1Vector Management Division, Defence Research and Development Establishment,Jhansi Road Gwalior, India.
2School of studies in Microbiology, Jiwaji University, Gwalior, India.
Corresponding author email: sachin.tikar@drde.drdo.in
Article Publishing History
Received: 05/12/2020
Accepted After Revision: 19/03/2021
Tick borne diseases (TBDs) have remained a major concern throughout the world. The cases of TBDs have increased in past few years. Moreover, new emerging pathogens are being detected from ticks which have led the scientists to reveal more on this field. Among various pathogens transmitted from ticks, Spotted Fever Group rickettsia (SFGR) represents the huge number of new and emerging infectious pathogens but unfortunately has been neglected. Therefore, the study was carried out to detect SFGR from ticks collected from Gwalior. This report is a description Rickettsia felis which is a potential human pathogen detected in adult Rhiphicephalus sanguineus tick collected from dogs in Gwalior, India. For SFGR screening in the ticks, partial conserved region of Citrate Synthase (gltA) and Outer Membrane Protein B (rOmpB) genes were targeted. Both of the genes were amplified and thus indicated the presence of the rickettsial pathogen and its associated risk. Five percent (5.19%) of the screened ticks were PCR positive for SFGR. PCR amplicons were sequenced further. Additionally, Immunoflourescence assay (IFA) test was also done to double sure the result obtained. The sequencing result of gltA gene showed 97.87 percent similarity to R. felis (MH194353) and for rOmpB gene there was 99.47 percent similarity to R. felis (MG451836). This is the first report of R. felis DNA from Rh. sanguineus ticks in India. Further studies are required for clarity on the distribution of the R. felis in ticks and its transmission to human being. Thus, a comprehensive awareness is much needed for tackling rickettsial pathogen and subsequent steps leading to an improved health management in India.
Rickettsia Felis. Rhiphicephalus Sanguineus. Citrate Synthase Gene. Outer Membrane Protein B. India. Ticks
Ghosh, Tikar S. N, Gupta M. K, Gupta A. D, Sukumaran D. Analysis on the Molecular Detection of Rickettsia felis in Rhiphicephalus sanguineus Ticks Found in India. Biosc.Biotech.Res.Comm. 2021;14(1).
Ghosh, Tikar S. N, Gupta M. K, Gupta A. D, Sukumaran D. Analysis on the Molecular Detection of Rickettsia felis in Rhiphicephalus sanguineus Ticks Found in India. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3twM6Zd”>https://bit.ly/3twM6Zd</a>
Copyright © Ghosh et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Since 20th century, ticks were known to transmit human infectious diseases transmitting variety of pathogens (Parola and Raoult, 2001). Novel SFGR species have been detected from different tick species around the globe (Noh et al., 2017; Rivera-Páez et al., 2018). Many researchers have conducted tick prevalence in different areas. Recently surveillance was carried out in India on the distribution of tick species to have insight on its vector ecology. Rh. sanguineus, also known as the brown dog tick are prevalent all over the world. They mostly parasitize dogs, but feeding on humans have also been reported (Palmas et al., 2001; Dantas-Torres, 2007; Ghosh et al., 2020).
Numerous pathogens such as Babesia canis, Ehrlichia canis, R. rickettsii, R. conorii are known to be transmitted by Rh. sanguineus causing diseases in humans as well as in animals (Brumpt et al., 1932; Parker et al., 1933; Groves et al., 1975). For better knowledge on SFGR and its diagnosis, much research is needed in Asia (Robinson et al., 2019). There are abundant literatures prevailing in the last decade which reported several native and new species of Rickettsia detected from ticks (Shao et al., 2020; Binetruy et al., 2020). In spite of these studies, we lack in the complete understanding of SFGR interaction with ticks for its transmission and its impact on Human as well (Tomassone et al., 2018). Keeping in view the role of ticks in passive detection of Rickettsia, we have conducted the study to detect the presence of SFGR in Rh. sanguineus, a potential vector for Rickettsia in and around Gwalior of India.
MATERIAL AND METHODS
Ticks were collected from dogs in Tekanpur location of Gwalior shown in figure 1. with geographical coordinates of 26° 22′ 0″ North, 78° 18′ 0″ East. Collected ticks were kept in vials with proper label and transported. Live unengorged ticks were sorted and were washed with 70% ethanol followed by distilled water, dried and viewed under Leica EZ4D and Leica M205A stereo zoom microscope attached with DFC500 camera. Ticks were morphologically identified as Rh. sanguineus using taxonomical keys which involved broader Capitulum, comma-shaped and elongated spiracles throughout, short palps (Walker et al., 2005). Identified ticks were preserved in 90% ethanol in -200C till use for DNA extraction. Each tick pool used for DNA extraction comprise of 2 ticks. DNA extraction was performed using DNeasy blood and tissue kit (Qiagen) according to the manufacturer’s instruction (Walker et al., 2005).
For qualitative analysis, the extracted DNA was checked by running it on 1% agarose gel electrophoresis. To corroborate the result of morphological identification, 16SrRNA and COI gene of tick was amplified using the primer and protocol of previously published literatures (Black et al., 1994). The sequences amplified were submitted to NCBI. Subsequently screening of Rh. sanguineus tick was done for Rickettsial pathogen. Partial regions of gltA and rOmpB genes (nested PCR) of SFG Ricketssia were amplified using primers and protocols generating product of 401bp; 511bp and 420bp respectively (Chitimia et al., 2010).
Rickettsia conorii DNA (Vircell) was taken as positive control whereas nuclease free distilled water as negative control (Labruna et al., 2004; Choi et al., 2005). The positive PCR amplicons were subsequently excised and purified using Qiagen gel extraction kit. Further characterization of the PCR products was done by Sanger’s sequencing (Genotypic technology, Bangalore). The recovered nucleotide sequences were compared with the available sequences in Genbank using BLAST and phylogenetic tree was made for evolutionary analysis of the SFGR detected. Furthermore, IFA test was done to support and corroborate the result obtained from PCR reaction. For IFA, a smear was made from triturated tick (pool of 2 ticks) and subsequently air dried (Choi et al., 2005).
The slides were fixed in Acetone at 4ºC for 20 minutes followed by washing step (3 times) in PBS. Thereafter, Rickettsia conorii Antibody (1: 128) was added over the smear made and incubated for 30 minutes at 37ºC in humidity chamber. Slides were washed with PBS followed by an addition of a drop of FITC conjugated secondary antibody and further incubated in humidity chamber for 30 minutes at 37ºC in dark. Afterwards the slides were washed in PBS. Two drops of mounting media were added to each slide and coverslip was placed removing any air bubbles. The slides were observed at 400X magnification in fluorescence microscope (Labruna et al., 2004; Choi et al., 2005).
Figure 1: Study Area
RESULTS AND DISCUSSION
Figure 2: All ticks collected from dogs were morphologically identified as Rh. sanguineus

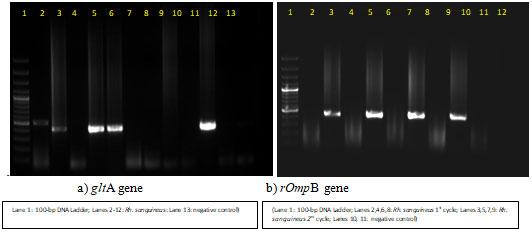
The taxonomical keys used involved broader Capitulum, ‘Simus’ pattern of punctuation, elongated spiracles throughout, short palps (Walker et al., 2005). Sequences for 16SrRNA and COI gene of ticks were successfully amplified and were submitted in NCBI with accession number MH765330 and MH765337 respectively. A total 77 tick DNA pool were used for screening of SFGR. The PCR result showed that, out of 77 tick pools screened, four pools were found positive for SFGR DNA with 5.19% positivity in ticks by both the genes (figure 3) (Walker et al., 2005).
Figure 3: Agarose gel electrophoresis of gltA and rOmpB gene PCR from screened tick species
PCR positive amplicons were sequenced. The sequencing result for gltA and rOmpB genes showed 97.87 percent identity to R. felis (MH194353) from Brazil and 99.47 percent identity with R. felis (MG451836) from Italy. The phylogenetic tree was made using maximum likelihood and Tamura-Nei model under 1000 bootstrap replicates in Mega X (figure 4 & 5) (Walker et al., 2005). Maximum likelihood (ML) tree inferred from rOmpB gene partial sequences of R. felis detected in the present study (*) and sequences from GenBank using MEGA X. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site (next to the branches)
Figure 4: Maximum likelihood (ML) tree inferred from rOmpB gene partial sequences of R. felis detected in the present study (*) and sequences from GenBank using MEGA X. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site (next to the branches)
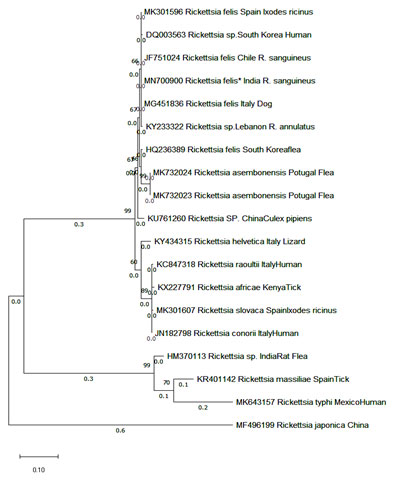
Figure 5: Maximum likelihood (ML) tree inferred from gltA gene partial sequences of R. felis detected in the present study (*) and sequences from GenBank using MEGA X. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site (next to the branches)

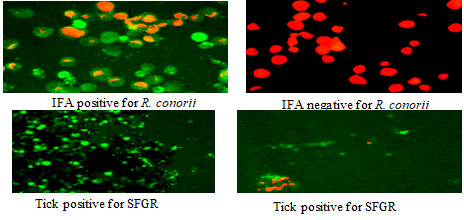
The gene sequences for R. felis were deposited in GenBank under accession number MN704850 for gltA gene and MN700900 for rOmpB gene. In case of IFA, 25 pools of ticks were screened, out of which 4 tick pools showed positive result (figure 6) (Walker et al., 2005).
Figure 6: Immunofluorescence (IFA) of ticks
felis has been proposed as a new member for the spotted fever group (SFG) of the genus Rickettsia. First human pathogenicity linked with R. felis was from Texas in 1994 (Schriefer et al., 1994; Bouyer et al., 2001). It is widely distributed all over the world and has become a major threat for human with its flexibility of adapting a broad array of ectoparasites and mammals including humans as well (Pérez-Osorio et al., 2008). In recent time R. felis was also reported from various mosquito species and was proposed experimentally that Anopheles gambiae can act as possible vector of R. felis (Dieme et al., 2015). Likewise, the spreading of chikungunya virus infection from the Indian Ocean to parts of world, Zika virus from French Polynesia to America (Parola et al., 2016).
Parola et al., (2016) raised concern on the possibility of R. felis outbreak from mosquitoes also. Although R. felis has been detected in mainly in cat flea, it has also been documented from several ticks, including Rh. sanguineus (Cardoso et al., 2006; Abarca et al., 2013). About thirty-nine species of ectoparasites have been linked with R. felis which includes numerous species of fleas, ticks, lice, and mosquitoes whereas Oliveira et al. (2008) confirmed that R. felis is circulated and maintained in different ectoparasites including Rh. sanguineus as well. Rh. sanguineus tick from China was also found to be positive with R. felis with a positivity rate of ten percent, whereas in our study it was five percent (Zhang et al., 2014; Brown et al., 2016).
Additionally, R. felis was detected from C. felis, Rh. sanguineus and Dermacentor nitens with a varying positivity rate of 22.22%, 3.22% and 1.75% respectively (Oliveira et al., 2020). These variations in the positivity rate of R. felis are affected by various factors such as using different methodologies used, the environmental condition of the vectors and the presence of Rickettsia reservoirs (Khrouf et al., 2014). Rh. sanguineus is the known vector of several pathogenic agents like Ehrlichia canis, Coxiella burnetii, R. rickettsii, and R. conorii which have a great zoonotic importance (Dantas-Torres et al., 2008). In India, positives cases for R. felis were reported in 11 patients from south eastern India whereas, flea borne rickettsia was also detected from cat flea Ceratophyllus fasciatus from Western Himalayan region, however its prevalence in arthropod vector was not studied (Chahota et al., 2015; Koralur et al., 2016).
In similar line, R. felis-like organism (Rickettsia sp. genotype RF2125) was reported from Delhi, Mumbai and Rajasthan of India and its high prevalence in flea associated with dogs as reservoir was implicated as important zoonotic threat (Hii et al., 2015). To support our result obtained from PCR, we have done IFA which is a known confirmatory technique as well. Because of greater sensitivity and specificity, PCR based method is preferred over direct detection method such as IFA (Massung et al., 1998). IFA results in existing study exhibited 16% tick positivity to SFGR. This confirms the existence of SFGR in Rh. sanguineus ticks from Gwalior. Thus, current study indicates that Rickettsial infections are circulated and maintained in ticks but are underrated and its real existence on public health is left unknown. Our result showed that R. felis is circulated in the ticks of Gwalior and it therefore becomes imperative to elucidate the status of SFGR in ticks associated with dog, mainly in Rh. sanguineus (Koralur et al., 2016; Oliveira et al., 2020).
Additionally, so far there is no published report of R. felis DNA detected either from patient or from any of tick vector in India. Our study is the first report of R. felis DNA from Rh. sanguineus ticks in India. Since Ctenocephalides felis is the only known biological vector for R. felis, the report of R. felis DNA in tick raises many questions about the role of Rh. sanguineus as vector and also the role of dogs as reservoir. R. felis has been documented to be transmitted through co-feeding of numerous arthropods, horizontally from infected to uninfected Arthropods, via direct contact or through the consumption of contaminated blood meals and also via mating, however its transmission route in India is not systematically studied. Although vector status of Rh. sanguineus in India is not established for R. felis, the biological risk associated with R felis could not be neglected. Taking all these facts into consideration, our study emphasizes the need of extensive research on association of R. felis with Rh. sanguineus ticks of India which may act as a potential vector and transmit this human pathogen thereby becoming a global threat (Nguyen et al., 2020).
CONCLUSION
This is the first report of presence of R. felis DNA in Rh. sanguineus ticks from Gwalior, India. There is a huge scarcity of information regarding TBDs in India, therefore these studies will help to elucidate and resolve the unsolved queries regarding TBDs. The result of the study also emphasizes on the presence of novel Rickettsia species in India which still needs to be explored from different tick vectors. Further studies are required to have more clarity on the distribution of the R. felis in ticks and its transmission to human being. These kinds of studies are required to fill the wide gaps in the broad areas of ticks and tick-borne diseases.
Conflict of Interest: There were no conflict of interest among the authors.
Funding: None
Ethical approval: Not applicable.
Author’s contribution: All the authors have contributed to the design and conceptualization of the study. The conduct of experiment and manuscript writing was done by Pooja Ghosh. The morphological identification and the idea behind the study were done by Sachin Tikar. Data analysis was done by Mahendra K. Gupta. Sample collection was done by Abhay D. Gupta and D. Sukumaran. All the authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
We are thankful to Director, DRDE, Gwalior, Madhya Pradesh (India) for taking keen interest and providing all the essential facilities to conduct this research. We are also thankful to National Training Centre for Dogs (NTCD), Tekanpur for providing ticks samples. The DRDE accession number for this manuscript is DRDE/VMD/01/2020.
REFERENCES
Abarca, K., López, J., Acosta-Jamett, G. and Martínez-Valdebenito, C., (2013). Rickettsia felis in Rhipicephalus sanguineus from two distant Chilean cities. Vector Borne Zoonotic Dis 13(8):607-9.
Binetruy, F., Buysse, M., Barosi, R. and Duron, O., (2020) Novel Rickettsia genotypes in ticks in French Guiana, South America. Scientific reports, 10(1):1-11.
Black, W.C. and Piesman, J., (1994) Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. PNAS 91 (21):10034-8.
Bouyer, D.H., Stenos, J., Crocquet-Valdes, P., Moron, C.G., Popov, V.L., Zavala-Velazquez, J.E., Foil, L.D., Stothard, D.R., Azad, A.F. and Walker, D.H., (2001) Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int J Syst Evol Micr 51(2):339-47.
Brown, L.D. and Macaluso, K.R., (2016) Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep 3(2):27-39.
Brumpt, E., (1932) Longevity of the Virus of Fièvre boutonneuse in R. sanguineus. Compete rendu des seances de la Societe de biologie 110(28):1199-202.
Cardoso, L.D., Freitas, R.N., Mafra, C.L., Neves, C.V.B., Figueira, F.C.B., Labruna, M.B., Gennari, S.M., Walker, D.H. and Galvão, M.A.M., (2006) Characterization of Rickettsia spp. circulating in a silent peri-urban focus for Brazilian spotted fever in Caratinga, Minas Gerais, Brazil. Cadernos de saude publica 22(3):495-501.
Chahota, R., Thakur, S.D., Sharma, M. and Mittra, S., (2015) Detection of flea-borne Rickettsia species in the Western Himalayan region of India. Indian J Med Microbiol 33(3):422-25.
Chitimia, L., Lin, R-Q., Cosoroaba, I., Wu, X-Y., Song, H-Q., Yuan, Z-G. and Zhu, X.Q., (2010) Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox1 and nad5 genes. Exp Appl Acarol 52(3):305-11.
Choi, Y-J., Jang, W-J., Kim, J-H., Ryu, J-S., Lee, S-H., Park, K-H., Paik, H.S., Koh, Y.S., Choi, M.S. and Kim, I.S., (2005) Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis 11(2):237-44.
Dantas-Torres, F., (2007) Rocky Mountain spotted fever. Lancet Infect Dis 7(11):724-32.
Dantas-Torres, F., (2008) The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol 152(3-4):173-85.
de Oliveira, J.C.P., Reckziegel, G.H., do Nascimento Ramos, C.A., Giannelli, A., Alves, L.C., de Carvalho, G.A. and Ramos, R.A.N., (2020) Detection of Rickettsia felis in ectoparasites collected from domestic animals. Exp Appl Acarol 81: 255–264.
Dieme, C., Bechah, Y., Socolovschi, C., Audoly, G., Berenger, J-M., Faye, O., Raoult, D. and Parola, P., (2015) Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. PNAS 112(26):8088-93.
Ghosh, P., Tikar, S.N., Gupta, M.K. and Sukumaran, D., (2020) Molecular Characterisation using 16S rRNA and COI Gene Sequences in Hard Ticks of Gwalior, India. Def Life Sci J 5(3): 173-179.
Groves, M.G., Dennis, G.L., Amyx, H.L. and Huxsoll, D.L., (1975) Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am J Vet Res 36(7):937-40.
Hii, S.F., Lawrence, A.L., Cuttell, L., Tynas, R., Abd Rani, P.A.M., Šlapeta, J. and Traub, R.J., (2015) Evidence for a specific host-endosymbiont relationship between Rickettsia sp. genotype RF2125 and Ctenocephalides felis orientis infesting dogs in India. Parasit Vectors 8(1):169.
Khrouf, F., M’Ghirbi, Y., Znazen, A., Jemaa, M.B., Hammami, A. and Bouattour, A. (2014) Detection of Rickettsia in Rhipicephalus sanguineus ticks and Ctenocephalides felis fleas from southeastern Tunisia by reverse line blot assay. J Clin Microbiol 52(1):268-274.
Koralur, M., Bairy, I., Varma, M., Athan, E. and Stenos, J. (2016) Spotted fever group and typhus fever group rickettsiosis in South Western India. IJID 45:180-1.
Labruna, M.B., Whitworth, T., Horta, M.C., Bouyer, D.H., McBride, J.W., Pinter, A., Popov, V., Gennari, S.M. and Walker, D.H., (2004) Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. JCM 42(1):90-8.
Massung,R.F., Slater, K., Owens, J.H., Nicholson, W.L., Mather, T.N., Solberg, V.B. and Olson, J.G., (1998) Nested PCR assay for detection of granulocytic ehrlichiae. JCM 36(4): 1090-1095.
Ng-Nguyen, D., Hii, S.F., Hoang, M.T.T., Nguyen, V.A.T., Rees, R., Stenos, J. and Traub, R.J., (2020) Domestic dogs are mammalian reservoirs for the emerging zoonosis flea-borne spotted fever, caused by Rickettsia felis. Sci Rep 10(1):1-10.
Noh, Y., Lee, Y.S., Kim, H.C., Chong, S.T., Klein, T.A., Jiang, J., Richards, A.L., Lee, H.K. and Kim, S.Y. (2017) Molecular detection of Rickettsia species in ticks collected from the southwestern provinces of the Republic of Korea. Parasites Vectors 10(1): 20.
Oliveira, K.A.D., Oliveira, L.S.D., Dias, C.C.A., Silva, Jr. A., Almeida, M.R., Almada, G., Bouyer, D.H., Galvão, M.A.M. and Mafra C.L., (2008) Molecular identification of Rickettsia felis in ticks and fleas from an endemic area for Brazilian Spotted Fever. MEM I OSWALDO CRUZ 103(2):191-194.
Palmas, C., Bortoletti, G., Conchedda, M., Contini, C., Gabriele, F. and Ecca, A.R., (2001) Study on immunobiology in ectoparasites of public health interest: Rhipicephalus sanguineus. Parassitologia 43(1):29-35.
Parker, R.R., Philip, C.B. and Jellison, W.L., (1933) Rocky Mountain Spotted Fever. Potentialities of Tick Transmission in Relation to Geographical Occurrence in the United States. Amer J Trop Med 13(4):341 -79.
Parola, P. and Raoult, D., (2001) Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis 32(6):897-928.
Parola, P., Musso, D. and Raoult, D., (2016) Rickettsia felis: the next mosquito-borne outbreak? Lancet Infect Dis 16 (10):1112-3.
Pérez-Osorio, C.E., Zavala-Velázquez, J.E., León, J.J.A. and Zavala-Castro, J.E., (2008) Rickettsia felis as emergent global threat for humans. Emerg Infect Dis 14(7):1019-23.
Rivera-Páez, F.A., Martins, T.F., Ossa-López, P.A., Sampieri, B.R. and Camargo-Mathias, M.I., (2018) Detection of Rickettsia spp. in ticks (Acari: Ixodidae) of domestic animals in Colombia. Ticks Tick Borne Dis 9(4):819-823.
Robinson, M.T., Satjanadumrong, J., Hughes, T., Stenos, J. and Blacksell, S.D., (2019) Diagnosis of spotted fever group Rickettsia infections: the Asian perspective. Epidemiology & Infection, 147.
Schriefer, M.E., Sacci, J.B., Dumler, J.S., Bullen, M.G. and Azad, A.F., (1994) Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol 32 (4):949-54.
Shao, J.W., Zhang, X.L., Li, W.J., Huang, H.L. and Yan, J., (2020) Distribution and molecular characterization of rickettsiae in ticks in Harbin area of Northeastern China. PLOS Neglected Tropical Diseases, 14(6), p.e0008342.
Tomassone, L., Portillo, A., Nováková, M., De Sousa, R. and Oteo, J.A., (2018). Neglected aspects of tick-borne rickettsioses. Parasites & vectors, 11(1): 263.
Walker, J.B., Keirans, J.E. and Horak, I.G., (2005) The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world. Cambridge University Press.
Zhang, J., Lu, G., Kelly, P., Zhang, Z., Wei, L., Yu, D., Kayizha, S. and Wang, C., (2014) First report of Rickettsia felis in China. BMC Infect 14(1):682.