*1Research and Development Department, Uttaranchal University, Dehradun, Uttarakhand, India
2Chemical Sciences Division, CSIR- Central Institute of Medicinal and Aromatic Plants, Lucknow, Uttar Pradesh, India
3Crop Protection Division, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, Uttar Pradesh, India
Corresponding author email: atulsrivastav.micro@gmail.com
Article Publishing History
Received: 21/10/2020
Accepted After Revision: 11/12/2020
Essential oils play a major role in the management of phytopathogenic fungal diseases and come forward as a replacement of chemical fungicides. Many of the medicinal and aromatic plants essential oils are used for its preservative, and pesticidal properties. The study of how essential oils affect the phytopathogenic fungal diseases is not widely studied by many researchers, specifically, the essential oils which we have taken. This compelled us to conduct an original research for Ageratum conyzoides, Zanthoxylum armatum, and Mentha arvensis and fungi Curvularia lunata. The essential oils of Ageratum conyzoides, Zanthoxylum armatum, and Mentha arvensis, were procured from fresh herbage sampled from the Lucknow region of India. However, A. conyzoides was also sampled from the Lakhimpur, Pantnagar, and Kanpur region of India.
These plant-based essential oils were extracted by the hydrodistillation method and were stored for further studies. The antifungal activity was examined by the use of poison food techniques. Hundred percent of fungal (Curvularia lunata) growth inhibition showed by the use of 0.2 mg/ml and 8 mg/ml concentration of A. conyzoides and Z. armatum essential oils respectively. C. lunata is the major cause of leaf spot/leaf blight disease on economically important crops and causing heavy loss of economy. M. arvensis essential oil showed greater antifungal activity as compared to its major compound menthol and DMO via poison food technique. Overall, the study revealed the antifungal activity of essential oils against C. lunata. Moreover, the essential oil of M. arvensis shows the higher inhibition as compare to the menthol/DMO against the C. lunata.
Ageratum Conyzoides, Zanthoxylum armatum, Mentha arvensis, Curvularia lunata, Essential Oil
Srivastava A. K, Gupta M, Singh R. P, Misra P. Analysis on Inhibiting Pathogenic Activity of Fungi Curvularia lunata by Essential Oils. Biosc.Biotech.Res.Comm. 2020;13(4).
Srivastava A. K, Gupta M, Singh R. P, Misra P. Analysis on Inhibiting Pathogenic Activity of Fungi Curvularia lunata by Essential Oils. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/38b5OSe
Copyright © Srivastava et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Foliar region of plant provides the favourable habitat for epiphytic microorganisms, many of which are capable to influence the growth of pathogens. More than 12% reduction of world crop yield reported due to pre- and post-harvest diseases and FAO proposed that about 20 to 40 percent of global crop yields losses per year by the plant pest and diseases (Agrios, 1997; FAO, 2020). The fungal disease causes major yield reduction and economic losses in agriculture and forestry of the world. Among them, the foliar disease is one of the major constraints.
Medicinal and aromatic plants (MAPs) have been commercially cultivated as a source of important medicinal constituents and essential oil in various parts of the world but adversely affected by many fungal diseases in the humid season. Fungal pathogens infect MAPs under the favorable condition and produce a serious threat to their commercial cultivators. In the present scenario, the infection of Curvularia spp. on the commercial fields of MAPs increases day by day which adversely affected the economic yield of the crops. Curvularia belongs to family Pleosporaceae, the largest family in the Pleosporales (Zhang et al., 2012; Hyde et al., 2013; Wijayawardene et al., 2014).
Leaf blight and leaf spot are the most common foliar diseases caused by the Curvularia spp., and posed to potential threats in many important crops. Curvularia lunata seems to infect a wide range of medicinal and aromatic crops such leaf spot of Zatropha curcus in Mexico, leaf blight of Ocimum basilicum, Leaf spot of Mentha arvensis, and leaf spot of Acorus calamus in India (Thakur et al., 1974; Cisnero-Lopez et al., 2012; Srivastava et al., 2015; Srivastava et al., 2019). The Indian trade of medicinal and aromatic products is approximately 120 million US$ per annum. India is the biggest producers of MAPs and potential supplier of essential oils to the world market (Tripathi et al., 2016).
The foliar diseases (leaf spot and leaf blight) cause the economic losses and reduced the shelf life and market values of the food commodities. Thus, it is essential to manage the foliar disease with biological features without any harm the quality of MAPs. Ageratum conyzoides L., is an annual herb belong to the family Asteraceae, also known as billygoat-weed, chick weed and white weed. It used as medication in Asia, Africa, and South America (Vera, 1993; Okunade et al., 2002; Singh et al., 2013; Moore et al., 2020).
The essential oil of A. conyzoides has the potential antimicrobial, antiaflatoxigenic and antioxidant activity (Patil et al., 2010; Osho et al., 2011). Zanthoxylum armatum DC. (Rutaceae) is a shrub, and widely distributed in Asian region. The leaf, seed and fruit essential oil of Z. armatum has antibacterial, antifungal and anti-inflammatory activity (Mehta et al., 1981; Guo et al., 2010; Sati et al., 2011). Mentha arvensis L. belongs to the family Lamiaceae, native to temperate regions of Europe, western and central Asia, east to the Himalaya and eastern Siberia and North America. A lot of antimicrobial activity recorded of M. arvensis. The major bioactive compounds of Mentha are menthol, menthone, eugenol and pulegone (Luca et al., 2011; Moghtader, 2013; Akbar et al., 2014).
Herbal-based approaches come forward for development of new strategies to manage the plant diseases. A lot of plants essential oil used in the management of plant diseases and reduced the fungal as well as bacterial growth. Some plants like Origanum vulgare, Cuminum cyminum, Eucalyptus citriodora, Thymus vulgaris, Cymbopogon citrates, Lavandula officinalis, and Zingiber officnalae have essential oils that are reported for its antifungal activity (Lee et al., 2007; Cosic et al., 2010; Saroj et al., 2018; Raveau et al., 2020). In this study, A. conyzoides, Z. armatum, and M. arvensis essential oil used to the management of fungal pathogen C. lunata, cause leaf spot/leaf blight disease. The comparative antifungal activity of Menthol, Dementhol (DMO), and M. arvensis essential oil against C. lunata also studied in this paper.
MATERIAL AND METHODS
Plants and essential oils: The fresh A. conyzoides plant sample was collected in the plastic bag from the Lakhimpur, Pantnagar, and Kanpur, Uttar Pradesh, India. Whereas Z. armatum, and M. arvensis plants were sampled from the CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, India. Sampled plants were taken quickly to laboratory and the fresh herbages were processed for essential oil extraction by the hydrodistillation method in the clevenger type apparatus (Maji et al., 2013). Extracted oils were purified and collected in to glass vial for further use.
In vitro screening of essential oils: Potato dextrose agar (PDA) plates have been prepared by adding different concentrations (0.05, 0.1, 0.15, 0.2 and 0.25 mg/mL) of essential oil {(A. conizoides (code L1, P1 and K2)}at 40-45°C. To ensure the proper mixing of essential oil, 0.05% Tween-80 was added. A 6 mm disc of C. lunata was placed on the PDA filled Petri plates. All Petri-plates were incubated at 27±1°C. Plates without essential oil served as a control. The effect of essential oil on the growth of C. lunata was measured after five days of incubation. Minimum inhibitory concentration (MIC) was calculated using poison food technique (Grove and Moore, 1962; Knobloch et al., 1989; Nene et al., 1993). Percentage of the growth inhibition was calculated as follows:
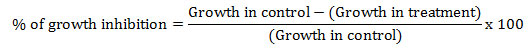
In vitro screening of chemical fungicides: By using the poison food technique, the fungicides Ridomil Gold and Bavistin are also tested for antagonistic activity against C. lunata in the concentration of 0.2, 0.4, 0.6, 0.8mg/mL. This study helps in the comparative study of chemical fungicide against selective essential oils.
RESULTS AND DSICUSSION
Management of fungal diseases on plants using synthetic chemicals is more in practice. However, they possessed hazardous effect on plant, environment and human health. Thus, an alternate approach, employing of natural products viz. essential oil and chemical substances of plant origin, gained attention for the in vitro management of diseases caused by a plant pathogen. Here Z. armatum, A. conyzoides, and M. arvensis screen for the antifungal activity against the growth of C. lunata by Poison food technique.
Screening of essential oils against the growth of Curvularia lunata: Our results on in vitro screening showed that essential oils of Ageratum conyzoides, Zanthoxylum armatum, and Mentha arvensis proved highest antagonistic activity against the Curvularia lunata cause leaf spot/leaf blight diseases.
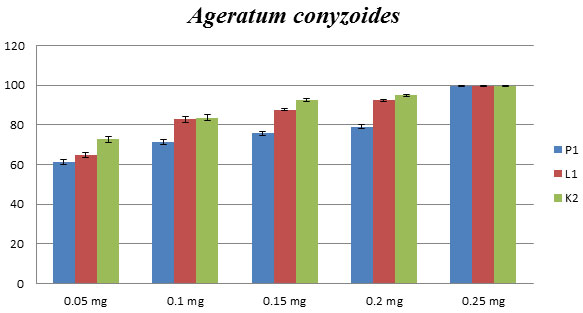
Ageratum conyzoides: Antifungal activity of Ageratum conyzoides, isolated from the different geographical location (Lakhimpur (L1), Pantnagar (P1), and Kanpur (K1) district of Uttar Pradesh) showed a similar type of growth inhibition. The concentration of 0.2mg has revealed a higher percentage (more than 90%) of inhibition as compared to others three concentrations of essential oils against C. lunata (Fig.1). The MIC was measured as 0.25 mg/mL, showed 100% of inhibition.
Figure 1: Effect of P1, L1 and K2 essential oils on phytopathogenic C. lunata. Percentage of growth inhibition measured using poison food technique. (P1, L1, and K2 shows that Ageratum conyzoides get from the different geographical location as Pantnagar, Lakhimpur and Kanpur respectively).
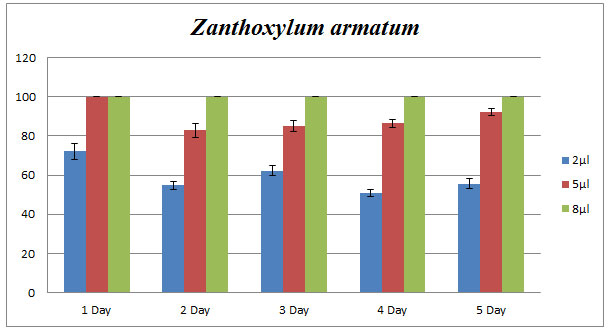
Zanthoxylum armatum: Z. armatum (8µl) showed 100% of inhibition of C. lunata by applying poison food technique while 5µl oil resulted in 80-100% of inhibition. Bar diagram revealed the percent of inhibition by applying 2, 5, and 8µl of essential oil continuously for five days (Fig.2).
Figure 2: Effect of Z. armatum essential oil on phytopathogenic C. lunata. Percentage of growth inhibition studied using poison food technique.
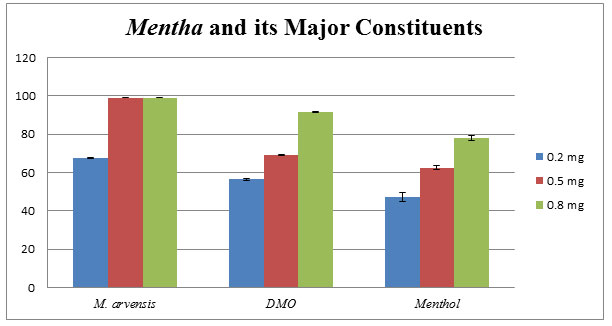
Mentha arvensis: Menthol is the major constituent of M. arvensis essential oil, discussed and proved its potential by various researchers as described in the review of literature section. Removal of menthol from M. arvensis essential oil, Dementhol or DMO remains present in the oil. Comparative analysis among menthol, DMO and M. arvensis oil; M. arvensis oils show greater antifungal activity against C. lunata as compared to menthol and DMO as denoted in the bar diagram (Fig.3).
Figure 3: Comparative effects of M. arvensis EO, DMO, and Menthol against phytopathogenic C. lunata. The mean of three independent experiments shows the percentage of growth inhibition using poison food technique.
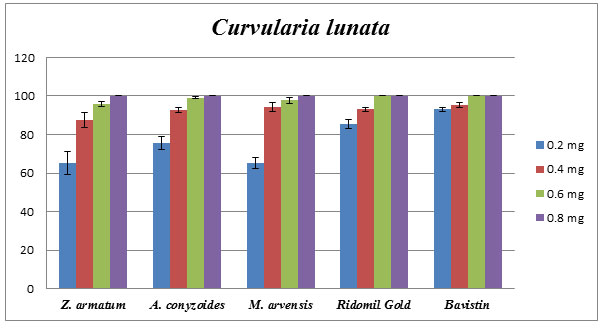
Comparative study of Essential oil vs. specific fungicide: From above result, it shows that Z. armatum (8mg/mL), A. conyzoides (0.25mg/mL) and M. arvensis (0.8mg/mL) showed 100% of inhibition against C. lunata by applying poison food technique. Results in the case of selective chemical fungicides such as Ridomil Gold and Bavistin demonstrated 100% of inhibition when applied with a concentration of approx 0.5 mg/mL against C. lunata. Therefore, selective essential oils showed results to be at par as selected chemical fungicides (Fig.4).
Figure 4: Comparative effect of Z. armatum, A. conyzoides, M. arvensis oils, Reidomil Gold, and Bavistin on phytopathogenic C. lunata. Percentage of growth inhibition established using poison food technique.
Many researchers have studied the antagonistic activity of essential oils and its compound against bacteria and fungus. Essential oils are the mixture of compounds so, it is most likely to show antimicrobial activity is not due to a single compound therefore it may be a synergistic/synchronous effect (Skandamis et al., 2001; Carson et al., 2002).
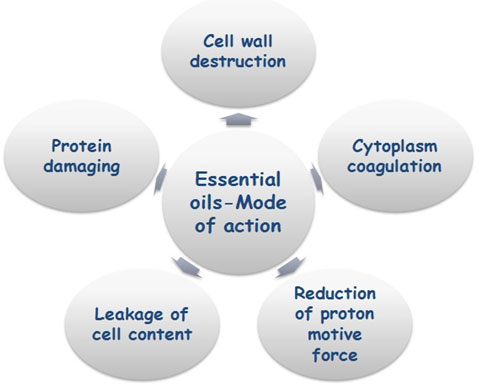
Proposed Mechanism of fungal inhibition by essential oil: The antifungal activity of essential oils and their components is based on the feature of essential oils that plays a critical role in antagonistic activity is hydrophobicity interaction with the cell membrane. It enables them to penetrate in the lipids of the cell membrane and mitochondria; disturbing the cell structure and causing cell lysis/cell bursting resulting into the leakage of ions and other cell contents (Knobloch et al., 1989; Sikkema et al., 1994; Oosterhave et al., 1995; Helander et al., 1998; cox et al., 2000; Lambert et al., 2001; Skandamis et al., 2001; Ultee et al., 2002; Carson et al., 2002; Pragadheesh et al., 2013). Figure 5 shows the mode of action of essential oils on the fungal cell.
Figure 5: Diagrammatic representation of essential oil actions on fungal cell
Expansion in population and food demand lead the development of new strategies for enhancing the agricultural crops resistant to any constraint. To avoid phytopathogenic diseases conventional pesticides applied by the farmers on the agricultural crop. However, conventional pesticides are cheap and lead to loss of nutrients in the soil and agricultural products as well (Singh et al., 2020). Thus, biological pesticides come in light, which is effective on plant diseases and sustain the quality of soil and plant products. Essential oil is the most effective biological pesticides used in the management of plant diseases. However chemical compositions of the essential oils are very complex because of the huge number of different groups of chemical constituents present in it. Thus, it is most likely that their antimicrobial activity is not due to one compound alone or by single mechanism but might be there are several targets in the cell (Skandamis et al., 2001; Carson et al., 2002; Raveau et al., 2020).
CONCLUSION
Fungi are the major causative organism of plant diseases on the MAPs as well as agricultural crops. The incidence of C.lunata fungi seems to be higher on the MAPs field shown in the recent years. Thus, the management of leaf spot/leaf blight diseases on sustainable basis should be necessary for save the economy of our country. In the present study, M. arvensis essential oil shows greater antifungal activity as compared to its major compound menthol and DMO via poison food technique. It means that there is a synergistic effect of menthol and another compound present in the oil to show greater antifungal activity while menthol or DMO show lesser activity. Along with M. arvensis, A. conyzoides and Z. armatum also exhibited significant pharmacological and biocidal activity.
Funding: This work was supported by CSIR-Central Institute of Medicinal and Aromatic Plants.
ACKNOWLEDGEMENTS
All the experiments were carried out in the Plant pathology department of CSIR- Central Institute of Medicinal and Aromatic Plants.
Conflict of interest statement: There is no conflict of interest.
REFERENCES
Agrios, G.N. (1997). Control of Plant Diseases. In: Plant Pathology, 4th Edition, Academic Press, San Diego, 200-216.
Akbar, S., Majid, A., Hassan, S., Rehman, A.U., Khan, T., Jadoon, M.A. and Rehman, M.U. (2014). Comparative in vitro activity of ethanol and hot water extracts of Zanthoxylum armatum to some selective human pathogenic bacterial strains. International Journal of Biosciences, 4(1), pp.285-291.
Carson, C.F., Mee, B.J. and Riley, T.V. (2002). Mechanism of action of Melaleuca alternifolia (Tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrobial agents and chemotherapy, 46(6), pp.1914-1920.
Cisneros-López, E., González-Quintero, J., Moreno-Medina, V.R. and Álvarez-Ojeda, J. (2012). First Report of Curvularia lunata on Jatropha curcas in Mexico. Plant disease, 96(2), pp.288-288.
Ćosić, J., Vrandečić, K., Poštić, J., Jurković, D. and Ravlić, M. (2010). In vitro antifungal activity of essential oils on growth of phytopathogenic fungi. Poljoprivreda (Osijek), 16(2), p.25.
Cox, S.D., Mann, C.M., Markham, J.L., Bell, H.C., Gustafson, J.E., Warmington, J.R. and Wyllie, S.G. (2000). The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (Tea tree oil). Journal of applied microbiology, 88(1), pp.170-175.
FAO, (2020). Keeping plant pests and diseases at bay: experts focus on global measures. (http://www.fao.org/news/story/en/item/280489/icode/#:~:text=FAO%20estimates%20that%20between%2020,by%20plant%20pests%20and%20diseases).
Grover, R.K. and Moore, J.D. (1962). Toximetric Studies of fungicides against brown rot organisms, Sclerotinia-Fructicola and S-Laxa. Phytopathology, 52(9), pp.876.
Guo, T., Deng, Y.X., Xie, H., Yao, C.Y., Cai, C.C., Pan, S.L. and Wang, Y.L. (2011). Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice. Fitoterapia, 82(3), pp.347-351.
Gustafson, J.E., Liew, Y.C., Chew, S., Markham, J., Bell, H.C., Wyllie, S.G. and Warmington, J.R. (1998). Effects of tea tree oil on Escherichia coli. Letters in applied microbiology, 26(3), pp.194-198.
Helander, I.M., Alakomi, H.L., Latva-Kala, K., Mattila-Sandholm, T., Pol, I., Smid, E.J., Gorris, L.G. and von Wright, A. (1998). Characterization of the action of selected essential oil components on Gram-negative bacteria. Journal of agricultural and food chemistry, 46(9), pp.3590-3595.
Hyde, K.D., Jones, E.G., Liu, J.K., Ariyawansa, H., Boehm, E., Boonmee, S., Braun, U., Chomnunti, P., Crous, P.W., Dai, D.Q. and Diederich, P. (2013). Families of dothideomycetes. Fungal diversity, 63(1), pp.1-313.
Knobloch, K., Pauli, A., Iberl, B., Weigand, H. and Weis, N. (1989). Antibacterial and antifungal properties of essential oil components. Journal of Essential Oil Research, 1(3), pp.119-128.
Lambert, R.J.W., Skandamis, P.N., Coote, P.J. and Nychas, G.J. (2001). A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of applied microbiology, 91(3), pp.453-462.
Lee, S.O., Choi, G.J., Jang, K.S., Lim, H.K., Cho, K.Y. and Kim, J.C. (2007). Antifungal activity of five plant essential oils as fumigant against postharvest and soil borne plant pathogenic fungi. The Plant Pathology Journal, 23(2), pp.97-102.
Luca, S.A. and Ciucanu, I. (2011). Solid-phase microextraction of menthol from peppermint. New Frontiers in Chemistry, 20(1), p.71.
Maji, D., Barnawal, D., Gupta, A., King, S., Singh, A.K. and Kalra, A. (2013). A natural plant growth promoter calliterpenone from a plant Callicarpa macrophylla Vahl improves the plant growth promoting effects of plant growth promoting rhizobacteria (PGPRs). World Journal of Microbiology and Biotechnology, 29(5), pp.833-839.
Mehta, M.B., Kharya, M.D., Srivastava, R. and Verma, K.C. (1981). Antimicrobial and anthelmintic activities of the essential oil of Zanthoxylum alatum Roxb. Indian Perfumer.
Moghtader, M. (2013). In vitro antifungal effects of the essential oil of Mentha piperita L. and its comparison with synthetic menthol on Aspergillus niger. African Journal of Plant Science, 7(11), pp.521-527.
Moore, D., Robson, G.D. and Trinci, A.P. (2020). 21st century guidebook to fungi. Cambridge University Press.
Nene, Y.L. and Thapliyal, P.N. (1993). Fungicides in plant disease control (No. Ed. 3). International Science Publisher.
Okunade, A.L. (2002). Ageratum conyzoides L.(asteraceae). Fitoterapia, 73(1), pp.1-16.
Oosterhaven, K., Poolman, B. and Smid, E.J. (1995). S-carvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Industrial Crops & Products, 1(4), pp.23-31.
Osho, A. and Adetunji, T. (2011). Antimicrobial activity of the essential oil of Ageratum conyzoides L. Asian J of Sci and Tech, 2(3), pp.001-005.
Patil, R.P., Nimbalkar, M.S., Jadhav, U.U., Dawkar, V.V. and Govindwar, S.P. (2010). Antiaflatoxigenic and antioxidant activity of an essential oil from Ageratum conyzoides L. Journal of the Science of Food and Agriculture, 90(4), pp.608-614.
Pragadheesh, V.S., Yadav, A., Singh, M. and Chanotiya, C.S. (2013). Characterization of volatile components of Zingiber roseum essential oil using capillary GC on modified cyclodextrins. Natural product communications, 8(2), p.1934578X1300800223.
Raveau, R., Fontaine, J. and Lounès-Hadj Sahraoui, A. (2020). Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods, 9(3), p.365.
Saroj, A., Srivastava, A.K., Nayak, A.K., Chanotiya, C.S. and Samad, A. (2018). Essential Oils in Pest Control and Disease Management. Phytochemistry: Marine Phytochemistry, Industerial Applications and Phytochemical Pesticides, 3, p.341e368.
Sati, S.C., Sati, M.D., Raturi, R., Badoni, P. and Singh, H. (2011). Anti-inflammatory and antioxidant activities of Zanthoxylum armatum stem bark. Global Journal of researches in engineering: J General Engineering, 5, p.86.
Sikkema, J., Weber, F.J., Heipieper, H.J. and Bont, J.A.D. (1994). Cellular toxicity of lipophilic compounds: mechanisms, implications, and adaptations. Biocatalysis, 10(1-4), pp.113-122.
Singh, S., Kumar, V., Datta, S., Dhanjal, D.S. and Singh, J. (2020). Plant Disease Management by Bioactive Natural Products. In Natural Bioactive Products in Sustainable Agriculture (pp. 15-29). Springer, Singapore.
Singh, S.B., Devi, W.R., Marina, A., Devi, W.I., Swapana, N. and Singh, C.B. (2013). Ethnobotany, phytochemistry and pharmacology of Ageratum conyzoides Linn (Asteraceae). Journal of Medicinal Plants Research, 7(8), pp.371-385.
Skandamis, P.N. and Nychas, G.J. (2001). Effect of oregano essential oil on microbiological and physico‐chemical attributes of minced meat stored in air and modified atmospheres. Journal of Applied Microbiology, 91(6), pp.1011-1022.
Srivastava, A.K., Kumar, A., Saroj, A., Singh, S., Lal, R.K. and Samad, A. (2015). New report of a sweet basil leaf blight caused by Cochliobolus lunatus in India. Plant Disease, 99(3), pp.419-420.
Srivastava, A.K., Saroj, A., Nishad, I. and Samad, A. (2019). First report of Acorus calamus leaf spot caused by Curvularia pseudobrachyspora in India. Plant Disease, 103(4), pp.767-767.
Thakur, R.N., Singh, K.P. and Husain, A. (1975). Curvularia leaf spot of Japanese mint in India. Indian journal of mycology and plant pathology.
Tripathi, H., Suresh, R., Kumar, S. and Khan, F. (2016). International Trade in Essential Oils and Resinoids: A Case Study of Past 18 Years. Journal of Medicinal and Aromatic Plant Sciences, 38(1-4), pp.1-28.
Ultee, A., Bennik, M. and Moezelaar, R. (2002). The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Applied and environmental microbiology, 68(4), pp.1561-1568.
Vera, R. (1993). Chemical composition of the essential oil of Ageratum conyzoides L. (Asteraceae) from Reunion. Flavour and fragrance journal, 8(5), pp.257-260.
Wijayawardene, N.N., Crous, P.W., Kirk, P.M., Hawksworth, D.L., Boonmee, S., Braun, U., Dai, D.Q., D’souza, M.J., Diederich, P., Dissanayake, A. and Doilom, M. (2014). Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Diversity, 69(1), pp.1-55.
Zhang, Y., Crou,s P.W., Schoch, C.L., Hyde, K.D. (2012). Pleosporales Fungal Diversity. 52. Pp. 1-225.