Department of Microbiology, SIES College of Arts, Science and Commerce, Mumbai, India
Corresponding author email: carolbraggs@gmail.com
Article Publishing History
Received: 28/04/2021
Accepted After Revision: 30/06/2021
Bacterial persisters are phenotypic variants that form under stress, cause recurrent infections and also possess antibiotic tolerance. The use of colloidal silver to combat persisters seems to be a promising alternative since bacterial tolerance towards metals or metal ions has not been reported. The objective of this study was to determine the effect of colloidal silver on E. coli K-12 NCIM 2665 persisters to Ampicillin. The study determined the effect of colloidal silver on E. coli persisters to Ampicillin. The combined action of Ampicillin and silver against persisters was determined by checkerboard assay. Tolerance of log phase population of E. coli K-12 NCIM 2665 to silver and whether persisters to silver are formed was also determined. The Fractional Inhibitory Concentration (ƩFIC) index for Ampicillin and colloidal silver was determined to be ≤ 1 which indicates an additive effect.
A five-log reduction in log phase population and two log reduction in antibiotic persisters was observed after one hour exposure to 16ppm silver; indicating the effectiveness of colloidal silver. Colloidal silver decreases the formation of Ampicillin persisters as well as prevents the survival of existing Ampicillin persisters. In order to combat recurrent bacterial infections, methods need to be found to reduce the formation of pathogenic bacterial persisters or to enhance the susceptibility of persisters to antibiotics. The results of the present study imply that colloidal silver can be used as an anti-persister strategy directly or in combination with an antibiotic.
Colloidal silver, E. coli K-12, Persister cells
Braggs C, Desouza A. Analysis on the Novel Approach of Using Colloidal Silver Against E. coli Persisters to Ampicillin. Biosc.Biotech.Res.Comm. 2021;14(2).
Braggs C, Desouza A. Analysis on the Novel Approach of Using Colloidal Silver Against E. coli Persisters to Ampicillin. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/3xAjsse“>https://bit.ly/3xAjsse</a>
Copyright © Braggs and Desouza This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The host immune system has a variety of mechanisms that can combat bacterial infections. However, pathogens like Pseudomonas aeruginosa, Mycobacterium tuberculosis, Helicobacter pylori, Candida albicans not only evade the host immune system, but also persist in the host even after antibiotic treatment resulting in recurrent infections (Jayaraman 2008; Dawson et al. 2011; Fauvart et al. 2011; Hong-Geller and Micheva-Viteva 2015; Fisher et al. 2016; Moorhouse et al. 2016).
One of the main factors that contribute to the recurrence of infection is persistence. Persistence was first observed as a phenomenon by Bigger when a population of S. aureus survived after treatment with Penicillin (Bigger 1944). Bacterial persisters are distinct physiological variants that exhibit tolerance to antimicrobial agents. Tolerance towards antimicrobials makes treatment of such recurrent infections difficult (Moorhouse et al. 2016).
Persistence differs from antibiotic resistance since resistant cells grow in the presence of higher doses of antibiotics while persister cells remain dormant. Multidrug tolerance of persisters is one of the major factors for recurrent infections and the inability of antibiotics to eliminate the pathogen (Waters and Bassler, 2005; Lewis, 2008; Moker et al. 2008; Lewis, 2010). Many antibiotics have been shown to be active only against dividing bacteria. Persister cells are known to greatly slow down essential cellular functions that are generally targeted by antibiotics such as transcription, translation, cell wall synthesis and DNA replication (Kwan et al. 2013).
New methods to enhance the susceptibility of persisters to antibiotics or to reduce the formation of pathogenic bacterial persisters are required to combat recurrent bacterial infections (Defraine et al. 2018). The methods for eliminating persisters can be categorized based on the mechanism of action -Direct killing of metabolically dormant persister cells, Sensitization of persisters to activate the target site for antibiotics, Stimulation of antibiotic influx and Use of methods that can interfere with or reduce the formation of persister cells, (Defraine et al. 2018).
Metal and metal ions exert bactericidal effect through multiple mechanisms in contrast to site specific action of an antibiotic. Metals are involved in high-affinity, energetically favourable, reactions with a number of target biomolecules (Harrison et al. 2005). Silver or silver ions have been known to possess a broad spectrum of strong inhibitory and microbicidal effects (Lansdown 2006). Silver ions react with proteins by combining with the thiol (–SH) group leading to their inactivation (Russell and Hugo 1994; Feng et al. 2000). Colloidal silver has been shown to be very effective as an antibacterial agent (Morrill et al. 2013; Domínguez et al. 2020). A colloid consists of solid particles (1 nm to 1000 nm) suspended in a liquid. Colloidal silver comprises of two distinctly different forms of silver; metallic silver particles and silver ions (Domínguez et al. 2020).
The total amount of silver that is reported as the silver concentration in parts per million (ppm) is the sum total of the silver contained as particles as well as ions. Colloidal silver has multiple modes of action which makes it more effective (Silverbiotics.com). The nano colloidal silver particles get attracted to the surrounding water molecules and become a part of the structure of the water. This makes the colloidal silver much more stable and bioavailable than other forms of silver. It has the ability to attract and accept multiple electrons compared to ionic silver which can accept only one electron. Treatment with colloidal silver also leads to an increase in production of Reactive Oxygen Species (ROS) further enhancing the killing of bacterial cells (Domínguez et al. 2020).
Overall, colloidal silver disturbs the structure of biomolecules resulting in cell death (Domínguez et al. 2020). The present study describes the effect of colloidal silver against E. coli persisters to Ampicillin. Though there are recent reports on methods to eliminate persisters this study is the first of its kind investigating the use of colloidal silver to combat persisters to antibiotics (Defraine et al. 2018; Khan et al. 2021).
MATERIAL AND METHODS
Bacterial strains and growth medium: E. coli NCIM 2665 (E. coli K-12) was obtained from NCCS Pune, India. Luria Bertani (LB) broth and Luria Bertani (LB) agar were used as growth media. Log phase culture was prepared by inoculating 500μl of a saline suspension (OD530 – 0.4) of overnight grown culture into 100ml of sterile LB broth and incubating for 3hrs at 37oC, 220 rpm. Log phase was achieved at OD530 – 0.5, as determined from growth curve experiments.
Antibiotics and Colloidal silver solution: Ampicillin sodium salt was obtained from Hi Media laboratory, India. Stock solutions of Ampicillin (10mg/ml) were prepared in sterile saline and stored at -18oC. The stock solution was freshly diluted before each experiment with sterile saline or sterile LB broth. Colloidal silver solution was obtained from Viridis Biopharma Pvt. Ltd, India. It was diluted in sterile distilled water or sterile LB broth.
Minimum Inhibitory Concentration (MIC): The MIC of Ampicillin for E. coli NCIM 2665 was determined by the broth dilution method according to Clinical and Laboratory Standards Institute (CLSI) Standards (CLSI 1991, 2015). The concentration range used was 2-10μg/ml of Ampicillin. Presence of growth was measured as Optical density (O.D) at 530nm after 24hours.The presence of E. coli NCIM 2665 persisters to Ampicillin was determined by dose dependent and time dependent kill curve.
Dose dependent kill curve: To determine the antibiotic concentrations that result in the survival of only drug-tolerant persister cells, killing curves were performed. Dose-dependent study was done using different concentrations (40x, 80x, 100x MIC) of Ampicillin against E. coli. Respective antibiotic concentrations were added to 10 ml of log phase culture and further incubated at 37oC, 220 rpm for one hour. The medium was separated by centrifugation at 8000 rpm for 10 mins and the cell pellet obtained was washed thrice with sterile saline and then suspended in 10ml sterile saline. Cell counts were determined in triplicates by drop plate technique on LB agar plates using 10μl of the dilutions (Miles et al. 1938; Moker et al. 2010).
Time dependent kill curve: The antibiotic concentration determined by the dose dependent kill curve was added to log phase culture and incubated at 37oC, 220 rpm for time intervals of 1, 2 and 3 hours. After the designated incubation time, samples were centrifuged at 8000rpm for 10 mins. The cell pellet was washed thrice with sterile saline, and then suspended in 10ml sterile saline. Cell counts were determined in triplicates by drop plate technique on LB agar plates using 10μl of the dilutions to determine the number of surviving persister cells.
Persister cells are obtained by exposure of log phase cultures to a certain concentration of antibiotics for a given time interval. Based on dose and time dependent kill curve experiments, the log phase cultures of E. coli were treated with 400μg/ml of Ampicillin for 1 hour. The high concentration of antibiotic would kill non persister population; resulting in survival of only persister cells. After the antibiotic treatment, the cells were pelleted by centrifugation at 8000rpm for 10 mins. The cell pellets were washed thrice with sterile saline and then suspended in sterile saline. Persister cell numbers were determined by drop plate technique using 10μl of the diluted cell suspension on LB agar plates. The number of colonies obtained represents the number of cells that survived the high antibiotic exposure for one hr and thus were considered as persisters.
Minimum Inhibitory Concentration (MIC) of Colloidal silver for E. coli NCIM 2665: MIC was determined by the broth dilution method according to CLSI Standards. The concentration range of colloidal silver used was 1- 10ppm. Presence of growth was measured as Optical density (OD) at 530nm after 24hours.
Determination of the effect of colloidal silver on formation of persister cells to Ampicillin: Silver at MIC concentration was added to the log phase culture in three different ways: i) 4ppm colloidal silver was added to culture at log phase followed by addition of 400μg/ml Ampicillin after 1 hr incubation, ii) 400μg/ml Ampicillin was added to culture at log phase followed by addition of 4ppm colloidal silver after 1 hr incubation, iii) 400μg/ml Ampicillin and 4ppm colloidal silver were added simultaneously at log phase. Each set was incubated at 37oC, 220 rpm for further one hour and persister cell count was performed in triplicates.
Synergistic action of Ampicillin and colloidal silver against persister cells: Synergistic action was determined using the checkerboard assay in microtitre plates (Balouiri et al. 2016). Persister cells to Ampicillin were obtained as per the earlier mentioned protocol and used as inoculum for the checker board assay. Stock solutions of both Ampicillin and colloidal silver were prepared in LB medium. The reaction volume in each well in the microtiter plate was set as 150μl of the given concentration of Ampicillin in LB medium + 150μl of the given concentration of colloidal silver in LB medium + 20μl of persister cell suspension (3×105cfu/ml).
The concentrations of Ampicillin used were 0-20μg/ml while that of colloidal silver were 0-5ppm. After incubation at 37oC for 24hr, the growth was determined by measuring optical density at 530nm and further confirmed by addition of 10μl of Triphenyl tetrazolium chloride TTC solution (1%w/v) to each well. Conversion of colourless TTC solution to red Formazan indicated growth. The Formazan intensity was measured at 490nm. According to the CLSI guideline, synergy between drugs is determined by calculating the Fractional Inhibitory Concentration (ƩFIC) index.
ƩFIC index represents the sum of the FICs of each drug tested, where the FIC is determined for each drug by dividing the inhibitory concentration of each drug when used in combination by the MIC of each drug when used alone. In the given study ƩFIC = FIC Ampicillin + FIC colloidal silver. The combination is considered synergistic when the ƩFIC is = 0.5; additive, when the ƩFIC is >0.5 to < 2, and antagonistic when the ƩFIC is = 2 (Balouiri et al. 2016).
Determination of persistence/tolerance of E. coli NCIM 2665 to colloidal silver: Log phase cultures of E. coli NCIM 2665 were treated with colloidal silver at concentration range of 4 – 20ppm and incubated at 37oC, 220 rpm for one hour. After incubation, the cell pellets obtained after centrifugation were rinsed with sterile saline thrice to remove traces of colloidal silver and suspended in sterile saline. Enumeration of surviving cell number was done in triplicates by the drop plate method.
Effect of colloidal silver on E. coli NCIM 2665 persisters to Ampicillin: E. coli NCIM 2665 persisters formed to 400μg/ml Ampicillin were inoculated in LB medium containing colloidal silver at concentration range of 4 – 20ppm. Exposure to colloidal silver was done for 1 hour. Enumeration of cell number was done in triplicates by drop plate method.
Statistical analysis: Graphs were prepared using Microsoft Excel 2016. Error bars in the graphs were expressed as standard error of the mean ± (SEM). For effect of colloidal silver on persister formation, mean values were compared within and between groups using one-way analysis of variance (ANOVA) followed by Bonferroni’s test using SPSS software (file version 1.0.0.118). P value less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Minimum Inhibitory Concentration (MIC) of Ampicillin: The MIC value of Ampicillin for E. coli NCIM 2665 as determined by the broth dilution method was found to be 5μg/ml. Dose dependent kill curve: Dose dependent study was done using different concentrations (40x, 80x, 100x MIC) of Ampicillin. Fig. 1 depicts the survival of E. coli population on exposure to the different concentrations of Ampicillin for 1 hr.
Increase in the Ampicillin concentration to 400μg/ml resulted in a rapid killing of the majority of the population followed by a plateau consisting of surviving drug-tolerant persister cells with further increase in drug concentration (Fig. 2). Concentration of Ampicillin for obtaining persister population was selected within the plateau region of the survival curve. With further increase in Ampicillin concentration beyond 400μg/ml there was no significant decrease in surviving population count. Hence, 400μg/ml of Ampicillin was used for the isolation of persister cells throughout the study.
Figure 1: Dose dependent kill curve. Log phase culture was treated with different concentrations of Ampicillin. Cell numbers are plotted as log CFU/ml. The values are average of three independent experiments. Error bars indicate ± SEM
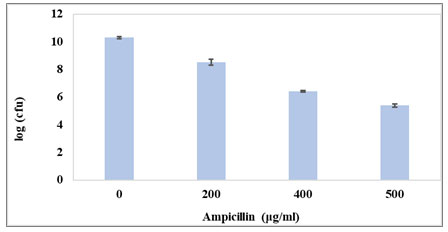
Figure 2: Percentage survival for dose dependent kill curve. Untreated control sample (0μg/ml) was taken prior to antibiotic treatment. Cell numbers are plotted as percentage survival at different concentration of Ampicillin.
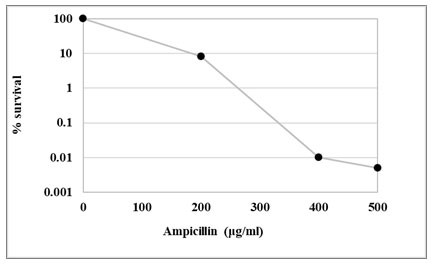
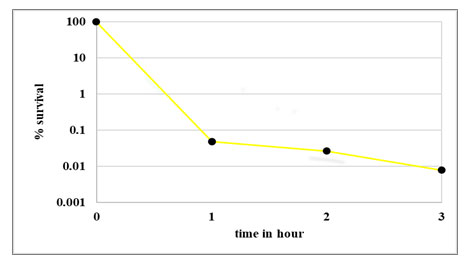
Time dependent kill curve: Survival of E. coli population on exposure to 400μg/ml Ampicillin at different time intervals (1hr, 2hrs and 3hrs) is presented in Fig. 3. The control was log phase culture incubated without any antibiotic for the same time period. The time kill curve in Fig. 4 shows a typical biphasic curve with rapid decrease in population at the end of 1 hour; followed by constant surviving population count with further increase in time of exposure to antibiotic. As exposure time of one hour resulted in maximum persister cell number; for further experiments, persister population was obtained by exposing log phase culture to 400μg/ml of Ampicillin for 1 hr at 37oC and 220rpm.
Figure 3: Ampicillin. Control indicates untreated culture. Test indicates log phase culture treated with 400μg/ml of Ampicillin and incubated for different time intervals. Cell numbers are plotted as log CFU/ml. The values are average of three independent experiments. Error bars indicate ± SEM.
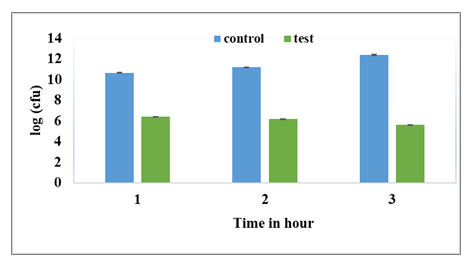
Figure 4: Percentage survival for time dependent kill curve at 400μg/ml Ampicillin. Untreated control sample (0μg/ml) was taken prior to antibiotic treatment. Cell numbers are plotted as percentage survival after 1, 2, 3 hours of exposure to 400μg/ml of Ampicillin
Minimum Inhibitory Concentration (MIC) of Colloidal silver: The MIC of Colloidal silver for E. coli NCIM 2665 determined by the broth dilution method was found to be 4ppm.
Effect of colloidal silver on formation of persisters to Ampicillin: Colloidal silver was seen to have an effect on formation of E. coli NCIM 2665 persisters to Ampicillin (Fig. 5).
Figure 5: Effect of colloidal silver on persister formation in E. coli NCIM 2665. Untreated control indicates log phase culture not treated with antibiotic. Persister control indicates log phase culture treated with only 400μg/ml Ampicillin. Antibiotic first indicates addition of Ampicillin at log phase followed by silver. Silver first indicates addition of silver at log phase followed by Ampicillin. Antibiotic + silver indicates addition of both simultaneously at log phase. Cell numbers are plotted as log CFU/ml. The values are average of three independent experiments. Error bars indicates ± SEM.
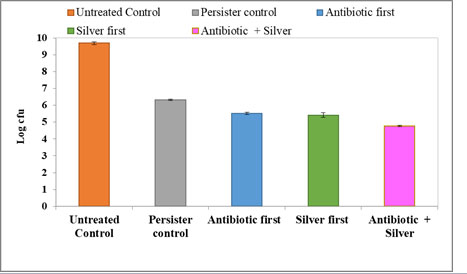
The number of persisters decreased under both conditions namely when silver was added prior to the addition of Ampicillin or when it was added after the antibiotic. Also, when Ampicillin and silver were added simultaneously at log phase, persister population decreased further significantly. Fig. 6 represents the percentage survival under the three different experimental conditions. The persister control is the number of cells that survived after exposure to 400μg/ml of Ampicillin for one hour.
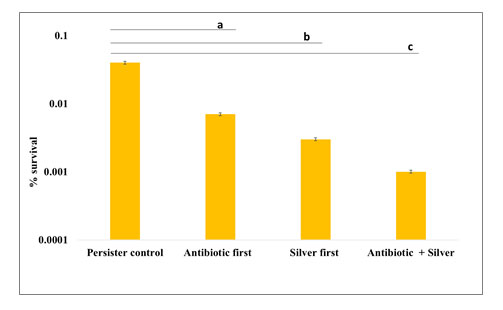
The percentage of persister cell survival in the presence of 400μg/ml of Ampicillin was found to decrease in the presence of silver under all three experimental conditions. It can be concluded that 4ppm colloidal silver was able to significantly decrease the formation of E. coli persisters to Ampicillin. The results obtained by the three experimental set ups (Fig. 6) were compared with the persister control and statistically evaluated using one way ANOVA followed by Bonferroni post hoc test. For all the three sets using silver, decrease in the population was found to be statistically significant (P<0.001).
Figure 6: Effect of colloidal silver on persister formation in E.coli NCIM 2665 (Percentage survival) Persister control indicates population that survived after treating log phase culture with 400μg/ml of Ampicillin only. For treatment methods – Antibiotic first, Silver first and Antibiotic + silver, the percentage survival was calculated in comparison to persister control. The values are average of three independent experiments. Error bars indicate ± SEM. a, b, c indicates P<0.001 compared to persister control for each treatment method respectively.
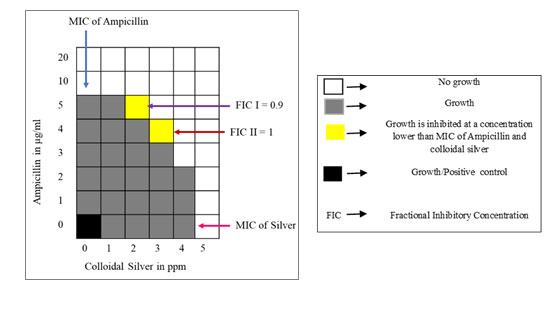
Synergistic action of Ampicillin and colloidal silver against persisters: The results of the microtitre plate checker board assay to determine the synergistic action of Ampicillin and colloidal silver on E. coli persisters to Ampicillin are represented in Fig. 7. Ampicillin and colloidal silver in combination inhibited the persisters at concentrations lower than their respective MIC concentrations. The ƩFIC was calculated for the two combinations showing inhibition as indicated in the figure.
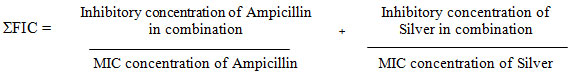
ƩFIC I = 5/10 + 2/5 = 0.9 ƩFIC II = 4/10 +3/5 = 1
A combined action is considered additive, if the ƩFIC lies between 0.5 to 2. Hence, colloidal silver and Ampicillin were found to exert an additive effect on E. coli persisters to Ampicillin. Colloidal silver significantly reduced the inhibitory concentration of Ampicillin.
Figure 7: Checkerboard assay for Ampicillin and colloidal silver against E. coli persister. Ampicillin and colloidal silver was used at 1-20μg/ml and 1-5ppm concentration respectively. Growth control indicates maximum growth and viability of persister cells. FIC was calculated for two combinations I & II where growth was inhibited at a concentration lower than MIC of Ampicillin and colloidal silver.
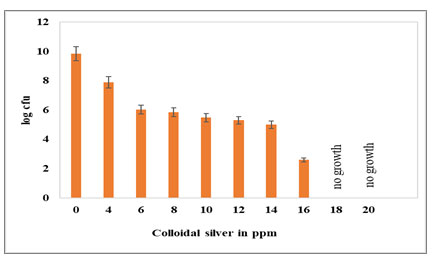
Determination of persistence/tolerance of E. coli NCIM 2665 to colloidal silver: As the MIC concentration of 4ppm of colloidal silver was used to study the inhibitory effect of colloidal silver on the formation of E. coli persister to Ampicillin, it was imperative to study the tolerance of E. coli to silver and whether persisters to silver get formed. Log phase cultures of E. coli were treated for 1 hr with colloidal silver ranging from 4ppm to 20ppm at intervals of 2ppm. E. coli NCIM 2665 was found to tolerate up to 16ppm of colloidal silver (Fig. 8); albeit a significant decrease in population.
At 16ppm, a five-log reduction was observed as compared to untreated control log phase population. There was total killing of cells on exposure to 18ppm for 1 hour. For isolation of persisters to Ampicillin; E. coli NCIM 2665 was exposed to 80xMIC (400μg/ml) and 100xMIC (500μg/ml) for 1 hr. However, E. coli was able to tolerate 1 hr exposure to only 16ppm of colloidal silver which is 4xMIC and 18ppm brought about total killing in 1 hr. It seemed evident that E. coli NCIM 2665 does not form persisters to colloidal silver.
Figure 8: Tolerance of E. coli NCIM 2665 to colloidal silver. Log phase culture was exposed to different concentrations of colloidal silver. No growth indicates growth is inhibited by colloidal silver. Cell numbers are plotted as log CFU/ml. The values are average of three independent experiments. Error bars indicate ± SEM.
Effect of colloidal silver on E. coli NCIM 2665 persisters to Ampicillin: Ampicillin persister cells were exposed to different concentrations of colloidal silver for 1 hr. Similar to the log phage E. coli population, the Ampicillin persister cells were also found to tolerate up to 16ppm of colloidal silver (Fig. 9). At 16ppm, a two-log reduction was observed as compared to untreated persister control while the log phase E. coli population showed a five log reduction at 16ppm (Fig. 8).
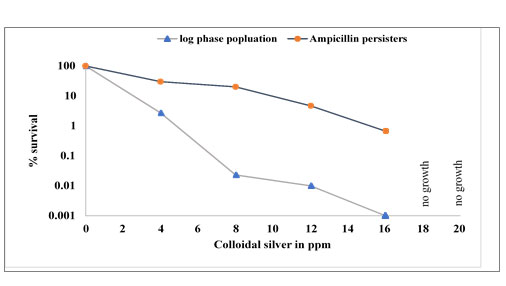
Similarly, total killing of the Ampicillin persisters was seen after 1 hr exposure to 18ppm of colloidal silver as was observed with log phase E. coli. Fig. 10 shows percentage survival of log phase population and Ampicillin persisters of E. coli in the presence of various concentrations (4ppm to 16 ppm) of colloidal silver. Both, the log phase E. coli population as well as the Ampicillin persisters were able to tolerate 16ppm colloidal silver for 1 hr. However, the log reduction was much greater in the case of normal cells as compared to the two-log reduction for persister population.
Figure 9: Effect of colloidal silver on E.coli Ampicillin persisters. Persister control indicates persister cells unexposed to colloidal silver. Persister cells were exposed to 4-20ppm of colloidal silver. Cell numbers are plotted as log CFU/ml. No growth indicates growth is inhibited by colloidal silver. The values are average of three independent experiments. Error bars indicate ± SEM.
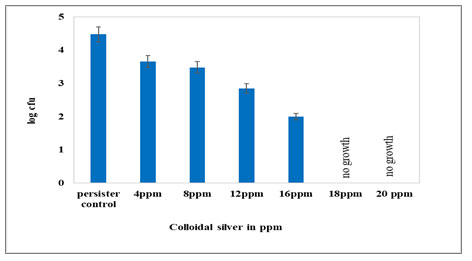
Figure 10: Survival percentage of log phase population and Ampicillin persisters of E.coli in the presence of colloidal silver. The population counts taken prior to the treatment with colloidal silver were considered as 100%. No growth indicates no viable cells or zero percent survival.
Bacterial persisters are phenotypic variants that form under stress and possess antibiotic tolerance (Bigger 1944; Lewis 2010; Fisher et al. 2017; Van den Bergh et al. 2017). Bacterial persistence is an emerging problem in addition to drug resistance. Recurrence of an infection indicates survival of the pathogen in the host for longer duration (Levin and Rozen 2006; Michiels et al. 2016; Meylan et al. 2018; Bartell et al. 2020). Increasing the dosage or duration of the antibiotics not only increases tolerance towards the drugs but also promotes persistence.
Different strategies to eliminate bacterial persister cells include the direct killing of metabolically dormant persister cells, sensitization of persister cells for the stimulation of antibiotic influx, and the use of molecules capable of interfering with or reducing the formation of persister cells (Gefen and Balaban 2009; Allison et al. 2011b; Feng et al. 2015; Kwan et al. 2015; Chowdhury et al. 2016; Orman and Brynildsen 2016; Liu et al. 2020; Khan et al. 2021). Also, using combination of a strong and weak metabolism-dependent antibiotic has shown to decrease persister population effectively (Zheng et al. 2020).
Polymyxin B, poly-L-lysine and phenothiazine have also known to reduce the persistent phenotype in Escherichia coli and Pseudomonas aeruginosa (Mohiuddin et al. 2020). Electrochemical methods combined with antibiotics have been suggested as an effective alternative for persister cell elimination (Sultana et al. 2016). The use of copper to control persisters has been recently shown to be effective (Moreira et al. 2020). Silver is known to enhance antibiotic activity against Gram-negative bacteria (Morones et al. 2005; Morones et al. 2013).
However, till date the use of silver against persisters is not known. Hence the use of colloidal silver in this study is a novel alternative approach to control bacterial persister cells (Moreira et al. 2020). In the present study, E. coli NCIM 2665 persisters were obtained at Ampicllin concentration of 400μg/ml. The cell population so obtained on exposure to 400μg/ml of Ampicillin for 1 hr were considered to be persisters since they exhibited a biphasic killing curve (Keren et al. 2004; Moker et al. 2010; Kamble and Pardesi 2020).
The MIC of colloidal silver used in the present study was found to be 4ppm for E. coli NCIM 2665. Effect of colloidal silver on persister formation was studied under antibiotic stress. Survival of persister population decreased with prior silver treatment but when silver and antibiotic were added simultaneously at log phase, persister population decreased significantly (P<0.001).
Checkerboard assay indicated additive effect and therefore the colloidal silver can be administered along with antibiotic. Colloidal silver decreased the survival of both log phase culture and Ampicillin persister population. Both log phase population and antibiotic persisters were able to tolerate 1 hour exposure to colloidal silver up to 16ppm. However, antibiotic persisters showed a higher percentage of survival compared to the non persister population which confirms their rigid persistence feature (Kamble and Pardesi 2020).
A drastic five log reduction in log phase population and two log reduction in antibiotic persisters at 16ppm of colloidal silver, indicates the its effectiveness. There was total killing of log phase culture as well as Ampicillin persisters at 18 ppm silver. Overall decrease in persisters observed after simultaneous addition of silver and Ampicillin may be attributed to the killing effect exerted by colloidal silver on log phase cells as well as on the antibiotic persisters once formed. Additive effect as seen in the checkerboard assay may also contribute to it. Colloidal silver may be modifying the physiological state of the cells or the state of the environment such that persistence is not promoted.
CONCLUSION
This study confirms that colloidal silver can be considered as a promising anti persistence strategy. It can be used either as the sole bactericidal agent or can be used to enhance the effectiveness of an antibiotic. The key advantage of colloidal silver besides its inhibitory effect is that persister cells to the colloidal silver do not seem to be formed.
Conflict of Interests: There was no conflict among the interests of the participating authors.
REFERENCES
Allison, K.R., Brynildsen, M.P. and Collins, J.J. (2011b). Heterogeneous bacterial persisters and engineering approaches to eliminate them. Curr. Opin. Microbiol. 14 (5), pp.593–598.
Balouiri, M., Sadiki, M. and Ibnsouda, S., (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6(2), pp.71-79.
Bartell, J. A., Cameron, D. R., Mojsoska, B., Haagensen, J., Pressler, T., Sommer, L. M., Lewis, K., Molin, S., and Johansen, H. K. (2020). Bacterial persisters in long-term infection: Emergence and fitness in a complex host environment. PLOS pathogens, 16(12), e1009112.
Bigger, J.W. (1944). Treatment of Staphylococcal infections with penicillin by intermittent sterilisation. The Lancet, 244(6320), pp.497–500.
Chowdhury, N., Wood, T.L., Martínez-Vázquez, M., García-Contreras, R. and Wood, T.K. (2016). DNA-crosslinker cisplatin eradicates bacterial persister cells. Biotechnol. Bioeng. 113 (9), pp.1984-1992.
Clinical & Laboratory Standards Institute. (CLSI.) (2018). M07: Dilution AST for Aerobically Grown Bacteria – CLSI. [online] https://clsi.org/standards/products/microbiology/documents/m07/ [Accessed Mar. 19, 2020].
Clinical & Laboratory Standards Institute. (CLSI.) (1999). M26AE: Bactericidal Activity of Antimicrobial Agents. [online] Available at: https://clsi.org/standards/products/microbiology/documents/m26/ [Accessed Mar. 19, 2020].
Defraine, V., Fauvart, M. and Michiels, J. (2018). Fighting bacterial persistence: Current and emerging anti-persister strategies and therapeutics. Drug Resistance Updates, 38, pp.12–26.
Domínguez, A. V, Ayerbe Algaba, R., Miró Canturri, A., Rodríguez Villodres, Á., and Smani, Y. (2020). Antibacterial Activity of Colloidal Silver against Gram-Negative and Gram-Positive Bacteria. Antibiotics (Basel, Switzerland), 9(1), 36.
Fauvart, M., De Groote, V.N. and Michiels, J. (2011). Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. Journal of Medical Microbiology, 60(6), pp.699–709.
Feng, J., Auwaerter, P.G. and Zhang, Y. (2015b). Drug combinations against Borrelia burgdorferi
persisters in vitro: Eradication achieved by using daptomycin, cefoperazone and doxycycline. PLOS One 10 (3), e0117207.
Feng, Q. L., Wu, J., Chen, G. Q., Cui, F. Z., Kim, T. N., and Kim, J. O. (2000). A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of biomedical materials research, 52(4), pp.662–668.
Fisher, R.A., Gollan, B. and Helaine, S. (2017). Persistent bacterial infections and persister cells. Nature Reviews Microbiology, 15(8), pp.453–464.
Gefen, O. and Balaban, N.Q. (2009). The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress. FEMS Microbiol. Rev. 33 (4), pp.704–717.
Harrison, J. J., Ceri, H., Roper, N. J., Badry, E. A., Sproule, K. M., and Turner, R. J. (2005). Persister cells mediate tolerance to metal oxyanions in Escherichia coli. Microbiology, 151(10), pp.3181–3195.
Hong-Geller, E. and Micheva-Viteva, S.N. (2015). Drug Discovery and Development – From Molecules to Medicine. [online] London, UK: IntechOpen Limited, pp.201–208. DOI: 10.5772/59404. Available from: https://www.intechopen.com/books/drug-discovery-and-development-from-molecules-to-medicine/targeting-bacterial-persistence-to-develop-therapeutics-against-infectious-disease
Jayaraman, R. (2008). Bacterial persistence: some new insights into an old phenomenon. Journal of Biosciences, 33(5), pp.795–805.
Kamble, E., and Pardesi, K. (2021). Antibiotic Tolerance in Biofilm and Stationery-Phase Planktonic Cells of Staphylococcus aureus. Microbial Drug Resistance 27:1, pp.3-12.
Khan, F., Pham, D., Tabassum, N., Oloketuyi, S. F., and Kim, Y. M. (2020). Treatment strategies targeting persister cell formation in bacterial pathogens. Critical reviews in microbiology, 46(6), pp.665–688.
Kwan, B. W., Valenta, J. A., Benedik, M. J., and Wood, T. K. (2013). Arrested protein synthesis increases persister-like cell formation. Antimicrobial agents and chemotherapy, 57(3), pp.1468–1473.
Kwan, B.W., Chowdhury, N., and Wood, T.K. (2015). Combatting bacterial infections by killing
persister cells with mitomycin C. Environ. Microbiol. 17 (11), pp.4406–4414.
Lansdown A. B. (2006). Silver in health care: antimicrobial effects and safety in use. Current problems in dermatology, 33, pp.17–34.
Levin, B.R., and Rozen, D.E. (2006). Non-inherited antibiotic resistance. Nat. Rev. Microbiol, 4 (7), pp.556–562.
Lewis K. (2008). Multidrug tolerance of biofilms and persister cells. Current topics in microbiology and immunology, 322, pp. 107–131.
Lewis, K. (2010). Persister Cells. Annual Review of Microbiology, 64(1), pp.357–372
Liu, Y., Yang, K., Jia, Y., Shi, J., Tong, Z. and Wang Z. (2020). Cysteine Potentiates Bactericidal Antibiotics Activity Against Gram-Negative Bacterial Persisters. Infect Drug Resist.;13 pp.2593-2599.
Meylan, S., Andrews, I. W., and Collins, J. J. (2018). Targeting Antibiotic Tolerance, Pathogen by Pathogen. Cell, 172(6), pp.1228–1238.
Michiels, J.E., Van den Bergh, B., Verstraeten, N., and Michiels, J. (2016). Molecular mechanisms and clinical implications of bacterial persistence. Drug Resist. Updates 29, pp.76–89.
Miles, A.A., Misra, S.S. and Irwin, J.O. (1938). The estimation of the bactericidal power of the blood. Epidemiology and Infection, 38(6), pp.732–749.
Mohiuddin, S. G., Hoang, T., Saba, A., Karki, P., and Orman, M. A. (2020). Identifying Metabolic Inhibitors to Reduce Bacterial Persistence. Frontiers in microbiology, 11, pp. 472.
Moker, N., Dean, C.R. and Tao, J. (2010). Pseudomonas aeruginosa Increases Formation of Multidrug-Tolerant Persister Cells in Response to Quorum-Sensing Signalling Molecules. Journal of Bacteriology, 192(7), pp.1946–1955.
Moorhouse, A.J., Rennison, C., Raza, M., Lilic, D. and Gow, N.A.R. (2016). Clonal Strain Persistence of Candida albicans Isolates from Chronic Mucocutaneous Candidiasis Patients. PLOS ONE, 11(2), p.e0145888.
Moreira, M. P., Gong, T., de Souza A.A., and Wood, T. K. (2020). Copper Kills Escherichia coli Persister Cells. Antibiotics.; 9(8) pp.506.
Morones, J. R, Winkler, J. A., Spina, C. S., and Collins, J.J. (2013). Silver enhances antibiotic activity against gram-negative bacteria, Science translational medicine, 5(190), pp.190ra81.
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramírez, J. T., and Yacaman, M. J. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16(10), pp.2346–2353.
Morrill, K., May, K., Leek, D., Langland, N., Jeane, L.D., Ventura, J., Skubisz, C., Scherer, S., Lopez, E., Crocker, E., Peters, R., Oertle, J., Nguyen, K., Just, S., Orian, M., Humphrey, M., Payne, D., Jacobs, B., Waters, R. and Langland, J. (2013). Spectrum of Antimicrobial Activity Associated with Ionic Colloidal Silver. The Journal of Alternative and Complementary Medicine, 19(3), pp.224–231.
Orman, M.A. and Brynildsen, M.P. (2016). Persister formation in Escherichia coli can be inhibited by treatment with nitric oxide. Free Radic. Biol. Med. 93, pp.145–154.
Russell, A. D., and Hugo, W. B. (1994). Antimicrobial activity and action of silver. Progress in medicinal chemistry, 31, pp.351–370.
Silverbiotics.com. (2020). Home. [online] https://silverbiotics.com/ [Accessed Mar. 19, 2020].
Sultana, S. T., Call, D. R., and Beyenal, H. (2016). Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility. NPJ biofilms and microbiomes, 2, 2.
Van den Bergh, B., Fauvart, M., and Michiels, J., (2017). Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev, 41(3), pp.219–251.
Waters, C. M., and Bassler, B. L. (2005). Quorum sensing: cell-to-cell communication in bacteria. Annual review of cell and developmental biology, 21, pp.319–346.
Zheng, E. J., Stokes, J. M., and Collins, J. J. (2020). Eradicating bacterial persisters with combinations of strongly and weakly metabolism-dependent antibiotics. Cell chemical biology, 27(12), pp.1544–1552.e3.


