure 4: Docked poses of STAT3 druggable cavity with xyloketal B.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction pink colored dashed lines,Asp 369 mediated pi cation interaction with the receptor STAT3.
Figure 5: Docked poses of VEGFA druggable cavity with xyloketal B.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Glu 144, Ile 142) mediates the hydrogen bond, then Pro 143,Tyr 220,Pro 157,Glu 137 formed the carbon hydrogen bond interaction with the receptor VEGFA.
Figure 6: Docked poses of SRC druggable cavity with xyloketal B. Interactions are shown as dashed lines between receptor residues and ligand atoms. Asp386 formed the carbon hydrogen bond interaction, Leu 407, Cys 277, Val 281, Phe 278 were the residues involved in pi-alkyl bonding pink colored dashed lines, then Lys 295 mediated pi cation interaction with the receptor SRC.
Figure 7: Docked poses of CDK2 druggable cavity with xyloketal B. Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 54) mediates the hydrogen bond, then His 121 formed the carbon hydrogen bond and residues Leu 124, Val 123, Arg 150 involves in pi-Alkyl interaction with the receptor CDK2.
Figure 8: Docked poses of ErbB4 druggable cavity with xyloketal B. Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Ala 749,Thr 860,Asp 861) mediates the hydrogen bond,Leu 794,Val 781,Leu 783,Val 732,Leu 850,Leu 864,Met 772 involves in pi-Alkyl interaction.Lys 751 mediates the pi-cation interaction with ErbB4.
Figure 9: Docked poses of MMP9 druggable cavity with xyloketal B. Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Arg 162,Gln 169) mediates the hydrogen bond, residues Val 167,His 203,Ile 198 involves in pi-Alkyl interaction with the receptor MMP 9.
Study on non- bonded interactions between CEP-1347 and anti- inflammatory targets :CEP-1347 is a indolocarbazole derivatives derived from Marine bacterial sps Nocardiopsis. It is considered as potent inhibitor of SAPK/JNK pathway which activated after neuronal toxic insults in neurons. In our studies CEP-1347 was assessed for their affinity with anti-inflammatory targets namely PGR, STAT3, MMP9, VEGFA, CDK2, ErbB4, SRC and MMP9.Pharmacophore features of this potent inhibitor showed better binding affinity and Figures 10,11,12,13,14,15,16 depicted the intermolecular interactions. Interaction mediated by hydrogen bonds, pi-cation, pi-alkyl and carbon hydrogen bonds. Interacting residues listed in Tables 11,12,13,14,15 and 16.Three different datasets on ischemia conditions such as ischemic stroke, cardio embolic stroke and haemorrhagic stroke were analysed. The control and diseased samples from each dataset are studied using techniques such as differential analysis of the genes, networking, pathway, functional analysis, hub genes validation, expression level studies using graph theory.
Figure 10: Docked poses of PGR druggable cavity with CEP 1347.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction,Asp 369 mediated pi cation interaction with the receptor STAT3.
Figure 11: Docked poses of STAT3 druggable cavity with CEP 1347.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction represented in pink colored dashed lines ,Asp 369 mediated pi cation interaction with the receptor STAT3.
Figure 12: Docked poses of VEGFA druggable cavity with CEP 1347.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction,Asp 369 mediated pi cation interaction with the receptor VEGFA
Figure 13: Docked poses of SRC druggable cavity with xyloketal B. Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction,Asp 369 mediated pi cation interaction with the receptor SRC.
Figure 14: Docked poses of CDK2 druggable cavity with xyloketal B.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction,Asp 369 mediated pi cation interaction with the receptor CDK2
Figure 15: Docked poses of ErbB4 druggable cavity with xyloketal B.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction,Asp 369 mediated pi cation interaction with the receptor ErbB4.
Figure 16: Docked poses of MMP9 druggable cavity with xyloketal B.Interactions are shown as dashed lines between receptor residues and ligand atoms. Residues highlighted in Green (Leu 438) mediates the hydrogen bond, then His 437, Ser 381, Leu 378 and Gly 373 formed the carbon hydrogen bond and residues Lys 383, Val 490, Ala 377 involves in pi-Alkyl interaction,Asp 369 mediated pi cation interaction with the receptor MMP9.
These analyses helped us to predict that, the potential anti-inflammatory targets, according to Human Protein Atlas, the proposed targets are FDA approved, such as PGR(Liu et al.,2012; Zhu et al.,2017; Gibson et al.,2009),STAT3(Wang et al., 2017;Liang et al., 2016), MMP9 (Chaturvedi et al.,2014; Christy,2013), VEGFA(Greenberg et al.,2013), CDK2 (Osuga et al.,2000), ErbB4 (Robert et al.,2013, Qian et al.,2018) and SRC plays vital role in the neuro immunology, neuronal development such as angiogenesis, apoptosis, motor performances, pathological events through activation of multiple signalling pathways, and by regulating many numbers of proteins that participate in the post-ischemic events to can attenuate the expressions(. The inter-molecular interaction study revealed that Xyloketal B has higher interactions with PGR, STAT3, VEGFA, and ErbB4 with dock score 66.019, 52.471, 28.394, 66.695 and CEP 1347 having higher interactions with PGR, STAT3, SRC, ErbB4, and MMP9 with dock score 70.491, 57.725,99.144, 121.495 and 62.72 respectively.
Table 7. Non- bonded interactions between Xyloketal B and SRC
| Ligand | Dock score | Amino acid
(VEGFA) |
Atom | Distance | |
| RECEPTOR | LIGAND | ||||
| Xyloketal B | 28.394 | GLU 144
ILE 142 PRO 143 PRO 157 TYR 220 GLU 137 PRO 157 |
HN
O HA O OH OE1 O |
O5
H51 O5 H36 H36 H37 H37 |
2.09583
1.68155 2.36209 2.44219 2.25501 2.59563 2.9771 |
Table 8. Non- bonded interactions between Xyloketal B and CDK2
| Ligand | Dock score | Amino acid
(CDK2) |
Atom | Distance | |
| Ligand | Receptor | ||||
| Xyloketal B | 65.243 | LEU 54
HIS 121 |
H51
H34 |
O
O |
1.86881
2.8994 |
Table 9: Non- bonded interactions between Xyloketal B and ErbB4
| Ligand | Dock score | Amino acid
(ErbB4) |
Atom | Distance | |
| Ligand | Receptor | ||||
| Xyloketal B | 66. 695 | THR 860
ASP 800 ASP 861 ALA 749 |
O1
O1 02 H51 |
HG1
HN HN O |
2.08283
2.52537 2.17772 2.57675 |
Table 10. Non- bonded interactions between Xyloketal B and MMP9
| Ligand | Dock score | Amino acid
(MMP9) |
Atom | Distance | |
| Ligand | Receptor | ||||
| Xyloketal B | 61.088 | ARG 162
GLN 169 |
H51
03 |
O
HE22 |
2.36905
2.77257 |
Table 11. Non- bonded interactions between CEP-1347 and PGR
| Ligand | Dock score | Amino acid
(PGR) |
Atom | Distance | |
| Ligand | Receptor | ||||
| CEP-1347 | 70. 491 | TYR 753
HIS 881 GLY 923 TYR 753 LEU 921 SER 757 THR 829 LEU 921 |
S1
O5 O7 H50 H57 O6 O4 H52 |
HH
HD1 HN O O HB2 HB O |
2.580589
2.79844 1.93441 1.72248 2.14658 2.51224 2.95172 2.06928 |
Table 12. Non- bonded interactions between CEP-1347 and STAT3
| Ligand | Dock score | Amino acid
(VEGFA) |
Atom | Distance | |
| Ligand | Receptor | ||||
| CEP 1347 | 36.199 | GLN 22
ASN 219 |
O4
H64 |
HE21
OD1 |
2.65442
2.9773 |
Table 13. Non- bonded interactions between CEP-1347 and VEGFA
| Ligand | Dock score | Amino acid
(STAT3) |
Atom | Distance | |
| Ligand | Receptor | ||||
| CEP-1347 | 57.725 | LYS 370
ASN 491 LYS 488 THR440 SER 372 LEU 438 LYS 370 |
O7
06 H50 H57 O3 H51 H52 |
HZ2
HD22 O OG1 HB2 O O |
1.65372
1.86214 1.76279 2.09157 2.83771 2.37833 2.59893 |
Table 14 Non- bonded interactions between CEP-1347 and SRC
| Ligand | Dock score | Amino acid
(SRC) |
Atom | Distance | |
| Ligand | Receptor | ||||
| CEP- 1347 | 99.144 | CYS 277
LYS 295 GLY 276 ASP 404 ARG 388 ARG 388 |
O4
O6 O4 H44 – – |
HN
HZ2 HA2 OD2 HH12 HH22 |
1.81155
1.62797 2.69631 2.85921 2.85918 2.69236 |
Table 15 Non- bonded interactions between CEP-1347 and CDK2
| Ligand | Dock score | Amino acid
(CDK2) |
Atom | Distance | |
| Ligand | Receptor | ||||
| CEP-1347 | 111. 05 | ASP 145
ILE 10 |
O5
H51 |
HN
O |
2.87654
3.06647 |
Table 16 Non- bonded interactions between CEP- 1347 and ErbB4
| Ligand | Dock score | Amino acid
(ErbB4) |
Atom | Distance | |
| Ligand | Receptor | ||||
| CEP-1347 | 121.495 | MET 799
GLN 797 GLY 725 GLY 725 |
O7
H57 O3 O6 |
HN
O HA2 HA2 |
1.91772
2.67839 2.92012 2.57601 |
Table 17 Non- bonded interactions between CEP- 1347 and MMP9
| Ligand | Dock score | Amino acid
(MMP9) |
Atom | Distance | |
| Ligand | Receptor | ||||
| CEP-1347 | 62.72 | ASP 800
ASP 800 LEU 718 LEU 788 |
H61
H56 H57 H69 |
OD2
OD2 O O |
2.5093
1.75975 2.90109 3.03434 |
Through Cavity plus results, we ensured that the ligands are docked on the druggable cavities (having positive amino acids) of the receptor which has led to higher number of hydrogen bonds based on drug actions. This indicates that the intermolecular interactions are strong between the targets and the lead compounds, high solubility, high permeability across the membranes leading to good bioavailability of the compounds. There are many experimental and clinical studies being performed on xyloketal B and CEP-1347 as an anti-oxidative, anti-apoptotic showing positive results, which gives us a possibility for bring better stroke recovery (Xiao et al.,2014).
CONCLUSION
Computational analysis of Xyloketal B and CEP 1347 and their interactions with anti-inflammatory targets such as PGR, STAT3, MMP9, VEGFA, CDK2, ErbB4, and SRC have shown higher binding affinity with strong favourable hydrogen bond interactions. Cerebral ischemia/reperfusion injury generates various cell apoptotic pathways, it’s evident that ischemia-stimulated oxidative stress as well as inflammation are primarily responsible for the following cell death by necrotic or apoptotic processes. Reactive oxygen species (ROS) control cell survival/death by enabling several cells signaling process and related pathways, particularly JAK2/STAT3 stimulation makes a significant contribution to cell apoptosis subsequent ephemeral focal cerebral ischemia. In generic expression of VEGF-A in ischemic stroke group resulted in a decrease state as compared with the non-stroke group, thus VEGF signaling process correspond to vital targets for treatment of stroke. CDK inhibitors blocks pRb phosphorylation and the increase in E2F1 levels and dramatically reduces neuronal death by 80%. These results suggest that CDKs are a promising therapeutic target for the treatment of ischemia. Here we have reported that the protective effects of Xyloketal B and CEP-1347 in brain injury and neuroinflammation by inhibiting STAT3 activation. PR is a key factor for endogenous neuroprotection; thus, they are deemed to be pharmacological target to treat stroke. The docking studies have shown that these compounds having anti-inflammatory, anti-apoptotic, anti-oxidative abilities can serve as treatment for ischemic conditions. Also, many experimental studies and clinical studies have been performed for reperfusion conditions, neurological disorders and extensive studies on stroke can be carried out for prevention and better recovery.
REFERENCES
Agrawal S ., Sachin Chaugule ., Madhavi Indap.(2018). Marine Pharmaceuticals: A New Wave of Anti-angiogenic Drugs, Journal of Oceanography and Marine Research., 6, article no. 1000174
Arboix, A., & Alió, J. (2010). Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Current cardiology reviews, 6(3), 150–161. https://doi.org/10.2174/157340310791658730
Brooks, B. R., Brooks, C. L., 3rd, Mackerell, A. D., Jr, Nilsson, L., Petrella, R. J., Roux, B., Won, Y., Archontis, G., Bartels, C., Boresch, S., Caflisch, A., Caves, L., Cui, Q., Dinner, A. R., Feig, M., Fischer, S., Gao, J., Hodoscek, M., Im, W., Kuczera, K., … Karplus, M. (2009). CHARMM: the biomolecular simulation program. Journal of computational chemistry, 30(10), 1545–1614. https://doi.org/10.1002/jcc.21287
Chandra, A., Li, W. A., Stone, C. R., Geng, X., & Ding, Y. (2017). The cerebral circulation and cerebrovascular disease I: Anatomy. Brain circulation, 3(2), 45–56. https:// doi.org/ 10.4103/bc.bc_10_17
Chaturvedi, M., & Kaczmarek, L. (2014). Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Molecular neurobiology, 49(1), 563–573. https://doi.org/ 10.1007/ s12 035-013-8538-z
Christy HJ, Priyadharshini L. Differential expression analysis of JAK/STAT pathway related genes in breast cancer. Meta Gene 2018;16:122-9.
Christy J. (2013) Computational analysis of disease associated nssnps in mmp2. Int J Pharm Bio Sci ;4:504-12
Fan L, Yu X, Huang Z, Zheng S, Zhou Y, Lv H, et al., (2017) Analysis of microarray-identified genes and MicroRNAs associated with idiopathic pulmonary fibrosis. Mediators Inflamm. doi: 10.1155/2017/1804240.
Ferreira, L. G., Dos Santos, R. N., Oliva, G., & Andricopulo, A. D. (2015). Molecular docking and structure-based drug design strategies. Molecules (Basel, Switzerland), 20(7), 13384–13421. https://doi.org/10.3390/molecules200713384
French, B. R., Boddepalli, R. S., & Govindarajan, R. (2016). Acute Ischemic Stroke: Current Status and Future Directions. Missouri medicine, 113(6), 480–486.
Gibson, C. L., Coomber, B., & Rathbone, J. (2009). Is progesterone a candidate neuroprotective factor for treatment following ischemic stroke?. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry, 15(4), 324–332. https://doi.org/10.1177/1073858409333069
Gong, H., Luo, Z., Chen, W., Feng, Z. P., Wang, G. L., & Sun, H. S. (2018). Marine Compound Xyloketal B as a Potential Drug Development Target for Neuroprotection. Marine drugs, 16(12), 516. https://doi.org/10.3390/md16120516
Greenberg, D. A., & Jin, K. (2013). Vascular endothelial growth factors (VEGFs) and stroke. Cellular and molecular life Sciences: CMLS, 70(10), 1753–1761. https://doi.org/ 10.1007/s00018-013-1282-8
Huang C, Zhang Z and Cui W (2019) Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease, Marine Drugs, 11 Apr 2019, 17(4)
Li A. P. (2001). Screening for human ADME/Tox drug properties in drug discovery. Drug discovery today, 6(7), 357–366. https://doi.org/10.1016/s1359-6446(01)01712-3
Liang, Z., Wu, G., Fan, C., Xu, J., Jiang, S., Yan, X., Di, S., Ma, Z., Hu, W., & Yang, Y. (2016). The emerging role of signal transducer and activator of transcription 3 in cerebral ischemic and hemorrhagic stroke. Progress in neurobiology, 137, 1–16. https://doi.org/ 10.1016/j.pneurobio.2015.11.001
Liu, A., Margaill, I., Zhang, S., Labombarda, F., Coqueran, B., Delespierre, B., Liere, P., Marchand-Leroux, C., O’Malley, B. W., Lydon, J. P., De Nicola, A. F., Sitruk-Ware, R., Mattern, C., Plotkine, M., Schumacher, M., & Guennoun, R. (2012). Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology, 153(8), 3747–3757. https://doi.org/10.1210/en.2012-1138
Maroney AC, Finn JP, Connors TJ, Durkin JT, Angeles T, Gessner G, Xu Z, Meyer SL, Savage MJ, Greene LA, Scott RW, Vaught JL.(2001). Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J Biol Chem. 276:25302-25308.
Meng, X. Y., Zhang, H. X., Mezei, M., & Cui, M. (2011). Molecular docking: a powerful approach for structure-based drug discovery. Current computer-aided drug design, 7(2), 146–157. https://doi.org/10.2174/157340911795677602
Myers, J. S., von Lersner, A. K., Robbins, C. J., & Sang, Q. X. (2015). Differentially Expressed Genes and Signature Pathways of Human Prostate Cancer. PloS one, 10(12), e0145322.https://doi.org/10.1371/journal.pone.0145322
Osuga, H., Osuga, S., Wang, F., Fetni, R., Hogan, M. J., Slack, R. S., Hakim, A. M., Ikeda, J. E., & Park, D. S. (2000). Cyclin-dependent kinases as a therapeutic target for stroke. Proceedings of the National Academy of Sciences of the United States of America, 97(18), 10254–10259. https://doi.org/10.1073/pnas.170144197
Ponnan, S. Gupta, M. Chopra, R. Tandon, S.A. Baghel, G. Gupta, A.K. Prasad, R.C. Rastogi,M. Bose, H.G. Raj, (2012) .2D-QSAR, Docking Studies, and In Silico ADMET Prediction of Polyphenolic Acetates as Substrates for Protein Acetyltransferase Function of Glutamine Synthetase of Mycobacterium tuberculosis, ISRN Struct Biol 2013, 1-12.
Qian, H., Dou, Z., Ruan, W., He, P., Zhang, J. H., & Yan, F. (2018). ErbB4 Preserves Blood-Brain Barrier Integrity via the YAP/PIK3CB Pathway After Subarachnoid Hemorrhage in Rats. Frontiers in neuroscience, 12, 492. https://doi.org/ 10.3389/ fnins. 2018.00492
Robert M. Mitchella, Megan J. Janssena, Irina Karavanovaa , Detlef Vullhorsta , Katrina Furtha, Anthony Makuskyc , Sanford P. Markeyc , and Andres Buonanno.(2013) ErbB4 reduces synaptic GABAA currents independent of its receptor tyrosine kinase activity, PNAS., 110, pp. 19603-19608.
Simats, A., García-Berrocoso, T., & Montaner, J. (2016). Neuroinflammatory biomarkers: From stroke diagnosis and prognosis to therapy. Biochimica et biophysica acta, 1862(3), 411–424. https://doi.org/10.1016/j.bbadis.2015.10.025
Wang XL, Qiao CM, Liu JO, Li CY. Inhibition of the SOCS1-JAK2-STAT3 Signaling Pathway Confers Neuroprotection in Rats with Ischemic Stroke. Cell Physiol Biochem. 2017;44(1):85–98. doi:10.1159/000484585
Xiao, A. J., Chen, W., Xu, B., Liu, R., Turlova, E., Barszczyk, A., Sun, C. L., Liu, L., Deurloo, M., Wang, G. L., Feng, Z. P., & Sun, H. S. (2014). Marine compound xyloketal B reduces neonatal hypoxic-ischemic brain injury. Marine drugs, 13(1), 29–47. https://doi.org/10.3390/md13010029
Xu, Y., Wang, S., Hu, Q., Gao, S., Ma, X., Zhang, W., Shen, Y., Chen, F., Lai, L., & Pei, J. (2018). CavityPlus: a web server for protein cavity detection with pharmacophore modelling, allosteric site identification and covalent ligand binding ability prediction. Nucleic acids research, 46(W1), W374–W379.
https://doi.org/ 10.1093/ nar/ gky380Zhu, X., Fréchou, M., Liere, P., Zhang, S., Pianos, A., Fernandez, N., Denier, C., Mattern, C., Schumacher, M., & Guennoun, R. (2017).A Role of Endogenous Progesterone in Stroke Cerebroprotection Revealed by the Neural-Specific Deletion of Its Intracellular Receptors. The Journal of neuroscience: the official journal of the Society for Neuro science, 37(45), 10998–11020. https://doi.org/10.1523/JNEUROSCI.3874-16.2017