1Advanced Level Institutional Biotech Hub, Debraj Roy College, Golaghat, Assam, India.
2Post Graduate Department of Life Sciences, Debraj Roy College, Golaghat, Assam, India.
Corresponding author email: chaya.610@gmail.com
Article Publishing History
Received: 05/03/2021
Accepted After Revision: 27/06/2021
Cymbopogon martinii (Palmarosa) is one of the lemongrass varieties native to India and Indochina. Due to the vast economical usage of its essential oil, this lemongrass species is cultivated all over the world. The Palmarosa oil has been reported to reveal remarkably good antiviral, antibacterial, antihelmintic, antifungal, antioxidant and cytotoxic properties. In the present study, the essential oil was extracted from the Cymbopogon martinii collected from Arunachal Pradesh, India by hydro-distillation process in Clevenger apparatus. The extracted essential oil was then evaluated for its antimicrobial activity against five test bacteria viz. Bacillus cereus, Bacillus subtilis, Listeria monocytogenes, Staphylococcus aureus and Escherichia coli by measuring their zone of inhibition. Three different concentrations of essential oil in acetone viz. 25 ppm, 50 ppm and 100 ppm were also evaluated for its repellent activity against Aedes aegypti mosquito by K & D module. Antimicrobial assay showed a high sensitivity against Bacillus cereus and Bacillus subtilis; a medium sensitivity against Listeria monocytogenes and Staphylococcus aureus with a diameter of inhibition17.67±1.38, 16.95±1.23, 14.92±0.68 and 12.10±0.52 respectively. However, no obvious effect was shown against the gram-negative bacteria Escherichia coli. The results of repellency activity revealed significant repellent activity of the oil in a dose-dependent manner. The highest concentration i.e. the 100 ppm concentration showed the highest repellent activity against the mosquitoes which decreased as the time increased. The results validated the antimicrobial and repellent properties of the C. martinii essential oil and showed its potency to be used both as a natural repellent and an antimicrobial agent in the future.
Aedes Aegypti, Antimicrobial, Cymbopogon Martinii, Essential Oil, Repellency
Azad S, Chetia C. Analysis on the Antimicrobial and Repellent Activities of Cymbopogon martinii Essential Oil. Biosc.Biotech.Res.Comm. 2021;14(2).
Azad S, Chetia C. Analysis on the Antimicrobial and Repellent Activities of Cymbopogon martinii Essential Oil. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <ahref=”https://bit.ly/3xgf3dp“>https://bit.ly/3xgf3dp</a>
Copyright © Azad and Chetia This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The genus Cymbopogon (Poaceae syn. Graminaceae) consists of around 180 plant species native to the tropics and sub-tropics of Asia, America and Africa (Akhila et al. 2009; Avoseh et al. 2015; Baruah et al. 2016). India includes 45 species of the genus which makes it one of the hubs of Cymbopogon diversity (Bhatnagar 2018). Generally popular for the high content of essential oil, the plants included in this genus are perennial, monocotyledonous and aromatic in nature. Cymbopogon martinii (Roxb.) Will. Watson, commonly known as Palmarosa, is one of the lemongrass species available in north-east India. The plant is traditionally utilized for the treatment of multiple ailments such as arthritis, rheumatism, alopecia, enterosis, lumbago, dermatitis, spasms, impotence, biliousness, wound, cancer of stomach, snakebite, sore, bleeding, and pains by different communities worldwide (Boulos 1983; Dubey and Luthra 2001; Jummes et al. 2020).
Anti-inflammatory, anti-diabetic and diuretic properties are also claimed to be possessed by the plant (Duke and DuCellier 2008). Besides, scientific studies on the plant revealed its bronchodilator, vasodilator and antispasmodic properties (Janbaz et al. 2014). The Palmarosa essential oil is rich in geraniol, geranyl acetate, linalool, β-myrcene, limonene, cymene, sabinene and various other chemical compounds, with antimicrobial properties (Raina et al. 2003; Jummes et al. 2020). This essential oil of Palmarosa is commercially important due to its use in food and flavor industries, high-grade perfumes, soaps, cosmetics, toiletry and tobacco products for its rose-like aroma (Raina et al. 2003; Omar et al. 2016; Jummes et al. 2020).
Scientific studies also suggested its applicability in perfumery, cosmetics and pharmaceutical industries (Jummes et al. 2020). Therefore, it is cultivated on large scale in many regions in India including North-East India. Modern pharmacological studies showed the anthelmintic, antiseptic, antifungal and insect repellent activities of the Palmarosa essential oil (Nirmal et al. 2007; Prasad et al. 2010; Caballero-Gallardo et al. 2012). Geraniol is an efficient plant based antimicrobial agent and insect repellent (Bard et al. 1988; Barnard and Xue 2004). The mosquitoes are the carrier of several vector-borne diseases including malaria, yellow fever, dengue fever and many other (AI-Shehri et al. 2020).
Palmarosa oil has reported to possess repellent activities against different mosquito species (Tyagi et al. 1998). In addition, several studies demonstrated and documented the antibacterial and antifungal activity of Palmarosa oil against a variety of bacterial and fungal strains (Prashar et al. 2003; Ahmad and Viljoen 2015; Castro et al. 2020; Mutlu-Ingok et al. 2020; Scotti et al. 2021). Still there are more important bacterial strains and mosquito species against which the effects of Palmarosa essential oil are not evaluated. Therefore, the present study was designed to determine the antimicrobial activity of Palmarosa oil against four gram positive bacterial strain viz. Bacillus cereus ATCC 11778, Bacillus subtilis ATCC 6051, Listeria monocytogenes ATCC BA-751, Staphylococcus aureus ATCC 25923 and one gram negative bacterial strain, Escherichia coli ATCC 25922 and repellant activities of the oil against the mosquito Aedes aegypti.
MATERIAL AND METHODS
From the forest division of Pasighat, Arunachal Pradesh in north-east India (179 m, 28˚03ʹ58ʺN, 95˚19ʹ36ʺE), fresh leaves of Cymbopogon martinii (Palmarosa) were collected during March-April, 2019. The plant material was identified by Dr. Pankaj Chetia, Assistant Professor, Department of Life Science, Dibrugarh University, Assam, India.
The fresh leaves (800 grams) were subjected to hydro-distillation by using Clevenger Apparatus for about 3 h to obtain the essential oil. The collected essential oil sample was then stored in sealed glass vials in refrigerator prior to experimentation. Strains of four gram-positive bacteria viz. Bacillus cereus ATCC 11778, Bacillus subtilis ATCC 6051, Listeria monocytogenes ATCC BA-751, Staphylococcus aureus ATCC 25923 and one gram-negative bacterium, Escherichia coli ATCC 25922 were collected from Centre for Biotechnology and Bioinformatics, Dibrugarh University. Three well isolated colonies of the same morphological type were selected from an agar plate culture and the strains were transferred into a tube containing 4-5 ml of Luria broth (LB) after attaining the logarithmic growth phase, and then incubated at 37˚C.
To obtain turbidity optically comparable to that of the 0.5 McFarland standards to obtain approximately 106 CFU/ml of TB, the turbidity of the actively growing broth culture was adjusted with sterile distilled water. Media plates were inoculated within 15 minutes of standardizing the inoculums to avoid changes in microbial inoculum density (Abalaka et al. 2012). The antimicrobial susceptibility test was carried out by agar well diffusion method (Das et al. 2013). Fresh bacterial culture of 100 μl (106 CFU/ml) was added to the molten Muller Hilton agar medium in the Petri plates homogenously and allowed to solidify. After that with the help of a sterile gel puncture, four wells are aseptically punched on each Petri plates with a diameter of 6-8 mm. Then a 10 µl of the extracted essential oil was introduced into the well by a micropipette under aseptic conditions. 20 μg/ml of chloramphenicol was set as positive control and double distilled water (DDW) was set as negative control (Das et al. 2013).
Depending upon the test microorganisms, the plates were incubated at 37˚C for 18-36 hours and then the diameter of growth of inhibition was measured in mm. The antimicrobial activity was evaluated following the rules of extremely sensitive (>20 mm), high sensitivity (15~20 mm), medium sensitivity (10~15 mm), low sensitivity (7~10 mm) and not sensitive (<7 mm) (Zhou et al., 2020). Triplicate analysis was performed for this experiment. Duration of protection time against mosquito (Aedes aegypti) bite was determined by K & D module (Islam et al., 2017). Three different concentrations of C. martinii essential oils were prepared with acetone viz. 25 ppm, 50 ppm and 100 ppm. A volume of 25 µL of each concentration were applied randomly to the marked area of volunteer’s thigh and allowed to air dry for 5 minutes. The module was then placed over the thigh and the mosquitoes were allowed to access the treated area by opening the sliding door (Zhou et al. 2020).
Ten females (5-7 days old, blood meal unfed) were randomly selected from a pool of 200 adults placed into three adjacent cells in the K & D module. Observation was done three times on each oil concentration and the number of mosquitoes landing and biting in each cell within a 5-minute exposure was recorded. A different set of mosquito population was used for the replications. Observations on the number of bites were recorded at 30 min, 1 hr, 2 hrs, 3 hrs, 4 hrs of post treatment.
RESULTS AND DISCUSSION
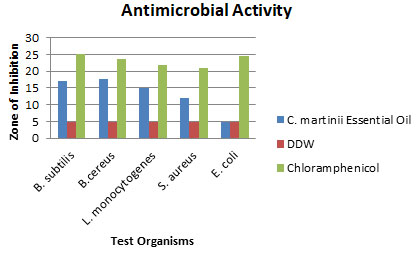
Assessment of Antimicrobial Activity: The antimicrobial activity of the essential oil of C. martinii against five test organisms was determined by measuring zones of inhibition (Table 1). The negative control showed no antimicrobial activity. In comparison to the positive control (20 μg/ml chloramphenicol) the essential oil showed a high sensitivity against Bacillus cereus and Bacillus subtilis; a medium sensitivity against Listeria monocytogenes and Staphylococcus aureus with a diameter of inhibition17.67±1.38, 16.95±1.23, 14.92±0.68 and 12.10±0.52 respectively. However, no obvious effect was shown against the gram-negative bacteria Escherichia coli. Several studies reported that essential oil showed less antimicrobial activity against gram-negative bacteria as the lipopolysaccharides in the outer membrane of these bacteria prevent the hydrophobic antimicrobial agents (Longbottom et al. 2004; Kavoosi and Rowshan 2013; Rashid et al. 2013). However, Scotti et al. (2020) found greater inhibitory effect of C. martinii essential oil against E. coli O157:H7 which also contradicts the results of Kim et al. (2016). This may be due to the higher concentrations of essential oil Scotti et al. (2020) used (Kim et al. 2016; Scotti et al. 2020).
Table 1. Zones of Inhibition of C. martinii essential oil, DDW and Chloramphenicol against five test organisms.
| Test organisms | Zone of Inhibition (mm±SD) | ||
| C. martinii Essential Oil | DDW | Chloramphenicol | |
| Bacillus subtilis(G⁺)ATCC 6051 | 16.95±1.23 | 5.00±0.00 | 25.19±2.31 |
| Bacillus cereus(G⁺)ATCC 11778 | 17.67±1.38 | 5.00±0.00 | 23.59±0.91 |
| Listeria monocytogenes(G⁺)ATCC BA-751 | 14.92±0.68 | 5.00±0.00 | 21.86±0.87 |
| Staphylococcus aureus(G⁺)ATCC 25923 | 12.10±0.52 | 5.00±0.00 | 21.00±0.00 |
| Escherichia coli(G–)ATCC 25922 | 5.00±0.00 | 5.00±0.00 | 24.53±1.45 |
(G⁺: Gram Positive; G–: Gram Negative
Figure 1: Zones of Inhibition of C. martinii Essential Oil, DDW and Chloramphenicol against five test organisms.
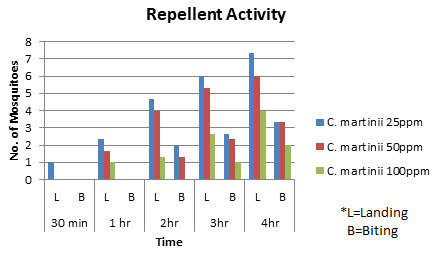
Assessment of Repellent Activity: The essential oil of C. martinii exhibited good repellent activity against Aedes aegypti mosquitoes (Table 2). The mosquitoes start landing in the cells in 30 minutes and start biting in 2 h at 25 ppm and 50 ppm concentrations. 100 ppm concentration of essential oil showed 100% repellency up to 2 h. The repellent activity of the essential oil was directly related to the concentrations of the oil as well as the time of exposure. The highest concentration i.e. the 100 ppm concentration showed the highest repellent activity against the mosquitoes which decreased as the time increased. The oil exhibited great repellent activity in a dose-dependent manner. Other authors also reported the dose-response relationship of repellency of essential oils (Caballero-Gallardo et al. 2011; AI-Shehri et al. 2020).
Luz et al. (2020) documented 337 essential oils from 225 plants that have been tested against A. aegypti and found more than 60% of these were active. Significant repellence profile of the essential oil of other Cymbopogon species such as C. nardus have been shown against Aedes aegypti mosquito (Muller et al. 2008; Songkro et al. 2012; Huang et al. 2015; Sajo et al., 2015; Harismah et al. 2017).
Table 2. Repellent activity of different concentrations of C. martinii essential oil against Aedes aegypti mosquitoes at different time intervals.
| Oil Extract | Concentration | 30 min | 1 hr | 2hr | 3hr | 4hr | |||||
| L | B | L | B | L | B | L | B | L | B | ||
| Cymbopogon martinii | 25ppm | 1 | 0 | 2.33 | 0 | 4.67 | 2 | 6 | 2.67 | 7.33 | 3.33 |
| 50ppm | 0 | 0 | 1.67 | 0 | 4 | 1.33 | 5.3 | 2.33 | 6 | 3.33 | |
| 100ppm | 0 | 0 | 1 | 0 | 1.33 | 0 | 2.67 | 1 | 4 | 2 | |
(L= Landing; B= Biting)
Figure 2: Repellent activity of different concentrations of C. martinii essential oil against Aedes aegypti mosquitoes at different time intervals.
CONCLUSION
In the present study, essential oil extracted from field collected leaves of Cymbopogon martinii from Arunachal Pradesh are evaluated for its antimicrobial and repellent activities. The findings revealed that the oil was extremely effective against gram-positive bacteria and less effective against gram-negative bacteria. The essential oil showed great repellent activity against Aedes aegypti mosquitoes in a dose-dependent manner. The chemical constituents of the C. martinii essential oil are responsible for these activities. However, further research is required to elucidate the molecular mechanisms underlying the noteworthy antimicrobial activity. The complex composition of the Palmarosa oil depending on geographical area of origin, extraction method etc. is the main limitation in its use. This entails the necessity for precise control of the individual batches.
ACKNOWLEDGEMENTS
We are thankful to the authorities of Defence Research Laboratory, Tezpur, Assam, India; Dibrugarh University, Dibrugarh, Assam, India and Debraj Roy College, Golaghat, Assam, India for giving administrative support to carry out this study. We are also thankful to Dr. Pankaj Chetia, Assistant Professor, Department of Life Sciences, Dibrugarh University for identifying the plant specimen of our research work.
Conflict of Interest
On behalf of all the authors, the corresponding author states no conflict of interests among themselves.
REFERENCES
Abalaka, M.E., Daniyan, S.Y., Oyeleke, S.B. and Adeyemo, S.O., (2012). The antimicrobial evaluation of Moringa oleifera leaf extract on selected bacterial pathogens. Journal of Microbiology Research, 2, 1-4.
Ahmad, A. and Viljoen, A., (2015). The invitro antimicrobial activity of Cymbopogon essential oil (lemongrass) and its interaction with silver ions. Phytomedicine, 22, 657-665.
AI-Shehri, A.M.G., AI-Johny, B.O., AL-Ghamdi, K.M.S., AL-Kenani, N., Anwar, Y., Alqarhi, T.F. and Ullah, I., (2020). Analysis of anti-mosquito and antimicrobial activities of the extract of Nigella sativa (Black seeds). Bioscience Biotechnology Research Communications, 13, 1938-1941.
Akhila, A., Bertea, C.M., Bigheli, A., Casanova, J., Khunkitti, W., Maffei, M.E., Mathur, A.K., Moyler, D.A., Pandey, A.K., Sumi, H., Tiwari, R. and Yatagai, C., (2009). Essential oil-bearing grasses: The genus Cymbopogon. Taylor & Francis Group, New York.
Avoseh, O., Oyedeji, O., Rungqu, P. and Nkeh-Chungag, B., (2015). Cymbopogon species: Ethnopharmacology, phytochemistry and the pharmacological importance. Molecules, 20, 7438-7453.
Baruah, J., Gogoi, B., Das, K., Ahmed, N.M., Sarmah, D.K., Lal, M. and Bhau, B.S., (2016). Genetic diversity study amongst Cymbopogon species from NE-India using RAPD and ISSR markers. Industrial Crops and Products, http://dx.doi.org/10.1016/j.indcrop.2016.10.022
Bard, M., Albrecht, M.R., Gupta, N., Guynn, C.J. and Stillwell, W., (1988). Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids, 23, 534–538.
Barnard, D.R. and Xue, R., (2004). Laboratory evaluation of mosquito repellents against Aedes albopictus, Culex nigripalpus, and Ochlerotatus triseriatus (Diptera: Culicidae). Journal of Medical Entomology, 41, 726–730.
Bhatnagar, A., (2018). Composition variation of essential oil of Cymbopogon spp. growing in Garhwal region of Uttarakhand, India. Applied and Natural Science Foundation, 10, 363-366.
Boulos, L., (1983). Medicinal Plants of North Africa. Algonac (Michigan, USA): Reference Publications.
Caballero-Gallardo, K., Olivero-Verbel, J. and Stashenko, E., (2012). Repellency and toxicity of essential oils from Cymbopogon martinii, Cymbopogon flexuosus and Lippia origanoides cultivated in Colombia against Tribolium castaneum. Journal of Stored Products Research, 50, 62-65.
Caballero-Gallardo, K., Olivero-Verbel, J. and Stashenko, E., (2011). Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. Journal of Agricultural and Food Chemistry, 59, 1690-1696.
Castro, J.C., Pante, G.C., Centenaro, B.M. and Ribeiro De, R.T., (2020). Antifungal and antimycotoxigenic effects of Zingiber officinale, Cinnamomum zeylanicum and Cymbopogon martinii essential oils against Fusarium verticillioides. Food Additives & Contaminants, 37, 1531-1541.
Das, D.C., De, S., Bhattacharya, S. and Das, M., (2013). Antibacterial activity and phytochemical analysis of Cardanthera difformis druce leaf extracts from West Bengal, India. International Journal of Phytomedicine, 5, 446-451.
Dubey, V.S. and Luthra, R., (2001). Biotransformation of geranyl acetate to geraniol during palmarosa (Cymbopogon martinii, Roxb.Wats. Var. motia) inflorescence development. Phytochemistry, 57, 675-680.
Duke, J.A. and DuCellier, J.L., (2008). Duke’s Handbook of Medicinal Plants of the Bible. Boca Raton, CRC Press.
Harismah, K., Vitasari, D., Mirzaei, M., Ahmad Fuadi, M. and Aryanto, Y.H., (2017). Protection capacity of mosquito repellent ink from citronella (Cymbopogon nardus L.) and clove leaf oils (Syzygium aromaticum) against Aedes aegypti. In: AIP Conference Proceedings, Pp 020023.
Huang, T.H., Tien, N.Y. and Luo, Y.P., (2015). An in vitro bioassay for the quantitative evaluation of mosquito repellents against Stegomyia aegypti (=aedes aegypti) mosquitoes using a novel cocktail meal. Medical and Veterinary Entomology, 29, 238-244.
Islam, J., Zaman, K, Tyagi, V., Duarah, S., Dhiman, S. and Chattopadhyay, P., (2017). Protection against mosquito vectors Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus using a novel insect repellent, ethyl anthranilate. Acta Tropica, 174, 56-63.
Janbaz, K.H., Qayyum, A., Saqib, F., Imran, I., Zia-Ul-Haq, M. and De Feo, V., (2014). Bronchodilator, vasodilator and spasmolytic activities of Cymbopogon martinii. Journal of Physiology and Pharmacology, 65, 859-866.
Jummes, B., Sganzerla, W.G., Goncalves da Rosa, C., Noronha, C.M., Nunes, M.R., Bertoldi, F.C. and Manique Barreto, P.L., (2020). Antioxidant and antimicrobial poly-ε-caprolactone nanoparticles loaded with Cymbopogon martinii essential oil. Biocatalysis and Agricultural Biotechnology, 23, 101499.
Kavoosi, G. and Rowshan, V., (2013). Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: Effect of collection time. Food Chemistry, 138, 2180–2187.
Kim, Y.G., Lee, J.H., Gwon, G., Kim, S.I., Park, J.G. and Lee, J., (2016). Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Scientific Reports, 6, 36377.
Longbottom, C.J., Carson, C.F., Hammer, K.A., Mee, B.J. and Riley, T.V., (2004). Tolerance of Pseudomonas aeruginosa to Melaleuca alternifolia (tea tree) oil is associated with the outer membrane and energy-dependent cellular processes. Journal of Antimicrobial Chemotherapy, 54(2), 386–392.
Luz, T.R.S.A., de Mesquita, L.S.S., do Amaral, F.M.M. and Coutinho, D.F., (2020). Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae. Acta Tropica, 212, 105705.
Mutlu-Ingok, A., Devecioglu, D., Dikmetas, D.N., Karbancioglu-Guler, F. and Capanoglu, E., (2020) Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules, 25, 4711-4760.
Muller, G.C., Junnila, A., Kravchenko, V.D., Revay, E.E., Butlers, J. and Schlein, Y., (2008). Indoor protection against mosquito and sand fly bites: a comparison between citronella, linalool, and geraniol candles. Journal of the American Mosquito Control Association, 24, 150-153.
Nirmal, S.A., Girme, A.S. and Bhalke, R.D., (2007). Major constituents and anthelmintic activity of volatile oils from leaves and flowers of Cymbopogon martinii Roxb. Natural product research, 21, 1217-1220.
Omar, E., Pavlovic, I., Drobac, M., Radenkovic, M., Brankovic, S. and Kovacevic, N., (2016). Chemical composition and spasmolytic activity of Cymbopogon nervatus (Hochst.) Chiov. (Poaceae) essential oil. Industrial Crops and Products, 91, 249-254.
Prasad, C.S., Shukla, R., Kumar, A. and Dubey, N.K., (2010). In vitro and in vivo antifungal activity of essential oils of Cymbopogon martinii and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses, 53, 123-129.
Prashar, A., Hili, P., Veness, R.G. and Evans, C.S., (2003). Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry, 63, 569-575.
Raina, V.K., Srivastava, S., Aggarwal, K.K., Syamasundar, K.V. and Khanuja, S.P.S., (2003). Essential oil composition of Cymbopogon martinii from different places of India. Flavour and Fragrance Journal, 18, 312-315.
Rashid, S., Rather, M.A., Shah, W.A. and Bhat, B.A., (2013). Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chemistry, 138, 693–700.
Sajo, M.E.J, Song, S.B., Bajgai, J., Kim, Y.J., Kim, P.S., Ahn, D.W., Khanal, N. and Lee, K.J., (2015). Applicability of citronella oil (Cymbopogon winteratus) for the prevention of mosquito-borne diseases in the rural area of Tikapur, far-western Nepal. Rural Remote Health, 15, 1-10.
Scotti, R., Stringaro, A., Nicolini, L., Zanellato, M., Boccia, P., Maggi, F. and Gabbianelli, R., (2021). Effects of essential oils from Cymbopogon spp. And Cinnamomum verum on biofilm and virulence properties of Escherichia coli O157:H7. Antibiotics, 10, 113-127.
Songkro, S., Hayook, N., Jaisawang, J., Maneenuan, D., Chuchome, T. and Kaewnopparat, N., (2012). Investigation of inclusion complex of citronella oil, citronellal and citronellol with β-cyclodextrin for mosquito repellent. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 72, 339-355.
Tyagi, B.K., Shahi, A.K. and Kauf, B.L., (1998). Evaluation of repellent activities of Cymbopogon essential oils against mosquito vectors of Malaria, Filariasis and Dengue Fever in India. Phytomedicine, 5, 324-329.
Zhou, W., He, Y., Lei, X., Liao, L., Fu, T., Yuan, Y., Huang, X., Zou, L., Liu, Y., Ruan, R. and Li, J., (2020). Chemical composition and evaluation of antioxidant activities, antimicrobial, and anti-melanogenesis effect of the essential oils extracted from Dalbergia pinnata (Lour.) Prain. Journal of Ethnopharmacology, doi: https://doi.org/10.1016/j.jep.2020.112731.


