1Department of Interdisciplinary Sciences, Centurion University of
Technology and Management, R.Sitapur, Odisha, India
2Department of Medical Lab Technology and Biotechnology, Paramedical
College, Durgapur, West Bengal, India
3Department of Biochemistry and Plant Physiology, Centurion University of
Technology and Management, R.Sitapur, Odisha, India
Corresponding author email: banerjeepradipto.123@gmail.com
Article Publishing History
Received: 17/09/2021
Accepted After Revision: 18/12/2021
The sphere of Nanotechnology encompasses most of our lives and houses biomedicine and biomedical advancements. Nanoparticles owing to their minuscule sizes and due to various physicochemical and electrical properties have been exploited in pharmaceutical industries, agriculture, packaging, cosmetic, food industries. Nanomedicine is a laboratory-designed molecular-level pharmaceutical material that has revolutionized diagnostic techniques and therapeutics. Nanoscience and nanotechnology and their wide applications have become spread field worldwide because nanomaterials have novel and unique properties. Nanotechnology involves understanding and manipulating materials normally in the size range of 1 to 100 nm, where they show completely novel physicochemical properties from their bulk counterpart. The capacity to study compounds at the molecular level has aided the hunt for materials with exceptional qualities for medical applications. Nanotechnology in recent days is applied in the designing of nano biosensors. Nanobiosensors are biological molecules immobilized onto the surface of a signal transducer.
The application of nano biosensors in the field of disease detection has increased in recent years which has influenced in research of cancer and biosensing. Due to the high surface area of nanoparticles, they are important in the production of nano biosensors with high levels of sensitivity and diminish the response times. However, a comprehensive review regarding the type, mode of function, and their application in various diseases is missing. Nano Deterministic lateral displacement technology that provided exosome splitting based on size differences has resulted in providing the much-needed boost to cancer research. The time taken for cancer screening has been reduced drastically. that This review aims to describe the utilization of nano deterministic lateral displacement technology, nano biosensors, and their applications in certain disease diagnoses.
Biomedical Applications, Cancer, Nanobiosensors, SARS-Cov-2, Signal Transduction
Adhikary K, Chatterjee A, Banerjee P. An Updated Review on Nanomaterials for Biomedical Advancements: Concepts and Applications. Biosc.Biotech.Res.Comm. 2021;14(4).
Adhikary K, Chatterjee A, Banerjee P. An Updated Review on Nanomaterials for Biomedical Advancements: Concepts and Applications. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3jS7O6U“>https://bit.ly/3jS7O6U</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, sources the original author and sources are credited.
INTRODUCTION
There are several definitions and applications for nanotechnology. All definitions, however, emphasize the design and evolution of highly ordered bottom-up nanostructured materials that respond to specific stimuli. Surface chemistry and physics “tune” the uses of nanoscale materials (Fang 2018; Chaturvedi et al. 2019). The atom concentration on the surface of these systems can account for up to 90% of their total mass, resulting in increased reactivity. In present days, visceral imaging technology and morphological diagnosis of tissues or cells are helpful in the primary detection of tumors or cancer.
The frequently used diagnosis techniques, such as X-ray, magnetic resonance imaging (MRI), computed tomography (CT), endoscopy, and ultrasound, can only examine cancer only when there is a visible change to the tissue. For detection of cancer, nanoparticles are used to diagnose biomarkers of cancer for example cancer-associated proteins, tumor cells, and exosomes (Zhang et al. 2019).
An important advantage for the application of tiny particles for cancer diagnosis is based on the large surface area to volume ratio compared to bulk materials and with this characteristic, surfaces of tiny particles can be compactly wrapped with antibodies, peptides, and other moieties (Zhang et al. 2019). The nanoparticles along with quantum-dots technology and gold nanoparticles and their combinatorial applications can be used in the diagnosis of cancer (Chandrasekaran et al. 2019).
Nanotechnology products have become increasingly useful in biomedicine and have led to the advent of a hybrid science named nanobiotechnology. Nanomaterials are used extensively in nanobiotechnology, including diagnostic, medication delivery systems, prosthetics, and implants. Because most biological processes are nanoscale, nanoscale materials fit nicely with biomedical equipment (He et al. 2018; Zhang et al. 2019). Inorganic and metal nanoparticles, carbon nanotubes, liposomes, and metallic surfaces are popular materials utilized to build nanotechnology goods. The above-mentioned keywords were searched on PubMed and a total of 45 papers were included in this mini-review. We focused on the use of nanoparticles in cancer detection, Nano-DLD technology, biosensors, and the assessment of toxicity levels in an in vivo model in this review study.
Nano-DLD (Deterministic Lateral Displacement) Technology: One of the most essential factors in combating diseases like cancer is early detection. As was observed in Hawkes (2019), one-year survival for patients with colorectal cancer dropped from 97.7% when detected at Stage 1 to 43.9% when detected at stage 4. Classical techniques of early detection fall short due to poor tumor specificity in addition to high toxicity. Besides, most of the approaches target rectification of damage rather than proactive screening (Hawkes 2019).
Lab on a chip technology has allowed easy separation of exosomes from body fluids (Chandrasekaran et al. 2019). The introduction of NanoDLD Pillar Array Technology allows splitting of exosomes based on their size. Upon entry of the fluid containing exosomes into the NanoDLD chip, smaller particles tend to move in a streamline manner whereas, bigger particles are deflected owing to their size (Smith et al. 2018).
Figure 1: Nano DLD pillar array separates particles based on the fluidic forces.
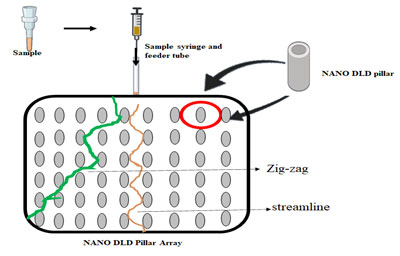
This technique allows for a gradient separation and the separated exosomes can be analyzed for biomarkers. Exosomes are nanoscopic particles that are released by both normal and tumor cells. These are powerful biomarkers, as they contain DNA, RNA, and proteins of the cell from which they are released. Due to their extremely small size, the traditional methods of isolation of exosomes fail to produce a high yield (Zhang et al. 2017; Wu et al. 2020; Dash et al. 2021).
Nano DLD pillar array separates particles based on the fluidic forces and the strategic placement of the pillars within the chip. Particles that have a smaller diameter than the space between two adjacent pillars will follow a streamlined path whereas those with larger diameters will follow a zig-zag route. To optimize a critical diameter, essential for optimum separation of particles, separation of whole-blood components was attempted without dilution. It was seen that when the pillars were tilted at a certain angle, optimum separation was achieved. However, this model fails to address the displacement of particles caused due to anisotropic permeability effect (Vernekar et al. 2017; Wu et al. 2020; Dash et al. 2021).
This issue was resolved by a comprehensive model that takes into account the displacement of small particles as well. This theory puts forward the notion that the trajectory of particles in the pillar array is largely dependent on the arrangement of the pillar arrays (Kim et al. 2017). To evaluate Nano DLD pillar technology as a point of care diagnostics, successful detection of albumin proteins at a concentration as low as 10ng/ml and nano-sized polymer vesicles at concentrations of 3.75ng/ml were successfully detected. This technology thus obviates the requirement of fluorescent labeling. The results obtained were in line with detection studies done with microcantilevers (Zeming et al. 2018).
The concentration of particles in the fluid plays a pivotal role in obtaining efficient separation. While working with blood, in the absence of anti-coagulating agents, clogging is a common issue in the microchannels, which ultimately renders the NANO DLD pillar array unusable. However, it was observed, that when the flow rate was controlled at 1ul/min and a voltage of 20V was passed as an anticoagulant, satisfactory purity levels were obtained up to12hours although the plasma purity dropped. In the absence of such voltage, the Nano DLD pillar array was unusable after 4 hours (Kang et al. 2018; Dash et al. 2021).
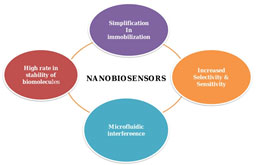
Nanobiosensors: Nanotechnology has highly influenced the field of biosensors, as their increased rate of sensitivity and selectivity. The emergence of nanotechnology design the materials to be prepared and fabricated in mini architecture at a tiny scale, producing tiny particles for successful assays with the integration of microfluidics devices (Chen et al. 2018). A biosensor is an analytical device that puts together a biologically active component with a signal transducer that can generate a computable signal tantamount to the concentration of chemical elements in any sample. Nano biosensors incorporate nanotechnology with biosensors (Jarockyte et al. 2020; Kveton et al. 2020; Sharifi et al. 2020).
Apart from this, nano biosensors played a key role in the detection of breast cancer as a portable device and also in the field of clinical medicine. The interrelationship of biomarkers and nanomaterials is efficient for the advancement of the performance of nano biosensors for all categories of diseases and also for treatment (Sharifi et al. 2020). Some of the major nano biosensors can be categorized based on whether nanoparticles, nanotubes, or nanowires have been used in the fabrication of the nano biosensors (Moon et al. 2020).
Figure 2: Application of nanotechnology in the advancement of nano biosensors
Nanoparticle-based biosensors include bulk acoustic wave devices (Liu et al. 2020). These devices use the acoustic properties of the analytes and amplify them to aid in detection. Nano biosensors fabricated with magnetic nanoparticles produce a magnetic flux that is detected under a microscope (SQUID microscope) (Reichert et al. 2018; Forsythe et al. 2018; Liu et al. 2020). In this technique antibodies against the epitope are attached to superparamagnetic particles and the magnetic flux is detected under the SUID microscope.
It was further reported that the unbound particles contributed very little to no noise in detection, thus a line can be drawn between bound and unbound magnetic labels. One of the most abundant utilization of nanotube-based biosensors is glucose biosensors that utilize carbon nanotubes and immobilizes glucose oxidase. Glucose oxidase breaks down glucose in the blood to gluconic acid, this is coupled with a redox reaction in the mediator that results in a release of electrons which are in turn detected by the electrode (Merinopoulos et al. 2020).
Nanoparticles made of metals are used to enhance detection along with sensor function. Further, they have been used to develop nano biosensors as their properties stand on principles of detection, therefore; they have been designed to perform as glucose biosensors. Nanoparticles mixed with solution suspension are then applied to sense glucose in various methods based on optical and electrochemical characteristics.
Various researchers in the past have concentrated on the magnetic properties of nanoscopic materials employed in biomedical applications, such as immobilized enzymes for biosensor development. According to previous studies, grapheme is a specialized sort of glucose biosensor that has generated encouraging results. They made a biosensor out of graphene and chitosan, which they drop-coated onto a glassy carbon electrode. Metal nanoparticles produced with graphene were subsequently employed as glucose biosensing with successful statistical findings (Lu et al. 2020).
Nanotechnology and SARS-CoV-2: The recent outbreak of the global pandemic that took the world by surprise was found to be caused by the Severe Acute Respiratory Syndrome Corona Virus2 (SARS-CoV-2). Nanotechnology has great application in the development of COVID-19 drug delivery as there are high advantages like the small shape and size and morphology of the nanoparticles (NP) helps the drug delivery to invincible sites and diminishes the immune response by reticular endothelial cells. Nanoparticles have a large surface-to-volume ratio which helps drug docking and loading. Though the nanoparticles can pass through cell membranes mostly anionic and it is only happening due to surface charge modification.
Nanotechnology in combination with large porous micro-particles (LPMP), solid lipid microparticles (SLMs) can be used to deliver drugs and can be adapted at antiviral drug therapy (Bhavana et al. 2020; McGrath et al. 2020; Zhu et al. 2020). The current technique for the detection of the virus is through reverse transcription polymerase chain reaction which is highly time-consuming given the rate of progression of the disease (Wong et al. 2020; Bhadra et al. 2020; Lu et al. 2020).
A novel technique using carboxyl polymer-coated magnetic nanoparticles allowed easy extraction and detection of SARS-CoV-2 RNA through reverse transcription-polymerase Chain Reaction. This technique combines both the lysis of viral particles along RNA binding and further reduces the time required for detection. The polymer-coated magnetic nanoparticles have high specificity for RNA binding, thereby producing a high throughput analysis (Zhao et al. 2020). However, magnetic nanoparticles require heavy instrumentation which poses problems in the portability area, but the higher output is available when used in a laboratory setup.
Such a problem can be averted when using techniques such as colorimetric or electrochemical assays (Tymm et al. 2020). However, Nanobiotechnology, over the past few decades has played a pivoting role not only in the early detection of tumorigenic cells but also in several infectious diseases. These include techniques like biochips, Nanoproteomics, Nanobiosensors, Nanoparticle-based immunoassays, etc (Ellwanger et al. 2019; Wong et al. 2020; Bhadra et al. 2020; Lu et al. 2020).
Diagnosis of Infectious disease: As per the reports published by World Health Organization on foodborne diseases, 1 out of 10 people fall ill every year (420000 die each year) from consumption of contaminated food, with children under the age of 5 being at high risk (125000 die each year) (Cissé 2019). Oral diseases like tooth caries, endodontic infections, etc are most common across the world. Prevalence of infection in the oral cavity and its effect on human lifestyle decreases the quality of everyday life which denotes an urge for effective therapy. The emergence and application of antimicrobial photodynamic therapy (APDT) on infection of oral disorder has been increased tremendously in past years (Qi et al. 2019; Lu et al. 2020).
In the United States alone Escherichia Coli have been reported to cause over 73,000 illnesses. Classical methods for the detection of E. coli require amplification to bring the sample up to the detection limit. However, antibody-conjugated nanoparticles have proven to be a highly sensitive and rapid immunological assay for the detection of bacterial cells without the requirement of amplification. This technique immobilizes several fluorescent dye molecules on each nanoparticle together on a silica matrix. Due to the presence of several surface antigens on the bacterium, numerous nanoparticles would bind to it resulting in amplified signal output. In a different approach, nanoparticles were found to have virucidal effects, owing to their size, surface area, and charge. Their small size allows effective membrane permeability within the foreign body and thus delivers virucidal drugs to the target (Singh et al. 2017; Russell et al. 2019; Lu et al. 2020).
In the modern science of treating infectious diseases, nanomedicine is a highly accepted approach for the advancement of nanotechnological systems in the field of disease diagnosis. For disease treatment, targeted therapy is the one of most enriched approaches to deliver the accurate quantity of therapeutic molecules for a long-term cure for the disease in the human body. For this advanced technology to apply and development of safer and more effective therapeutic nanoparticles is crucial and this is the main goal of nanomedicine.
With proper design and decoration of the nanoparticle surface with polyethylene glycol (PEG), acetyl groups, or protein moieties (arginine-glycine-aspartate or RGD) peptide, albumin), retention time can be altered (Shreffler et al. 2019). Last few years, the application of nanoparticle dependent vaccines has gained high acceptance in the field of vaccine technology and immunological experiments. To get the proper results in immune responses nanotechnology has no comparison (Yetisgin et al. 2020).
The nanocarriers improve vaccination administration by preventing antigens from being degraded prematurely by proteolytic enzymes, enhancing antigen presentation by antigen-presenting cells (APCs), controlling release, and being safe for human usage. It was also shown that providing viral and bacterial antigens in combination with gold nanoparticles improved immune responses in the host against influenza, immunodeficiency virus, foot and mouth disease, and TB. Mycobacterium tuberculosis is successfully reduced in infected rats using plasmid DNA encapsulated in gold nanoparticles that encode mycobacterial hsp65 antigen.
Some research has been done using hollow mesoporous silica, nanotube, and spherical forms of carbon nanoparticles as adjuvants to improve immunogenicity and deliver protein and antigenic peptides for viral infection. Some nanoparticles are based on silica which has utilization in the introduction on their surface with a proper functional group to access target cells for vaccine administration. One of the vast applications of inorganic nanoparticles is that they have low production cost, safer administration, and high reproducibility (Pati et al. 2018; Yetisgin et al. 2020).
Nanoparticles toxicity analysis toward its in vivo application: The toxicity of nanoparticles increases with their size. This is partly because tiny nanoparticles are more easily taken up into the cell or even near the nucleus (Serpooshan et al. 2019). Because their cellular absorption is restricted at the same dose, larger nanoparticles may be less harmful. A few aspects should be thoroughly investigated to assess and forecast probable nanoparticle toxicity in vivo applications (Chen et al. 2018; Donahue et al. 2019; Shao et al. 2020). To begin with, in vitro cytotoxicity studies should be utilized with caution to extrapolate predicted outcomes in vivo research. Nanoparticles in an in vivo system would be subjected to far more complex perturbations due to a large number of proteins and tiny biomolecules present.
Nanoparticles can be destroyed, absorbed by phagocytic cells, or transported away from the target location by the lymphatic system because of these nearby biomolecules. Assay responses acquired in a well-controlled environment, such as a culturing plate, may not necessarily match those obtained in an in vivo setting. As a result, drawing any inferences from the in vitro assay for nanoparticle responses in an in vivo system will be insufficient until at least animal model investigations are completed (Cao et al. 2021).
Second, the limits of present cytotoxicity or inflammatory reactions of cells to nanomaterials should be carefully noted, and more effort should be put into developing tools for better assaying nanoparticles. Traditional tests established for chemical poisons or microparticles have been used in studies of in vitro cytotoxicity and the inflammatory response to nanoparticles (Lih et al. 2018). These studies don’t go into detail on how specific cells react to nanoparticles. Furthermore, because cells might behave differently depending on the assays used, the examination of these test data is prone to inaccuracy. The following are the limitations of existing cytotoxicity and immune response tests for nanoparticle evaluation. For starters, cells cannot be retrieved after a single test readout (Schaerli 2018), limiting the options for tracking changes in a cell’s activity over time. Second, the results of the experiments are averaged over all of the cells present.
As a result, the reactions of a single cell to the nanoparticles cannot be recorded separately from the experiment. Third, nanoparticles within a cell may interfere with the fluorescence signal generated by the test dye. Nanoparticles can also interact with dyes and/or bind to them, changing their absorption and/or fluorescence. Nanoparticles can also adsorb to proteins and other biomolecules in the cell culture medium, interfering with the particles’ usual interactions with cells (Faruqu et al. 2018). Furthermore, nanoparticles can attach to cytokines generated by cells, reducing the positive signal in an assay artificially.
The technique of flow cytometry is widely employed in biological response experiments, although it necessitates the removal of cells from the cell culture plate, which may affect the cells’ mortality. Finally, there have been no multiplexed investigations of nanoparticles in the same well as single cells. Because of these constraints, a solid assay that overcomes the aforementioned issues with traditional assays and can examine biological responses to nanoparticles in a multiplexed, high-throughput way is urgently needed (Jeon et al. 2019).
To measure cytotoxic and inflammatory reactions to nanoparticles in a multiplexed manner, cutting-edge single-cell test approaches have been created. The multiplexed analytical approach will be employed in numerous nanoparticle safety investigations. Time-dependent investigation of a single cell’s reactions to nanoparticles may reveal the mechanism of nanoparticle toxicity; these single-cell studies will be utilized in conjunction with standard bulk experiments (Yi et al. 2019). The methodologies outlined will enhance nanotoxicological investigations and their uses mentioned in table 1. The larger nanotechnology community demonstrating the feasibility of a high-throughput, multiplexed analytical tool for investigating the safety of nanoparticles at the single-cell level (Jonghoon and Nam Sun 2011; Gibellini et al. 2020).
Table 1: Few important nanomaterials and their applications in the field of medical diagnosis and treatment involving use in drug delivery.
| Nanomaterial | Applications |
| Calcium Carbonate Nano Particles coated with Hyaluronic Acid activated by Glucose | In Oral delivery of Insulin
(Liu et al. 2017) |
| Low molecular weight protamine nanoparticle with paclitaxel; enzyme activated | In Glioblastoma Therapy
(Gu et al. 2013) |
| Nanocarrier containing Histidine-4 polyamidoamine dendrimer linked with disulfide bonds to Polyethelene glycol and Transferrin | As Anticancer drug delivery.
(Shi et al. 2020) |
| Alginate and cystamine based nanogels | As anticancer drug delivery
(Xu et al. 2018) |
| Melanin like nanoparticles | In imaging of tumors
(Zhou et al. 2020) |
| Nanoparticles containing hyaluronic acid, chitosan, and lipoic acid are linked together. | In Breast Cancer therapy
(Mutlu-Agardan et al. 2020)
|
| Polyaniline based nanoparticle-containing gold nanocomposite activated through electrical signals | In the detection of Chronic Kidney Disease
(Shaikh et al. 2019) |
CONCLUSION
The study suggests that the exosome nanoscopic particles used as powerful biomarkers for malignant cells, as they contain DNA, RNA, and proteins of the cell from which they are released. Nano DLD pillar technology applied in diagnostics approach and successfully detects albumin proteins in living cells. Numerous nanoparticles can bind bacterium surface antigens, resulting in amplified signal output. By using different nanomaterials the recent outbreak of the global pandemic that took the world by surprise was found to be caused by the SARS-CoV-2 coronavirus.
The current technique for detection of the virus is through a reverse transcription-polymerase chain reaction which is highly time-consuming given the rate of progression of the disease. It’s tough to predict the future of any key technology. On the one hand, there is a common propensity to underestimate a technology’s influence and rate of development. As other kinds of nanotechnology grow more common, the prominence of nanoparticles as the most potentially harmful variety may shift. Nanoparticles are becoming more widespread, and their properties are becoming more understood.
Conflict of Interest: Authors declare no conflict of interest to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Aman, R., Mahas, A., and Mahfouz, M., (2020). Nucleic Acid Detection Using CRISPR/Cas Biosensing Technologies. ACS Synth. Biol. 9, 1226–1233.
Bhadra, S., Maranhao, A. C., Paik, I., et al. (2020). One-Enzyme Reverse Transcription qPCR Using Taq DNA Polymerase. Biochemistry, 59(49), 4638–4645.
Bhavana, V., Thakor, P., Singh, S. B., et al. (2020). COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life sciences, 261, 118336.
Cao, Y., Li, S., and Chen, J. (2021). Modeling better in vitro models for the prediction of nanoparticle toxicity: a review. Toxicology mechanisms and methods, 31(1), 1–17.
Chandrasekaran, A. R., Punnoose, J. A., Zhou, L., et al. (2019). DNA nanotechnology approaches for microRNA detection and diagnosis. Nucleic acids research, 47(20), 10489–10505.
Chaturvedi, V. K., Singh, A., Singh, V. K., et al. (2019). Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Current drug metabolism, 20(6), 416–429.
Chen, L., Hwang, E., and Zhang, J. (2018). Fluorescent Nanobiosensors for Sensing Glucose. Sensors (Basel, Switzerland), 18(5), 1440.
Chen, L., Liu, J., Zhang, Y., et al. (2018). The toxicity of silica nanoparticles to the immune system. Nanomedicine (London, England), 13(15), 1939–1962.
Cissé G. (2019). Food-borne and water-borne diseases under climate change in low- and middle-income countries: Further efforts needed for reducing environmental health exposure risks. Acta tropica, 194, 181–188.
Cui, F., Zhou, Z., and Zhou, H.S., (2020). Molecularly Imprinted Polymers and Surface Imprinted Polymers Based Electrochemical Biosensor for Infectious Diseases. Sensors 20.
Dash, M., Palaniyandi, K., Ramalingam, S., et al. (2021). Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochimica et biophysica acta. Biomembranes, 1863(2), 183490.
Donahue, N. D., Acar, H., and Wilhelm, S. (2019). Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Advanced drug delivery reviews, 143, 68–96.
Ellwanger, J. H., Kaminski, V. L., and Chies, J. (2019). Emerging infectious disease prevention: Where should we invest our resources and efforts?. Journal of infection and public health, 12(3), 313–316.
Fang, R. H., Kroll, A. V., Gao, W., et al. (2018). Cell Membrane Coating Nanotechnology. Advanced materials (Deerfield Beach, Fla.), 30(23), e1706759.
Faruqu, F. N., Xu, L., and Al-Jamal, K. T. (2018). Preparation of Exosomes for siRNA Delivery to Cancer Cells. Journal of visualized experiments :JoVE, (142), 10.3791/58814.
Forsythe, R., McBride, O., Robson, J., et al. (2018). Magnetic resonance imaging using ultrasmall superparamagnetic particles of iron oxide for abdominal aortic aneurysm: a risk prediction study. NIHR Journals Library.
Gibellini, L., De Biasi, S., Porta, C., et al. (2018). Magnetic resonance imaging using ultrasmall superparamagnetic particles of iron oxide for abdominal aortic aneurysm: a risk prediction study. NIHR Journals Library.
Gibellini, L., De Biasi, S., Porta, C., et al. (2020). Single-Cell Approaches to Profile the Response to Immune Checkpoint Inhibitors. Frontiers in immunology, 11, 490.
Gu, G., Xia, H., Hu, Q., et al. (2013). PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials 34, 196–208.
Hawkes, N. (2019). Cancer survival data emphasise importance of early diagnosis. BMJ (Clinical Research Ed.).
He, X., Deng, H., and Hwang, H. M. (2019). The current application of nanotechnology in food and agriculture. Journal of food and drug analysis, 27(1), 1–21.
Hillman, Y., Lustiger, D., and Wine, Y., (2019). Antibody-based nanotechnology. Nanotechnology 30, 282001.
Jarockyte, G., Karabanovas, V., Rotomskis, R., et al. (2020). Multiplexed Nanobiosensors: Current Trends in Early Diagnostics. Sensors (Basel, Switzerland), 20(23), 6890.
Jeon, J., Choi, N., Chen, H., et al. (2019). SERS-based droplet microfluidics for high-throughput gradient analysis. Lab on a chip, 19(4), 674–681.
Jonghoon C, and Nam Sun W.(2011). Nanoparticles in Biomedical Applications and Their Safety Concerns, 29, p.486.
Kang, D. H., Kim, K., and Kim, Y. J. (2018). An anti-clogging method for improving the performance and lifespan of blood plasma separation devices in real-time and continuous microfluidic systems. Scientific Reports.
Kim, S. C., Wunsch, B. H., Hu, H., et al. (2017). Broken flow symmetry explains the dynamics of small particles in deterministic lateral displacement arrays. Proceedings of the National Academy of Sciences of the United States of America.
Kveton, F., Blsakova, A., Kasak, P., et al. (2020). Glycan Nanobiosensors. Nanomaterials (Basel, Switzerland), 10(7), 1406.
Li, X., Qin, Z., Fu, H., et al. (2021). Enhancing the performance of paper-based electrochemical impedance spectroscopy nano biosensors: An experimental approach. Biosens. Bioelectron. 177, 112672.
Lih, E., Kum, C. H., Park, W., et al. (2018). Modified Magnesium Hydroxide Nanoparticles Inhibit the Inflammatory Response to Biodegradable Poly(lactide- co-glycolide) Implants. ACS nano, 12(7), 6917–6925.
Liu, D., Jiang, G., Yu, W., et al. (2017). Oral delivery of insulin using CaCO 3 -based composite nanocarriers with hyaluronic acid coatings. Mater. Lett. 188, 263–266.
Liu, Y. L., Chen, J. J., Ahmad, F., et al. (2020). A Novel Approach to Accumulate Superparamagnetic Particles in Aqueous Environment Using Time-Varying Magnetic Field. IEEE transactions on bio-medical engineering, 67(6), 1558–1564.
Liu, Y., Cai, Y., Zhang, Y., et al. (2020). Materials, Design, and Characteristics of Bulk Acoustic Wave Resonator: A Review. Micromachines, 11(7), 630.
Lu, R., Wu, X., Wan, Z., et al. (2020). A Novel Reverse Transcription Loop-Mediated Isothermal Amplification Method for Rapid Detection of SARS-CoV-2. International journal of molecular sciences, 21(8), 2826.
McGrath, B. A., Brenner, M. J., Warrillow, S. J., et al. (2020). Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. The Lancet. Respiratory medicine, 8(7), 717–725.
Merinopoulos, I., Gunawardena, T., Stirrat, C., et al. (2020). Diagnostic Applications of Ultrasmall Superparamagnetic Particles of Iron Oxide for Imaging Myocardial and Vascular Inflammation. JACC. Cardiovascular imaging, S1936-878X(20)30633-1. Advance online publication.
Moon, D., Cha, Y. K., Kim, S. O., et al. (2020). FET-based nano biosensors for the detection of smell and taste. Science China. Life sciences, 63(8), 1159–1167.
Mutlu-Agardan, N.B., Sarisozen, C., and Torchilin, V.P., (2020). Cytotoxicity of Novel Redox Sensitive PEG2000-S-S-PTX Micelles against Drug-Resistant Ovarian and Breast Cancer Cells. Pharm. Res. 37, 65.
Oudot, M., Neige, P., Shir, I.B., et al. (2020). The shell matrix and microstructure of the Ram’s Horn squid: Molecular and structural characterization. J. Struct. Biol. 211, 107507.
Pati, R., Shevtsov, M., and Sonawane, A. (2018). Nanoparticle Vaccines Against Infectious Diseases. Frontiers in immunology, 9, 2224.
Qi, M., Chi, M., Sun, X., et al. (2019). Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. International journal of nanomedicine, 14, 6937–6956.
Reichert, P., Deshmukh, D., Lebovitz, L., et al. (2018). Thin-film piezoelectrics for bulk acoustic wave (BAW) acoustophoresis. Lab on a chip, 18(23), 3655–3667.
Reis, A. F., Vestphal, M., Amaral, R., et al. (2017). The efficiency of polymerization of bulk-fill composite resins: a systematic review. Brazilian oral research, 31(suppl 1), e59.
Russell, M. W., Jerse, A. E., and Gray-Owen, S. D. (2019). Progress Toward a Gonococcal Vaccine: The Way Forward. Frontiers in immunology, 10, 2417.
Schaerli Y. (2018). Bacterial Microcolonies in Gel Beads for High-throughput Screening. Bio-protocol, 8(13), e2911.
Serpooshan, V., Sheibani, S., Pushparaj, P., et al. (2018). Effect of Cell Sex on Uptake of Nanoparticles: The Overlooked Factor at the Nanobio Interface. ACS Nano, 12(3), 2253–2266.


