1Institute of Biotechnology and Genetic Engineering (IBGE), University of Sindh, Jamshoro-76080, Pakistan.
2Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan-60600, Pakistan
Corresponding author email: rao.ikram@yahoo.com
Article Publishing History
Received: 29/03/2020
Accepted After Revision: 17/05/2020
The Bacillus subtilis (k1) is the SAFE microorganism with enourmous extracellular productions and it has ability to grow under large versatile nutrient conditions. Among the agriculture wastes, the rotten potatoes and sugar beets are being carbon-rich natural renewable agro-wastes. In compliance with current study, B. subtilis was fermented for 18 hrs on LB growth medium supplemented with 12.5 % extracts (v/v) of rotten potaoes and sugar beets i.e. LB₀ [1 % Bacto-trypton, 0.5 % NaCl, 0.5 % yeast extract in dH₂O (w/v)], LB₁ (⅛ LB₀), LB₂ (LB₁, peels of potatoes), LB₂ₐ (LB₁, peeled potatoes), LB₃ (LB₁, peels of sugar beet) and LB₃ₐ (LB₁, peeled sugar beet). The significantly higher cell growth rate was observed in LB₃ and LB₃ₐ cultures. Maximum reducing sugars observed in LB₄ while fructose contents in LB₃ (3.214±0.077 mg ml⁻1) and LB₃ₐ (2.971±0.044 mg ml⁻1) cultures. Simultaneously, higher enzymatic activities (p ≥ 0.05) showed by amylases on LB₂ₐ and LB₃ₐ, while xylanases on LB₂ₐ and pectinases on LB₃ cultures. For instance, pectinases activities remarkably exceeded at 50°C in LB₃ cultures maintained for 30 min. Overall, B. subtilis (k₁) can grow on both rotten potatoes and sugar beets with hug numbers extracellular productions, while sugar beets based cultures gave the best returns in bacterial sub-merged fermentation for the industrial enzymes productions.
Bacillus Subtilis, Potatoes, Sugar Beets, Glucose, Antioxidants, Free Prolines, Pectinase, Physical Conditions
Haq I-U, Nazir K, Yasin G, Ali S, Dua M. Analysis of Extra-Cellular Productions of Bacillus Subtilis Sub-Merged Fermentation Cultures Supplemented with Rotten Potatoes and Sugar Beats. Biosc.Biotech.Res.Comm. 2020;13(2).
Haq I-U, Nazir K, Yasin G, Ali S, Dua M. Analysis of Extra-Cellular Productions of Bacillus Subtilis Sub-Merged Fermentation Cultures Supplemented with Rotten Potatoes and Sugar Beats. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3eop3r9
Copyright © Haq et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The agricultures wastes are the unwanted materials produced from agro-based activities. It has been used as feed for the animals and fish. Even many are unsuitable for direct animal consumption so they are treated mechanically and chemically for their conversion into edible form (Jayathilakan et al., 2012). These costless waste materials are aggregated from the agriculture land to kitchen during its processing. With passage of time, these are converted to rotten form before its consumption or to get its end product (Obi et al., 2016). It depemds on the rate of production to its consumption over the time frame. The potato and sugar beets are the crops, which are observed into rotten form in the vegetable markete including the wastes excised during their processings (Wadhwa and Bakshi, 2013). The agriculture waste are comprised on mainly cellulose, hemicelluloses and lignin chemically. Utilization of these renewable carbon resources depends on their degratdation methods (Yahya et al., 2015; Emadian et al., 2017).
The narural degradation of agricultural wastes in the soil has been remained effecteive for the control of soil erosion and revegetation (Anastasi et al., 2005) and it is slower process. The chemo-mechanical degradation of the agriculture wastes is expensive and could be hazardous for human health, while biodegradation is cheap and manageable method. The soil born organisms are composting the organic wastes into organic fertilizers of soil through biological processes (Gautam et al., 2010). It means that a variety of microorganisms are able to grow on the agriculture wastes including Bacillus subtilis (An et al., 2018).
The agriculture wastes of crops contain sucrose (sugarcane, sugar beets), starch materials (corn, potatoes) and lignocellulosic materials (wood, grasses) are renewable lignocellulosic wastes available at lowest costs (Jenkins and Alles, 2011). However, their accumulation is causing various environmental problems, while these are potentially valuable sources to produce many value-added products like as fructose, glucose, ethanol, organic acids, food additives and enzymes (Pandey et al., 2016). Meanwhile, the type of production depends on the chemical composition of lignocellulosic source and the applied fermentation microorganism (Salazar et al., 2016; Tramontina et al., 2020).
The value added-renewable lignocellulosic agro-industrial waste can be used as cellular growth energy source and other secondary metabolites productions (Rocha et al., 2014). Meanwhile, efficient bio-tools for the management of agro-wastes are the microbial enzymes. The microorganisms are producing a variety of cellulases, proteinases and pectinolytic enzymes (Jayani et al., 2010; Tripathi et al., 2014). These are significantly eco-friendly industrial enzymes (Singh et al., 2009). Especially Bacillus subtilis are potential fermentation microorganism have been using in the global food, textile industries to composting processes (Nawawi et al., 2017). Perhaps the higher enzyme production costs are the major constraint for their commercialization. Though, selection of cheap carbon source, high yielding bacterial strains and optimal fermentation conditions significantly can reduce the enzyme production costs (Liu and Kokare, 2017; Tramontina et al., 2020).
In present study is aimed to analyse the net production of reducing sugars especially fructose, glucose and extracellular enzymes. These productions with Bacillus subtilis might be helpful for the determination of its abilities and efficiencies for the saccharification of lignocellulosic rich agriculture wastes of rotten potatoes and sugar beets. This work could be useful for the production of stable commercial organic compounds and industrial enzymes able to with-stand against the hazardous industrial conditions.
MATERIAL AND METHODS
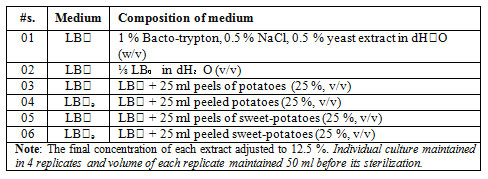
Preparation of inoculum:The inoculum of Bacillus subtillis (k1) prepared from its glycerol stock. For that it is mobilized in 2 ml LB₀ (TY) medium (Table 1), which was incubated at 37°C with constant shaking speed 250 rpm for overnight (De Vries et al., 2004). The 100 µl of above B. subtilis cultre sub-cultured in 5 ml LB₀ medium. It was again incubated under same physical circumstances for 30 minutes (Table 1). Now this master culture is inoculated in the agro-based waste fermentation medium (Table 1) with final OD600 upto 0.02.
Preparation of sugar beets and potatoes based agro-fermentation culture :The sugar beets and potatoes based fermentation cultures heightened in liquid nutrient LB₀ medium (5 g l-1 yeast extract, 5 g l-1 NaCl, 10 g l-1 Bacto-trypton, pH 7.0). Exact 12.5 % potatoes and sugar beets extracts (peels and peeled-potatoes and sugar beets) sustained in ⅛ strength of LB₀ mediu, while the LB₀ medium itself was considered as standard positive control for the growth of fermentation organism as well as ⅛ LB₀ medium as essential nutrient deficit control medium (Table 1). All of these cultures sterilized at 121°C for 20 minutes than cool down before inoculation at the room temperature.
Preparation of agro-fermentation substrate:The old and rotten potatoes and sugar beets collected from the nearby local vegetable market (Fig 1). These rotten potatoes and sugar beets were washed with running tap-water to clean dust or soil propely. Their skin or peels were separated with fine knife than peels and peeled stuff of both potatoes and sugar beets weighted exactly 50 g. These weighed material crushed with grinder grinder in 50 ml sterilized dH2O (w/v). After that the grinded mixture centrifuged at 4,000 rpm at room temperature for 10 min. The supernatant preserved at 4°C for next use in the agro-fermentation medium preparation while its pallet was discarded .
Harvesting of Bacillus fermentation culture:The inculated cultures incubated for 18 hours at 37°C with constant shaking at 250 rpm. After 18 hrs of incubation of the fermentation cultures, they were harvested. Before the collection of culture for harvest, their OD600 was measured. These cultures were centrifuged for 10 minutes at 7,000 rpm. The supernatants of the cultures transferred to the clean dark-colored glass-bottles, while its pallet was discarded. The supernatant of each culture stored at 4°C for next it was used as a sample for the measurements of different biochemical and activities of enzyme outcome.
Biochemical analysis of fermented supernatant:A number of biochemical testes performed on the fermented supernatant of Bacillus subtilis. Like as the total sugars anlysed by mixing 1 ml supernatant with 2.50 ml concentrated H₂SO₄ and 5 μl 80 % phenol in a clean and dried glass test tube. This mixture was allowed to stand at room temperature for minimum 10 min than its absorbance was read at OD485 (Dubois et al., 1956). Similarly, reducing sugars were also measured by mixing 1 ml sample with 2.0 ml DNS (3, 5-Dinitrosalicylic acid) reagent than mixture was heated for 15 min in boiling water-bath. After that its OD540 was read (Aakanchha and Richa, 2020). Furthermore, total protiens were also determined with following Lovrien and Matulis (2004) method. Exact 2.5 ml alkaline copper reagents mixed with 1 ml supernatant. The mixture was mixed thoroughly and incubated at room temperature for 10 min than 0.25 ml folin reagents (1:1, w/v) poured into the wall of the test tube and than its OD750 was read. Meanwhile the free prolines (Schweet, 1954), glycinebetaine (Grieve and Grattan, 1983), total flavonoids (Woisky and Salatino, 1998), total phenolics (John et al., 2014), ascorbic acid (Tabata and Morita, 1997) and antioxidants (Prieto et al., 1999) were also analyszed in the same Bacillus culture supernatants by following the respective reported methods.
The phosphate contents analyzed by mixing the 0.5 ml ammonium molybdate with 3 ml concentrated H2SO4 then 4 ml test sample added. After that 1 ml 0.05 M sodium sulphate also added. Its OD was read at 715 nm (Mahadevaiah et al., 2007). The methionines were also analyzed by mixing 0.2 ml 5 N NaOH with 1 ml test samples than 0.02 ml 10% Nitro-Prusside was added. After 10 min, 0.4 ml 3% aqeous glycine poured to the reaction mixture. It was incubated for 10 min at room temperature than finally 0.4 ml ortho-phosphoric acid added. Absorbance was read against blank at 540 nm after 5 min of incubation at room temperature (Gensch and Higuchi, 1967; Lavine, 1943). The citric acid quantified with titration method (Aguiar et al., 2005) and its quantity was calculated by applying this formula; Aceti acid (g 100 ml-1) = Volume of NaOH used*(0.03)*20.
The total fructose contents were quantified in the supernatant by mixing 3.5 ml 30 % HCl. The mixture was incubated in ice-bath for 10 min than 1 ml sample added. After 5 min of incubation in ice-bath 0.5 ml resorcinol thiourea was added. After that the reaction mixture was incubated at 80°C for 10 min than it was cool down under running tap water and its absorbance was read at 520 nm (Arsenault and Yaphe, 1966).
Measurments of enzyme activity: The supernatant of agro-fermented cultures of Bacillus subtilis was used as a crude enzyme mixture for the analysis of different enzymes activities. For the analysis of the amylases activities, its 1 ml used crude enzyme mixture mixed with 1 ml 0.5% starch fresh solution. The reaction mixture was incubated for 15 min at 37°C and than 2 ml DNS added to develop product complex and to stop the enzyme reaction. The absorbance of the reaction mixture was read at OD540 against blank (Mulimani and Lalitha, 1996). For determination of xylanases activity, 1 ml supernatant mixed with its 1 ml of xylanase substrate (0.5 % xylose) and reaction mixture incubated at 60°C. After 15 min, 2 ml DNS was added to stop the reaction and OD540 was noted (Bailey et al., 1992). Similary, other activities including the lipases (Espinosa-Ramírez et al., 2014), proteases (Anson, 1938) and pectinases (Miller, 1959) activities also measured. Alongwith the pectinases activities their thermostability also determined at different teparatures. Exact 1 ml 0.5 % pectin substrate mixed with 1 ml B. subtilis culture supernatant than incubated for 30 min at different temperatures (i.e 37°C, 25°C and 50°C). The 2 ml DNS reagent added in reaction and than its was kept in boiling water bath for 5 min. When it was cool down to room temperature, its OD540 was read.
Statistical analysis of data:The collected data of the studty was subjected to ANOVA (analysis of variance) and DMR (Duncan’s multiple range) tests at 5 % (p ≥ 0.05) for the data significance analysis (Snedecor and Cochran, 1983). These statistical analysis were analysed with computer based software “COSTAT” package (CoHort Software, Berkeley, USA).
RESULTS AND DISCUSSION
In the world, ligno-cellulosic plant biomass is an abundant renewable carbon complex. It is available as the great potential natural resource for bioconversion into various value-added bioproducts. These deposited agricultural residues (lignocellulosic) wastes are not properly disposed by the farmers that’s why it is being hazardous for environment and well as human health. These natural substrates with immense free energy potential are used to nurture the growth of fermentation organism in this study. Earlier, it has been demonstrated successfully about the implementation of these agro-renewable cost effective wastes for the screening of different hydrolytic fermentation microorganisms (Nagar et al., 2012; Varghese et al., 2017). Furthermore, it can also provide the plant organ based selective microbial growth for optimization of fermentation conditions and its respective extracellular products (Haq et al., 2017).
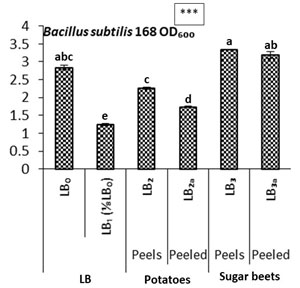
In this experiment, different LB-medium based cultures supplemented with lignocellulosic wastes of potatoes and sugar beets prepared to study the growth and extra-cellular productions of Bacillus subtilis (k1) (Table 1). There LB-medium contained valued nutrion to boost the optimal B. subtilis growth, which was compared with LB-deficit (⅛ LB0) to its different forms supplemented maintained with different regions of rotten potato and sugar-beet tubers. The comparative cell growth rates of B. subtilis (LBo to LB3a) drastically increased in LB₃ and LB3a cultures and seems to be comparable with LB1 standard medium. Meanwhile, the lowest cell multiplication rate observed in LB₁ (nutrient deficit) medium (Fig 1), while overall these raised cultures (LB2, LB2a, LB3 and LB3a) showed higher cell multiplication. Along the series of cultures from LB₀ to LB₂, relatively it was higher than LB₁. It means that the cultures supplemented with peels and peeled-off potatotes and sugar beets are growth supportive carbon containing agriculture wastes, which could serve as good nutrient-medium for the growth of B. subtilis and also for other fermentation organisms (Tin Lee, 2016). The Bacillus subtilis has already been identified as the faster organic food-waste hydrolyzer (Ale et al., 2015), it remains most effected if the wastes are treated with high temperatures (Kwon et al., 2014; Lim et al., 2017).
Figure 1: The agro-wastes used for Bacillus subtilus (k₁) growth as carbon source in sub-merged fermentation cultures [rotten potatoes (a) and sugar beets (b)].
Table 1. Composition of different bacterial nutrient media used for Bacillus subtilus (k₁) growth supplemented with potatoes and sugar beets as fermentation substrate.
The capabilities of the Bacillus subtilis to reduce the agriculture wastes remains differential from medium to medium. It is known to be attractive Bacillus to show up higher growth rate in agro-wastes, reductions to reducing sugars, biosynthesis of different metabolites and secretion of many hydrolyzing enzymes with GRAS status (Table 2, Figure 2). These properties are most attractive for industrial point of view for including its abilities for differential and stable multi-enzymes production, which can degrade diversed forms of substrates under diversed environmental conditions (Parrado et al., 2014). The B. subtilis is a soil growing microorganism able to solubilize phosphate (Chen et al., 2006; Chatli et al., 2008). It means that they are well adapted for colonization with plants (Reva et al., 2004; Allard-Massicotte et al., 2016). So it means that B. subtilis is being worth to grow on plant in narure and also useful for various industrial productions with agricultural wastes utilization.
Table 2: Comparative biocomponents and enzyme productions in various nutrient cultures of Bacillus subtilis (k1) supplemented with potatoes and sugar beets as carbon sources.
| #s. | Characters/Parameters | LB₀ | LB₁ (⅛LB₀) | LB₂ | LB₂ₐ | LB₃ | LB₃ₐ | Data significance |
| 01. | Total sugars (mg ml⁻¹) | cd3.664±0.045 | d1.832±0.034 | bc3.847±0.055 | c3.752±0.036 | a4.188±0.045 | b3.949±0.051 | *** |
| 02. | Reducing sugars (mg ml⁻¹) | c2.387±0.050 | e1.392±0.077 | bc2.831±0.058 | d1.758±0.048 | a3.214±0.077 | b2.971±0.044 | *** |
| 03. | Total proteins (mg ml⁻¹) | b8.743±0.033 | e1.799±0.056 | c8.221±0.054 | d7.792±0.052 | a9.684±0.103 | bc8.630±0.028 | *** |
| 04. | Fructose (mg ml⁻¹) | de0.722±0.003 | e0.147±0.004 | a1.078±0.003 | d0.789±0.003 | ab1.058±0.002 | c1.011±0.002 | *** |
| 05. | Citric acid (mg ml⁻¹) | ab0.086±0.003 | cd0.050±0.003 | abc0.087±0.004 | a0.091±0.002 | bcd0.063±0.003 | de0.049±0.003 | ** |
| 06. | Ascorbic acid (mg ml⁻¹) | d9.44±0.108 | f3.030±0.105 | c12.49±0.429 | de9.075±0.108 | a14.67±0.114 | b13.28±0.130 | *** |
| 07. | Proline (mg ml⁻¹) | cd1.007±0.006 | e0.639±0.005 | ab1.161±0.011 | a1.338±0.006 | bc1.122±0.005 | ab1.260±0.004 | *** |
| 08. | Glycinebetaine (mg ml⁻¹) | cde0.131±0.003 | f0.087±0.004 | def0.129±0.002 | cd0.140±0.005 | bc0.169±0.003 | a0.198±0.004 | ** |
| 09. | Phenolics (mg ml⁻¹) | ab7.264±0.037 | e1.892±0.040 | a7.450±0.022 | bc6.403±0.062 | b6.669±0.092 | cd6.081±0.023 | *** |
| 10. | Flavonoids (mg ml⁻¹) | bc1.862±0.060 | cde1.142±0.025 | def1.273±0.013 | bcd1.387±0.032 | a4.799±0.037 | b4.451±0.040 | *** |
| 11. | Antioxidants (mg ml⁻¹) | a0.865±0.013 | d0.242±0.002 | ab0.671±0.008 | bcd0.575±0.005 | a0.673±0.003 | bc0.591±0.006 | *** |
| 12. | Phosphates (mg ml⁻¹) | a0.984±0.003 | d0.345±0.007 | ab0.964±0.005 | ab0.967±0.004 | abc0.958±0.006 | bcd0.939±0.002 | ** |
| 13. | Methionine (mg ml⁻¹) | c0.181±0.005 | e0.090±0.002 | cd0.123±0.003 | de0.115±0.006 | b0.223±0.003 | a0.240±0.004 | *** |
| Note: Each parameter is presented with mean values of 4-replicates with their standard error and significances at 0.05 level (5%) with a,b,c,d … letters for DMR test. | ||||||||
Figure 2: Comparative cell growth rates of Bacillus subtilis (k1) on various nutrient cultures supplemented with potatoes and sugar beets as carbon sources (Each graph is presented with mean values and their standard error from 4-replicates with significances at 0.05 level (5%) and with a,b,c,d … letters for DMR test).
Among the other metabolites, production of total sugars sharply high in LB₃ , LB₂ and LB₂ₐ, while it is declined in LB₀. The reducing sugars and total proteins observed higher in LB₃ culture. The production of fructose noted higher in LB₂ and LB₃, citric acid in LB₂ₐ, ascorbic acid LB2 and LB₃ (Table 2) cultures. The free prolines and glycinebetain were observed higher in LB₂ and LB₂ₐ, while phenolics in LB₂ and LB₀ cultures. Production of flavonoids increased in LB₃, antioxidants and methionine in LB₃ cultures.
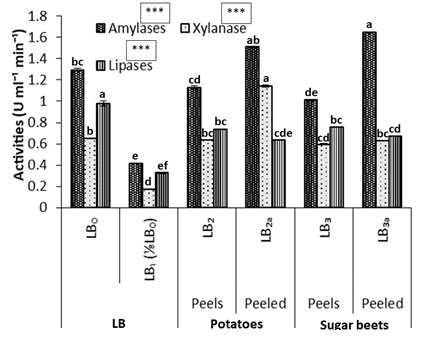
Figure 3: Comparative crude enzyme activities in the supernatant of Bacillus subtilis (k1) culture supplemented with potatoes and sugar beets as carbon sources. Each graph is presented with mean values and their standard error from 4-replicates with significances at 0.05 level (5%) and with a,b,c,d … letters for DMR test.
The B. subtilis has showed the production of various extra-cellular enzymes, which can lead to degrade the agro-based substrates (Meng et al., 2014). Similarly, amylases, xylanases, lipases and pectinases are also produced among other extra-cellular productions in the cultures when rotten rotten potatoes and sugar beets are used as carbon source. Both are rich in starch, which may be the basic source for the production of fructose/glucose syrup. Meanwhile, carbohydrates based agricultural products like starch from potato as well as sugar beets occur abundantly. The amylases have shown very drastic activities among the peeled-potatoes and sugar beets, while xylanases and pectinases are vey well performing in the cultures supplemented with peels of both agro-wastes as the carbon source. This differential formate of activities of various enzymes is due to the presence of their respective substrate in the plant orgn (Haq et al., 2017 and 2018).
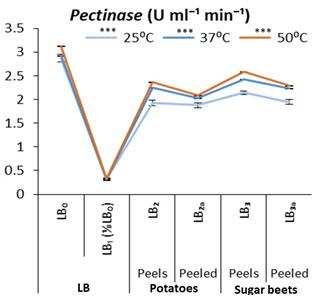
Figure 4: Comparative pectinases stability at different temperatures produced by Bacillus subtilis (k1) on different nutrient cultures supplemented with potatoes and sugar beets as carbon sources (Each graph is presented with mean values and their standard error from 4-replicates with significances at 0.05 level (5%) and with a,b,c,d … letters for DMR test).
From the results as shown in figure 3 about the pectinases production on various cultures and its stability at under different temrature conditions for 30 min. Maximum activities of pectinases observed at 50°C in LB₀, which is declined in LB₁ (nutrient deficit medium) then slightly increased in LB₂ and LB₃, while it was relatively lower in LB₂ₐ and in LB₃ₐ cultures. Almost similar but directly proportional pattern of activies observed among the cultures at 37°C and 25°C (Fig 3). Overall, pectinases showed best activities at 50°C in all cultures and it could be suggested that the production of pectinases are higher where its substrate is present in the medium (Amin et al., 2013). Similarly, the pectinases of A. fumigates and P. italicum remain stable even upto 60°C while remained highest active at 50°C (Phutela et al., 2005).
CONCLUSION
The rotten vegetables especially potatoes and sugar beets are rich with starch and other carbohydrates i.e. cellulose and lignin. These renewable agriculture wastes are available as the cheapest carbon source for the growth of fermentation microorganisms for the production of industrial hydrolytic enzymes. The Bacillus subtilis (k1) is one safe fermentation organism can grow on these substrates with aboundent prosuction of industrial enzymes especially amylases, pectinases and xylanases. The same setup could be best for the production of glucose syrup as the potatoes and sugar beets are rich in starch, which is major key source of sucrose.
ACKNOWLEDGEMENT
We are thankful to the University of Sindh, Jamshoro for the provison of financial support to complete this work. Authors are also grateful to the supporting staff of the laboratory at the respective institutes for their timely help.
Conflict of interest: Not any confilict among the authors on this study.
REFERENCES
Aguiar, A., Alexandre De, R., Nascimento, A., Puls, L., Adílson, F., and Gonçalves, R. (2005). Determination of organic acids and ethanol in commercial vinegars determinação de ácidos orgânicos e etanol em vinagres comerciais. Brazilian Journal of Food Technology, 1, pp 25-31.
Ale, C. E., Otero, M. C., and Pasteris, S. E. (2015). Freeze-drying of wine yeasts and oenococcus oeni and selection of the inoculation conditions after storage. Journal of Bioprocessing and Biotechniques, 5(8), pp 1-8. https://doi.org/10.4172/2155-9821.1000248
Allard-Massicotte, R., Tessier, L., Lécuyer, F., Lakshmanan, V., Lucier, J., Garneau, D., Caudwell, L., Vlamakis, H., Bais, H. P. and Beauregard, P. B. (2016). Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio 7(6), e01664-16. doi:10.1128/mBio.01664-16.
Altan, B. A., Submitted, D., Fulfillment, P., and Major, B. (2004). Isolation and molecular characterization of extracellular lipase and pectinase producing bacteria from olive oil mills. Biotechnology and Bioengineering, 115(2), pp 453-463.
Amid, M., Manap, Y., and Zohdi, K. (2014). Purifcation and characterisation of thermo-alkaline pectinase enzyme from hylocereus polyrhizus. European Food Research and Technology, 239(1), 21-29. https://doi.org/10.1007/s00217-014-2188-x
Amin, F., Bhatti, H. N., Ahmad Bhatti, I., and Asgher, M. (2013). Utilization of wheat bran for enhanced production of exo-polygalacturonase by penicillium notatum using response surface methodology. Pakistan Journal of Agricultural Sciences, 50(3), 469-477.
An, B., Park, M. K., and Oh, J. H. (2018). Food waste treatment using Bacillus species isolated from food wastes and production of air-dried Bacillus cell starters. Environmental Engineering Research, 23(3), pp 258-264. https://doi.org/10.4491/eer.2017.116
Anastasi, A., Varese, G. C., and Marchisio, V. F. (2005). Isolation and identification of fungal communities in compost and vermicompost. Mycologia, 97, pp 33-44. https://doi.org/10.3852/mycologia.97.1.33
Anson, M. L. (1938). The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. Journal of General Physiology, 22(1), pp 79-89. https://doi.org/10.1085/jgp.22.1.79
Arsenault, G. P., and Yaphe, W. (1966). Fructose-resorcinol-hydrochloric acid test for detection and determination of acetaldehyde. Analytical Chemistry, 38(3), pp 503-504. https://doi.org/10.1021/ac60235a036
Bailey, M. J., Biely, P., and Poutanen, K. (1992). Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology, 23(3), pp 257-270. https://doi.org/10.1016/0168-1656(92)90074-J
Chatli, A. S., Beri, V., and Sidhu, B. S. (2008). Isolation and characterisation of phosphate solubilising microorganisms from the cold desert habitat of Salix alba Linn. in trans Himalayan region of Himachal Pradesh. Indian Journal of Microbiology, 48(2), pp 267-273. https://doi.org/10.1007/s12088-008-0037-y
Chen, Y. P., Rekha, P. D., Arun, A. B., Shen, F. T., Lai, W. A., and Young, C. C. (2006). Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Applied Soil Ecology, 34(1), pp 33-41. https://doi.org/10.1016/j.apsoil.2005.12.002
Datta Tripathi, G., Javed, Z., and Singh, A. K. (2014). Pectinase production and purification from Bacillus subtilis isolated from soil. Pelagia Research Library Advances in Applied Science Research, 5(1), 103-105.
De Vries, Y. P., Hornstra, L. M., De Vos, W. M., and Abee, T. (2004). Growth and sporulation of Bacillus cereus ATCC 14579 under defined conditions: temporal expression of genes for key sigma factors. Applied and Environmental Microbiology, 70(4), pp 2514-2519. https://doi.org/10.1128/AEM.70.4.2514-2519.2004
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), pp 350-356. https://doi.org/10.1021/ac60111a017
Emadian, S. M., Onay, T. T., and Demirel, B. (2017). Biodegradation of bioplastics in natural environments. In Waste Management, 59, pp 526-536. https://doi.org/10.1016/j.wasman.2016.10.006
Espinosa-Ramírez, J., Pérez-Carrillo, E., and Serna-Saldívar, S. O. (2014). Maltose and glucose utilization during fermentation of barley and sorghum lager beers as affected by β-amylase or amyloglucosidase addition. Journal of Cereal Science, 60(3), pp 602-609. https://doi.org/10.1016/j.jcs.2014.07.008
Gautam, S. P., Bundela, P. S., Pandey, A. K., Awasthi, M. K., and Sarsaiya, S. (2010). Composting of municipal solid waste of Jabalpur city. Global Journal of Environmental Research, 4(1), pp 43-46.
Gensch, K. ‐H, and Higuchi, T. (1967). Kinetic investigation of reversible reaction between methionine and iodine. Improved iodometric determination of methionine. Journal of Pharmaceutical Sciences, 56, pp 177-184. https://doi.org/10.1002/jps.2600560205
Grieve, C. M., and Grattan, S. R. (1983). Rapid assay for determination of water soluble quaternary ammonium compounds. Plant and Soil, 70, pp 303-307. https://doi.org/10.1007/BF02374789
Haq, I.-U., Maher, A., Gill, N. P. Urooj, F., Qadir, G., and Ali, K. (2018). Growth of Bacillus subtilis and production of acetic acid with rotten potato: Used as substrate. International Journal of Pharmaceutical Sciences and Research, 9(10), pp 4229-4235. https://doi.org/10.13040/IJPSR.0975-8232.9(10).4229-35
Haq, I.-U., Choudhary, A. G., Mahar, A., Gill, N. P., and Yameen, M. (2017). Stable activity of extra-cellular xylanases and its phylogeny in different Bacillus species. International Journal of Biosciences, 11(5), pp 2222-5234. https://doi.org/10.12692/ijb/11.5.309-318
Jain, A., and Jain, R. (2020). Quantitative Analysis of Reducing Sugars by 3, 5-Dinitrosalicylic Acid (DNSA Method). In: Basic Techniques in Biochemistry, Microbiology and Molecular Biology. Springer Protocols Handbooks. Humana, New York, NY.
Jayani, R. S., Shukla, S. K., and Gupta, R. (2010). Screening of bacterial strains for polygalacturonase activity: Its production by Bacillus sphaericus (MTCC 7542). Enzyme Research, 2010, pp 1-5. https://doi.org/10.4061/2010/306785
Jayathilakan, K., Radhakrishna, K. S.K., and Bawa, A. S. (2012). Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. Journal of Food Sciences and Technology, 49(3): 278-293.
Jenkins, R., and Alles, C. (2011). Field to fuel: Developing sustainable biorefineries. Ecological Applications, 21(4), pp 1096-1104. https://doi.org/10.1890/09-0677.1
John, B., Sulaiman, C. T., George, S., and Reddy, V. R. K. (2014). Total phenolics and flavonoids in selected medicinal plants from Kerala. International Journal of Pharmacy and Pharmaceutical Sciences, 74(3), pp 258-60.
Kwon, B. G., Na, S.-H., Lim, H.-J., Lim, C.-S., and Chung, S.-Y. (2014). Slurry phase decomposition of food waste by using various microorganisms. Journal of Korean Society of Environmental Engineers, 36(5), pp 303 – 310. https://doi.org/10.4491/ksee.2014.36.5.303
Lavine, T. F. (1943). An iodometric determination of methionine. Journal of Biological Chemistry, 151, pp 281-297.
Li, S., Yang, X., Yang, S., Zhu, M., and Wang, X. (2012). Technology prospecting on enzymes: Application, marketing and engineering. Computational and Structural Biotechnology Journal, 2(3), pp 1-11. https://doi.org/10.5936/csbj.201209017
Li, Z. M., Jin, B., Zhang, H. X., Bai, Z. H., Xue, W. T., and Li, H. Y. (2008). Purification and characterization of three alkaline endopolygalacturonases from a newly isolated Bacillus gibsonii. The Chinese Journal of Process Engineering, 8(4), pp 768-773.
Lim, E. S., Lee, J. E., Kim, J. S., and Koo, O. K. (2017). Isolation of indigenous bacteria from a cafeteria kitchen and their biofilm formation and disinfectant susceptibility. LWT – Food Science and Technology, 77, pp 376e382. https://doi.org/10.1016/j.lwt.2016.11.060
Liu, X., and Kokare, C. (2017). Microbial enzymes of use in industry. In Biotechnology of Microbial Enzymes: Production, Biocatalysis and Industrial Applications. https://doi.org/10.1016/B978-0-12-803725-6.00011-X
Lovrien, R., and Matulis, D. (2004). Assays for total protein. Current Protocols in Protein Science, 1(1), pp 413-424. https://doi.org/10.1002/0471140864.ps0304s01
Mahadevaiah, Kumar, M. S. Y., Galil, M. S. A., Suresha, M. S., Sathish, M. A., and Nagendrappa, G. (2007). A Simple spectrophotometric determination of phosphate in sugarcane juices, water and detergent samples. E-Journal of Chemistry, 4(4), pp 467-473. https://doi.org/10.1155/2007/576560
Meng, F., Ma, L., Ji, S., Yang, W., and Cao, B. (2014). Isolation and characterization of Bacillus subtilis strain BY-3, a thermophilic and efficient cellulase-producing bacterium on untreated plant biomass. Letters in Applied Microbiology, 59, pp 306-312. https://doi.org/10.1111/lam.12276
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 89 (7) , pp 3959-3965. https://doi.org/10.1021/ac60147a030
Mulimani, V. H., and Lalitha, J. (1996). A rapid and inexpensive procedure for the determination of amylase activity. Biochemical Education, 24(4), pp 234-235. https://doi.org/10.1016/S0307-4412(96)00093-3
Nagar, S., Mittal, A., and Gupta, V. K. (2012). A cost effective method for screening and isolation of xylan degrading bacteria using agro waste material. Asian Journal of Biological Sciences, 5, pp 384-394. https://doi.org/10.3923/ajbs.2012.384.394
Nawawi, M. H., Mohamad, R., Tahir, P. M. and Saad W. Z. (2017). Extracellular xylanopectinolytic enzymes by Bacillus subtilis ADI1 from EFB’s compost. International Scholarly Research Notices, 2017, 7831954.
Obi, F., Ugwuishiwu, B., and Nwakaire, J. (2016). Agricultural waste concept, generation, utilization and management. Nigerian Journal of Technology, 35(4), 957-964. https://doi.org/10.4314/njt.v35i4.34.
Pandey, A., Negi, S. and Soccol, C. R. (2016). Current developments in biotechnology and bioengineering: Production, isolation and purification of industrial products. In book: Bioprocessing for Biomolecules Production, pp.195-209.
Parrado, J., Rodriguez-Morgado, B., Tejada, M., Hernandez, T., and Garcia, C. (2014). Proteomic analysis of enzyme production by Bacillus licheniformis using different feather wastes as the sole fermentation media. Enzyme and Microbial Technology, 57, pp 1–7. https://doi.org/10.1016/j.enzmictec.2014.01.001
Phutela, U., Dhuna, V., Sandhu, S., and Chadha, B. S. (2005). Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Brazilian Journal of Microbiology, 39, pp 602-604. https://doi.org/10.1590/S1517-83822005000100013
Prieto, P., Pineda, M., and Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry, 269(2), pp 337–341. https://doi.org/10.1006/abio.1999.4019
Reva, O. N., Dixelius, C., Meijer, J., and Priest, F. G. (2004). Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiology Ecology, 48(2), 249-259. https://doi.org/10.1016/j.femsec.2004.02.003.
Rocha, G. J. de M., Nascimento, V. M. and da Silva, V. F. N. (2014). Enzymatic bioremediation of effluent from sugarcane bagasse soda delignification process. Waste and Biomass Valorization, 5, 919-929.
Salazar, M. M., Grandis, A. P. S., Neto, J. L, Camargo, E. L. O., Alves, A., Rodrigues, J. C., Squina, F. Cairo, J. P. F., Buckeridge, M. S.. Hahn, M. G., Pereira, G. A. G. (2016). Eucalyptus cell wall architecture: clues for lignocellulosic biomass deconstruction. Bioenergy Research, 9(3), 969-979. doi: 10.1007/s12155-016-9770-y.
Schweet, R. S. (1954). The quantitative determination of proline and pipecolic acid with ninhydrin. The Journal of Biological Chemistry, 208(2), pp 603-613.
Singh Nee Nigam, P., and Pandey, A. (2009). Pectinolytic Enzymes. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues. https://doi.org/10.1007/978-1-4020-9942-7
Snedecor, G., and Cochran, W. (1983). Statistical Methods (6th Ed.). Oxford and IBH.
Tabata, M., and Morita, H. (1997). Spectrophotometric determination of a nanomolar amount of ascorbic acid using its catalytic effect on copper(II) porphyrin formation. Talanta, 44(2), pp 151-157. https://doi.org/10.1016/S0039-9140(96)02010-3
Tin Lee, C. (2016). Physico-chemical and biological changes during co-composting of model kitchen waste, rice bran and dried leaves with different microbial inoculants. Malaysian Journal of Analytical Science, 20(6), pp 1447 – 1457. https://doi.org/10.17576/mjas-2016-2006-25
Tramontina, R., Paiva, L. B. B., Sousa, A. S., Alves, R., Zetty-Arenas, A. M., Nascimento, V. M., Rabelo, S. C., Azzoni, S. F., Ruller, R. and Squina, F. M. (2020). Designing a cocktail containing redox enzymes to improve hemicellulosic hydrolysate fermentability by microorganisms. Enzyme and Microbial Technology, 135, 109490.
Varghese, L. M., Agrawal, S., Sharma, D., Mandhan, R. P., and Mahajan, R. (2017). Cost-effective screening and isolation of xylano-cellulolytic positive microbes from termite gut and termitarium. Biotechnology, 7, pp 108-115. https://doi.org/10.1007/s13205-017-0733-6
Wadhwa, M. and Bakshi, S. P. M. (2013). Utilization of fruit and vegetable wastes as livestock feed and as substrates for generation of other value-added products. FAO website (www.fao.org/publications).
Woisky, R. G., and Salatino, A. (1998). Analysis of propolis: Some parameters and procedures for chemical quality control. Journal of Apicultural Research, 37(2), pp 99-105. https://doi.org/10.1080/00218839.1998.11100961
Yahya, M. A., Al-Qodah, Z., and Ngah, C. W. Z. (2015). Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. In Renewable and Sustainable Energy Reviews, 46, pp 218-235. https://doi.org/10.1016/j.rser.2015.02.051
Zohdi, N. K., and Amid, M. (2013). Optimization of extraction of novel pectinase enzyme discovered in red pitaya (Hylocereus polyrhizus) peel. Molecules, 18(11), pp 14366-14380. https://doi.org/10.3390/molecules181114366

![Figure 1. The agro-wastes used for Bacillus subtilus (k₁) growth as carbon source in sub-merged fermentation cultures [rotten potatoes (a) and sugar beets (b)].](http://bbrc.in/wp-content/uploads/2020/11/Vol_13No_2_ANA_IKA_Fig1.jpg)