1Department of Zoology, Gauhati University, Guwahati, Assam, India
2Department of Zoology, Cotton University, Guwahati, Assam, India
Corresponding author email: titikkshadas.89@gmail.com
Article Publishing History
Received: 15/04/2021
Accepted After Revision: 10/06/2021
Blood parameters and the bioaccumulation of metals in different organs are the most important tools that can be used as effective and sensitive index to monitor physiological and pathological changes in fishes. In this study the effects of sublethal concentration of sodium arsenite were studied to evaluate heavy metal toxicity stress symptoms in fish blood. The relevant aspects of the therapeutic potential of aqueous garlic extract (AGE) against arsenic induced toxicity in the fresh water fish Channa punctatus have been evaluated. It was observed that haemoglobin content and red blood cells decreased significantly while the white blood cells and clotting time increased for the arsenic treated fishes. However, the haematological parameters were found to be stabilised in the revival group treated with garlic.
The accumulated arsenic in liver, gill, and kidney tissues of Channa punctatus was estimated by determining total arsenic concentration through Atomic Absorption Spectroscopy. The highest total arsenic content in the liver tissue of the fishes exposed to arsenic was 29.25 ± 0.15 ppb while the AGE treated group was found to be 27.96 ±0.13 ppb. In the gill tissue arsenic treated group accumulated 36.31 ± 0.21 ppb and arsenic + AGE treated group accumulated 34.94 ± 0.12 ppb of total arsenic. The total arsenic concentration in the kidney tissue was 21.8 ± 0.23 ppb for the arsenic treated group and arsenic content reduced to 20.27 ± 0.02 ppb for arsenic + AGE. Accumulation of arsenic found in liver, gill and kidney in the present study confirms the fact that arsenic toxicity was responsible for the physiological changes in the fishes. The results of the present study also signify that the haematological disturbances are caused by arsenic and its consequences could be reverted to a large extent by using aqueous garlic extract.
AAS, Arsenic, Garlic, Haematological Parameter.
Das T, Goswami M. Ameliorative Effects of Aqueous Garlic Extract on the Haematology of Arsenic-Induced Channa punctatus. Biosc.Biotech.Res.Comm. 2021;14(2).
Das T, Goswami M. Ameliorative Effects of Aqueous Garlic Extract on the Haematology of Arsenic-Induced Channa punctatus. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/2QNVEk3“>https://bit.ly/2QNVEk3</a>
Copyright © Das and Goswami This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Fishes are reasonably sensitive to the changes in their adjoining environment. Therefore, fish health is assumed as a reflection of the physical condition of a specific aquatic ecosystem. The freshwater fish Channa punctatus has been used as a bioindicator in toxicological studies since it is a very common species and it has higher tolerance for any stressed condition. Several studies conducted on mice, rats and chickens have reported that an oral garlic oil supplement affords significant protection against the toxicity of hepatotoxins including heavy metals (Senapati et al. 2001; Arora et al. 2004; Olaganathan and Patterson 2013).
Haematological parameters are one of the most important tools that can be used as effective and sensitive index to monitor physiological and pathological changes in fishes. The blood parameters of different fishes exposed to different water pollutants and toxicants, such as metals, biocides, pesticides, chemical industrial effluents, etc. had been used as sensitive indicator of stress for the toxicity evaluation studies of these pollutants (Singh et al. 2008; Podeti and Benarjee 2017). The aquatic biotope, fish species, age, sexual maturity and health status of the fishes were found to affect the haematological parameters (Radu et al. 2009; Patriche et al. 2011). Appreciable changes were observed in haematological parameters and biochemical profiles of the lithium induced Channa punctatus and Oreochromis niloticus (ThangaMalathi and Anuradha 2020).
The relationship of blood characteristics to the habitat and adaptability of the fish to the environment can be understood by working on the haematological and biochemical parameters (Podeti and Benarjee 2017). In order to understand the fish physiology and pathology different investigators had estimated the various blood parameters in fishes (Va´zquez and Guerrero 2007; Satheeshkumar et al. 2012). Blood constituents of fishes are directly or indirectly influenced by the quality of water, temperature, food availability and physiological status of the fishes (Dheer et al. 1988; Bala et al. 1994; Iqbal et al. 1997). It was also observed that many factors including environmental factor influence the haematological parameters of the Channa punctatus.
When the fish, Channa punctatus was treated with both copper and chromium a fall in RBC count, Hb% and PCV% along with acute anaemia was noticed (Pandey 1977; Singh 1995). The oxygen carrying capacity of blood, the iron content of the blood and number of red blood cells in fishes apparently depend on the life history, stage, habitat and environmental conditions (Singh et al. 2008). It was observed that acute sub-lethal concentrations of lead, copper and zinc can produce haemolytic anaemia which associated with the decrease in Hb%, PCV% value and the number of erythrocytes in Colisa fasciatus and Oreochromis mossambicus (Soiveo and Nikinmaa 1981; Sampath et al. 1998).
In the last few decades, the concentrations of heavy metals in fish had been extensively studied in different parts of the world (Elnabris et al. 2013). Most of these studies concentrated mainly on the heavy metals in the edible part (fish muscles). However, other studies reported the distribution of metals in different organs like the liver, kidneys, heart, gonads, bone, digestive tract and brain. It was observed that arsenic has the tendency to accumulate in bottom sediments (Smedley and Kinniburgh 2002). Assimilation of metals in fish takes place through ingestion of food, ingestion of material suspended in water; absorption on the tissue and membrane surfaces and exchange of ions dissolved in water across lipophilic membranes (e.g. gills).
Distribution of metal in different tissues depends on the mode of exposure (i.e., dietary exposure) and can be considered as an indicator of pollution (Alam et al. 2002). Therefore, bioaccumulation of metals has been considered as an index of the pollution status of the related water body and used as a tool to study the biological role of the metals present at elevated levels in aquatic organisms, particularly fish (Tariq et al. 1991; Mehra and Chadha 2020). Further, any alteration in biochemical parameters can have serious outcomes in the form of various diseases in both the animal and its consumers (Prakash and Verma 2020).
For determining the total arsenic (As) concentration in different fish tissue, several techniques such as inductively coupled plasma mass spectro-photometry (ICP-MS), electrothermal atomic absorption spectro-photometry (ETAAS) has been used (Gong et al. 2002). The generation of hydride is also an important process for the determination of As (Moretto and Cadore 2004). Microwave digestion processes have also been used in these purposes due to the advantages of this technique, which include the speed of digestion and less risk of contamination during the process.
Atomic absorption spectrometry through low injection with hydride generation has been used successfully for the determination of arsenic in biological samples (Ybanez et al. 1992; Navarro et al. 1992; Soylak et al. 2004) and it is possible to use for a few microlitres of sample. In order to determine the total arsenic concentration in different fish tissues, it is essential to assure complete mineralization of the samples using non-destructive technique (Jalbani et al. 2007). Bioaccumulation and concentration level of other heavy metals such as chromium, cadmium, lead etc. in the riverine water and edible fish such as Channa punctatus were also analyzed by using Atomic Absorption Spectrophotometer (Idrees et al. 2020).
Fishes are relatively situated at the top of the aquatic food chain; therefore, they can normally accumulate heavy metals from food, water, and sediments and they are considered as a good indicator of heavy metal contamination in water (Yilmaz et al. 2007; Zhao et al. 2012; Voegborlo et al. 2012).However, to the best of our knowledge, bioaccumulation studies on the Channa punctatus under the influence of any therapeutic agent such as aqueous garlic extract (AGE) are not available in the literature.
Hence, the present study aims to investigate the effect of arsenic in fresh water teleost Channa punctatus and to assess the impact of aqueous garlic extract on the revival of arsenic toxicity by evaluating the changes in the haematological parameters. Atomic absorption spectroscopy (AAS) was done to determine the total arsenic concentration accumulated in the liver, gill, and kidney tissues of Channa punctatus under the influence of Overborrow aqueous garlic extract (AGE).
MATERIAL AND METHODS
In the present study Channa punctatus was selected as an experimental animal. Healthy and disease free Channa punctatus were collected from local markets in Guwahati. Their weight was approximately in between 25-45 gm and average length was 14 cm. Fishes were brought to ambient laboratory condition. Fishes were disinfected with a dip of 2% potassium permanganate (KMnO4) solution the fishes were acclimatised in aquaria (75 30 60 cm) for two weeks before initiation of experiment. The fishes were fed everyday with fish food (Tokyu pallete) during acclimatization period. Following the standard procedure given by APHA the physicochemical parameter of the test water was monitored (Das and Goswami 2020).
Sodium Arsenite (NaAsO2), molecular weight- 129.91 Merck, India (Ltd.) was procured for performing the experiment. A stock solution was prepared by adding 5 mg of sodium arsenite to 100 ml of distilled water. The test concentration was prepared by diluting the stock solution with appropriate amount of distilled water. Each experimental aquaria contained 8 litres of water each. Sub-lethal doses of 2.5 ppm/litre were prepared by adding 20 ppm in 8 litres of water for each experimental setup arsenic (Das and Goswami 2020).
Physicochemical parameters of the water used in test solution were maintained according to the standard procedures given by APHA, 2005. The control group of fishes were kept in similar conditions without adding sodium arsenite. The second group of fishes were exposed to sub-lethal dose of arsenic, the third group was exposed simultaneously to arsenic and AGE. The fourth group was exposed to only garlic extract without arsenic (Das and Goswami 2020).
To prepare aqueous solution of garlic extract the outer layer of garlic (Allium sativum L.) cloves were removed, and crushed mechanically in a mortar-pestle with 100 ml of autoclaved distilled water for 1 gm of garlic. The homogenate was shaken for 20 minutes, filtered successively through gauze and 0.22 micron membrane filter to obtain the aqueous garlic extract (AGE) (Chowdhury et al. 2008; Das and Goswami, 2020). 10 ml of AGE / litre or 80 ml of AGE in 8 litres was added in each aquaria during the experimental procedure.
Fish specimens from the three groups were collected for haematological study. Blood samples were collected by piercing the caudal peduncle using plastic disposable syringe fitted with a needle which was moistened with heparin. To estimate the RBC from the fish blood Acid haematin technique has been used. Blood collected was quickly drawn into the cleaned and dried RBC pipette up to 0.5 or 1 mark. The extra blood was wiped off the outer surface of the pipette and immediately erythrocyte or red blood cell (RBC) fluid was drawn up to the 101 mark.
The contents of the pipette were thoroughly mixed by rotating with thumb and fingers for 5 minutes. The blood was drawn up to 1 mark to make dilution 100-fold. Diluted blood solution was filled up between the cover slip and the counting chambers of the haemocytometer by the capillary action. The haemocytometer was allowed to stand for few minutes and transferred carefully on the plate form of a microscope. The erythrocytes were counted in the four corner squares and in the central square. The cells were enumerated by using the Neubauer haemocytometer (Singh et al. 2008).
Blood collected was quickly drawn into the cleaned and dried WBC pipette and sucked the blood in the pipette up to 0.5 mark without any air bubble. The extra blood was wiped off the outer surface of the pipette and immediately diluting fluid was drawn up to the 11 mark. The contents of the pipette were thoroughly mixed by rotating with thumb and fingers for 5 minutes. It provides 20 times dilution of blood. The first one or two drops were discarded and then filled the Neubauer counting chamber.
Diluted blood solution was filled up between the cover slip and the counting chambers of the haemocytometer by the capillary action. The haemocytometer was allowed to stand for 3-5 minutes to settle the leucocytes. Haemocytometer was carefully transferred on the platform of a microscope. They were counted in four corners of 1 square mm in the central ruled area on both the sides of the counting chamber of the haemocytometer.
The haemoglobin concentration was evaluated to study the stress induced due to arsenic exposure on C. punctatus. Haemoglobin (gm/percent) was determined using haemometer (Humtsoe et al. 2007). 0.1 N HCl was filled up to lowest mark (20 % mark). Blood from the control and treated fish was collected and sucked into the Hb pipette up to 20 micron litre (µl). The blood was carefully sucked to avoid any air bubbles.
The blood was then transferred from the blood pipette into the acid at the bottom of the graduated tube and was shacked thoroughly. The mixture was allowed to stand for 10 minutes so that the haemoglobin gets converted to the dark brown coloured acid haematin. The solution was diluted by adding few drops of distilled water carefully and mixing with reaction mixture until the colour matches with that of the standard tube (Humtsoe et al. 2007).
The level of the fluid at its lower meniscus was noted and the reading corresponding to this level on the scale was recorded in g/dl. (Humtsoe et al. 2007). The heart of the control and treated fish were pierced by a needle to ensure free flow of blood and the time when the wound was made was recorded by starting a stopwatch. The first drop of blood was rejected and the second drop of free flowing blood was allowed to fill readily the capillary tube by capillary action. After every half a minute about 1 cm of the capillary tube was broken down so as to find whether fibrin string was formed or not. When the fibrin string appeared in the capillary piece then the total time required for clotting was noted down by stopping the stop-watch (Singh et al. 2008).
After a period of 15 days, surviving fish from experimental and control groups were sacrificed for the estimation of arsenic in the liver, gill and kidney tissue. Total arsenic concentration in the tissues was determined on a Varian Atomic Absorption Spectrophotometer (AAS), Model No.: Spectra AA 220 FS (Netherland). Analysis of heavy metal was carried out following the standard methodology of Angerer and Schaller (1988) and Zwart and Trivedi (1995).
RESULTS AND DISCUSSION
The 96 hours LC50 of sodium arsenite for Channa punctatus was found to be 25 ppm through probit analysis and it is discussed in details elsewhere (Das and Goswami 2020). The present toxicity studies carried out on 10 % of the 96 hour LC50 value (i.e 2.5 ppm) which was selected as sublethal concentration of sodium arsenite for 15 days exposure to the fish. In the present study, exposure of fish to sub-lethal concentration of arsenic for 15 days caused significant alterations in haematological parameters of freshwater fish, Channa punctatus. The changes observed in various haematological parameters, such as RBC, WBC, Hb% and clotting time of blood in the control and experimental groups were recorded (Table 1) (Das and Goswami 2020).
Table 1: Haematological profile of C. punctatus exposed to (10%) sub lethal concentrations of Arsenic and Aqueous Garlic Extract (AGE) for 15 days.
| Haematologic-al Parameter | Control Group | Arsenic Treated Group (10% of LC 50) | Arsenic + AGE Treated Group | AGE Treated Group |
| RBC (*106/mm3) | 2.71 ± 0.08 | 1.79 ± 0.02 * | 2.06 ± 0.04 * | 2.71 ± 0.03 |
| WBC (*103/mm3) | 58.08 ± 0.13 | 73.96 ± 0.14 * | 67.89 ± 0.19 * | 58.08 ± 0.14 |
| Hb gm (%) | 10.54 ± 0.06 | 8.25 ± 0.07 * | 9.24 ± 0.06 * | 10.47 ± 0.06 |
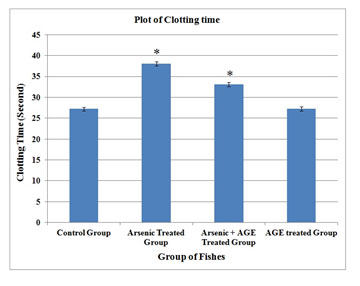
| Clotting Time (Second) | 27.20 ± 0.15 | 38.03 ± 0.16 * | 33.05 ± 0.10 * | 27.23 ± 0.13 |
In the control group of fishes in the present experimental set up RBC was found to be (2.71±0.08) 106/mm3, WBC (58.08±0.13) 103/mm3, haemoglobin content (10.54±0.06) gm % and clotting time (27.20±0.15) second. In the group which was exposed to sub-lethal concentration (10% of LC50 value) of sodium arsenite (Das and Goswami 2020). RBC counts were found to decrease to (1.79±0.02) 106/mm3, while WBC counts increased to (73.96±0.14) 103/mm3.The values were significant at p< 0.05. Haemoglobin was found to decrease to (8.25±0.07) % and clotting time was raised to (38.03±0.16) second.
When the fishes were treated with arsenic and garlic extract after 15 days of exposure, RBC was found to be (2.06±0.04) 106/mm3, WBC decreased to (67.89±0.19) 103/mm3. Haemoglobin content was found to increase to (9.24±0.06) gm % and clotting time was reduced to (33.05±0.10) second. The values were significantly different from control values (p< 0.05) (Table 1). When the data were plotted for each parameter significant change in each group was observed (Figure 1-4) (Das and Goswami 2020).
Figure 1: RBC profile of C. punctatus exposed to (10%) sub lethal concentrations of arsenic and Aqueous Garlic Extract (AGE) for 15 days along with control group. Values are significant at P < 0.05. (* indicates that the values are significantly different at P < 0.05 level compared to the values of control group determined by one way ANOVA analysis).
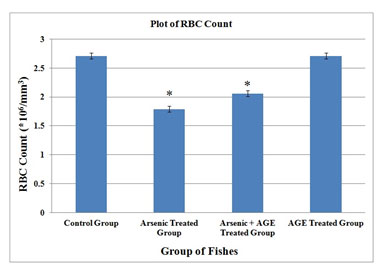
Figure 2: WBC profile of C. punctatus exposed to (10%) sub lethal concentrations of arsenic and Aqueous Garlic Extract (AGE) for 15 days along with control group. Values are significant at P < 0.05. (* indicates that the values are significantly different at P < 0.05 level compared to the values of control group determined by one way ANOVA).
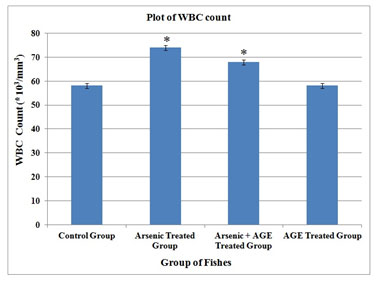
Figure 3: Haemoglobin profile of C. punctatus exposed to (10%) sub lethal concentrations of arsenic and Aqueous Garlic Extract (AGE) for 15 days along with control group. Values are significant at P < 0.05. (* indicates that the values are significantly different at P < 0.05 level compared to the values of control group determined by one way ANOVA).
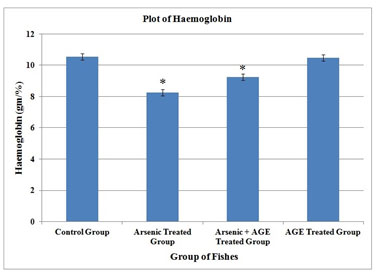
Figure 4: Clotting time profile of C. punctatus exposed to (10%) sublethal concentrations of arsenic and Aqueous Garlic Extract (AGE) for 15 days along with control group. Values are significant at P < 0.05. (* indicates that the values are significantly different at P < 0.05 level compared to the values of control group determined by one way ANOVA).
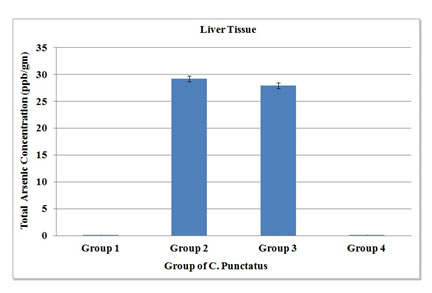
Considering the role of arsenic in the field of eco-toxicology, the present study had been undertaken to understand the accumulation of arsenic, when sodium arsenite was induced to fishes in laboratory conditions. The remedial measure with garlic extract was also aimed. So, liver, gill and kidney tissues from the four groups were taken for the present experimental set up (Table 2, 3 & 4). Accumulation of arsenic in treated group had given a very significant result. In control and only garlic treated group had shown that there was negligible amount of arsenic in the liver tissue, but it rose to 29.25 ± 0.15 ppb in arsenic treated group. However, the arsenic + AGE treated group had shown minimal decrease in arsenic accumulation (27.96 ± 0.13 ppb) (Figure 5).
Table 2. Total arsenic concentration in the liver tissue of four different groups of Channa punctatus. Values are represented as mean ± MSE, n=5, Values are significant at P < 0.05.
| Accumulation of total Arsenic (micro gram/gm or ppb) in Liver | |||
| Control Group
(Group 1) |
Treated Group (Sodium arsenite)
(Group 2) |
Treated Group (Sodium arsenite+AGE)
(Group 3) |
Treated Group (Only AGE)
(Group 4) |
| 0.01 ± 0.003 | 29.25 ± 0.15 | 27.96 ±0.13 | 0.02 ± 0.002 |
Table 3. Total arsenic concentration in the Gill tissue of four different groups of Channa punctatus. Values are represented as mean ± MSE, n=5, Values are significant at P < 0.05.
| Accumulation of total Arsenic (micro gram/gm or ppb) in Gill | |||
| Control Group
(Group 1) |
Treated Group (Sodium arsenite)
(Group 2) |
Treated Group (Sodium arsenite+AGE)
(Group 3) |
Treated Group (Only AGE)
(Group 4) |
| 0.02± 0.001 | 36.31 ± 0.21 | 34.94 ± 0.12 | 0.02± 0.004 |
Figure 5: Total arsenic concentration in liver tissue of four groups of C. Punctatus (Group 1-control, Group 2- Treated with sodium arsenite, Group 3- Treated with sodium arsenite + AGE, Group 4 – Treated with only AGE).
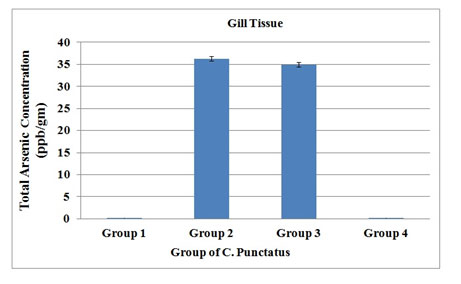
The total arsenic content accumulated in the gill tissues was found to be 36.31 ± 0.21 ppb while there was very little decrease in arsenic concentration in the arsenic +AGE (34.94 ± 0.12) treated group. Normal control and garlic treated group had shown no accumulation of arsenic (Figure 6).
Figure 6. Total arsenic concentration in Gill tissue of four groups of C. Punctatus (Group 1- control, Group 2- Treated with sodium arsenite, Group 3- Treated with sodium arsenite + AGE, Group 4 – Treated with only AGE).
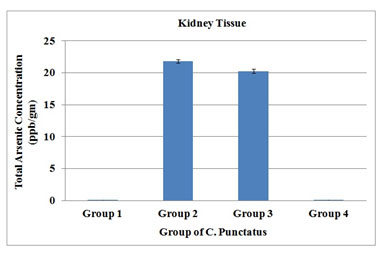
Arsenic concentration when analysed in kidney tissue from different groups of Channa punctatus, it was found that total arsenic accumulated was 21.8 ± 0.23 ppb in the kidney in the sodium arsenite treated group, whereas in the sodium arsenite + AGE treated group, arsenic content decreased to 20.27 ± 0.02 ppb. Control group and garlic treated group had shown no arsenic in their tissues (Figure 7).
Table 4. Total arsenic concentration in the kidney tissue of four different groups of Channa punctatus. Values are represented as mean ± MSE, n=5, Values are significant at P < 0.05.
| Accumulation of total Arsenic (micro gram/gm or ppb) in kidney | |||
| Control Group
(Group 1) |
Treated Group (Sodium arsenite)
(Group 2) |
Treated Group (Sodium arsenite+AGE)
(Group 3) |
Treated Group (Only AGE)
(Group 4) |
|
0.019 ± 0.001 |
21.8 ± 0.23 |
20.27 ± 0.02 |
0.02± 0.001 |
Figure 7. Total arsenic concentration in kidney tissue of four groups of C. Punctatus (Group 1- control, Group 2- Treated with sodium arsenite, Group 3- Treated with sodium arsenite + AGE, Group 4 – Treated with only AGE).
A comparative account of arsenic content of tissues in four different Groups showed that the total arsenic accumulated was highest in the gill tissue as it had the maximum accumulation followed by liver and kidney tissues (Figure 8).
Figure 8. A comparison of total arsenic concentration in liver, gill and kidney tissues of four groups of C. Punctatus (Group 1- control, Group 2- Treated with sodium arsenite, Group 3- Treated with sodium arsenite + AGE, Group 4 – Treated with only AGE).
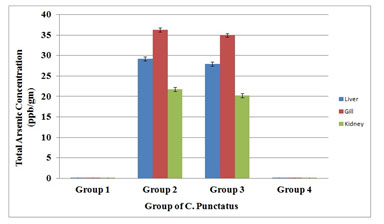
Similar trend was also observed in the sodium arsenite + AGE treated group but the amount of arsenic accumulated was less than in the sodium arsenite treated group. A negligible amount of arsenic content is observed in different tissues of control group of fishes and the group of fish treated with only AGE. This negligible value in the control and AGE treated group proved that fishes were collected from water bodies free from any arsenic contamination.
It was observed here that Hb% and RBC decreased significantly while WBC and clotting time increased for arsenic treated group. Similar alternations of these parameters were also observed in the group of fishes exposed simultaneously to arsenic and AGE. Although it was found that changes of the haematological parameters in the Arsenic + AGE treated group was less significant. The exposure of Channa punctatus to sub-lethal concentration of arsenic significantly decreased Hb% and RBC count leading to anaemia and it can be considered as early manifestation of acute and chronic intoxication of heavy metals. Anaemia under arsenic induced stress may also be due to the injury in the blood cell and disruption in the heamoglobin synthesis (Mckim et al. 1970; Gross et al. 1975).
Many Researchers previously reported similar results with significant reduction of RBC and Hb% content in fishes exposed to different heavy metals (Goel et al. 1985; Goel and Sharma 1987; Pamila et al. 1991). Joshi et al. (2002) stated that impaired intestinal absorption of iron exposure could decrease the RBC and Hb%. Pamila et al. (1991) suggested that the reduction in haemoglobin in fish blood exposed to heavy metal could be due to the inhibitory effect of the toxic substance on the enzyme system responsible for synthesis of haemoglobin. Christensen et al. (1972) stated that the changes in the haematological parameter could be understood in terms of the reduction of oxygen consumption in fish resulting in death due to heavy metal contamination (Christensen et al. 1972; Joshi et al. 2002).
According to Vanitha et al. (2017) the reduced RBC content in the blood under the effect of arsenic may be due to inhibition of erythropoiesis or by the destruction of blood cells. Saravanam et al. (2011) reported that disruption of haemopoietic processes and accelerated disintegration of erythrocyte cell membrane caused the reduction of haemoglobin content in toxicant exposed fishes. In this study, the observed decrease in RBC count and haemoglobin content in the arsenic treated C. punctatus might have resulted from destruction of RBC’s due to erythroblastosis leading to anaemia (Saravanam et al. 2011; Vanitha et al. 2017).
White blood cells are involved in the regulation of immunological functions and increase in their number under the exposure of any toxicant is a protective response in fish to stress conditions (Mishra and Niyogi, 2011). It was stated that increase in the white blood cell counts might be the indication of damages of body tissues due to infection, severe physical stress and as well as leukemia. In the present work white blood cell counts were found to be increased for arsenic treated group. Similar findings were also observed by other researchers in the fishes exposed to different heavy metals. In the present study white blood cell counts were found to be increased for both arsenic treated and arsenic + AGE treated group (Nath and Banerjee, 1995; Mazon et al. 2002; Singh et al. 2008; Vanitha et al. 2017).
The increase of WBC count for the arsenic + AGE treated group is less as compared to the arsenic treated group. In the present study it was observed that C. punctatus under sublethal exposure of arsenic exhibits a long clotting time. When a lesion is made in any blood vessel a clot is formed as the end product of blood coagulation. Prothrombin, a blood clotting substance is present in high percentage in fish blood (Vanitha et al. 2017). A substance called thrombosthenin released by the platelet is responsible for clot retraction (Pandey and Shukla, 2005). Analogous results could also be seen in Labeo rohita exposed to copper sulphate, and Catla catla exposed to cadmium (Vincent et al. 1996; Sinha et al. 2000). Singh et al. (2008) studied the impact of copper on haematological profile of freshwater fish, Channa punctatus.
The exposure of fish to sub-lethal concentration of copper for 15, 30 and 45 days caused significant alterations in haematological parameters of Indian freshwater fish, Channa punctatus. Hb% and RBC decreased significantly after 15, 30 and 45 days of exposure periods, respectively, in comparison with control. On the contrary, WBC were found significantly increased after 15, 30 and 45 days of exposure, as compared to control. Exposure of fish to copper showed a significant decrease in the haemoglobin (Hb) content, red blood cells (RBC) at the end of 45th day as compared to control. Whereas the white blood cells (WBC) increased from, clotting time (CT) from, erythrocyte sedimentation rate and mean corpuscular volume increased significantly with increase in exposure periods. Amsath et al. (2017) studied the effect of Arsenic on haematological parameters of freshwater air breathing fish, Channa punctatus (Bloch) (Pragnya et al. 2020).
Exposure of fish to 10% sub-lethal concentration of arsenic for 10, 20 and 30 days caused significant alterations in hematological parameters in C. punctatus along with development of lesion in epidermis. The exposure of C. punctatus to 10% sub lethal concentrations of arsenic trioxide for 10, 20 and 30 days showed significant decrease in RBC count and Hb%. While WBC count increased significantly following arsenic exposure (Amsath et al. 2017). Jha et al. (2017) studied the toxicological effects of arsenic exposure on haematology of fresh water fish Channa punctatus.
In their investigation the effect of subchronic exposure of arsenic induces significant alteration in hematological parameters when compared to control group. RBC count decreased corresponding increase in the exposure level, whereas, WBC count was increased at all concentration of arsenic treatment suggesting dose dependent response. Arsenic exposure may cause anaemic conditions and from their study it was found fish treated with subchronic doses of arsenic showed low Hb level resulting in anaemic behaviour. Mukherjee et al. (2015) studied arsenic toxicity on the Indian Murrel, C. punctatus at the haematological level that was under taken to assess the induction of stress on the fishes in controlled laboratory condition (Pragnya et al. 2020).
The exposure of C. punctatus to sub lethal concentration of sodium arsenite exhibited significant decrement in RBC count that might have led to anaemia. Anaemia under arsenic induced stress may be due to blood cell injury. White blood cell counts were found to increase significantly following arsenic exposure. The number of lymphocytes in arsenic exposed blood of fresh water teleost increased in compared to control (Mukherjee et al. 2015; Pragnya et al. 2020). The eosinophils have been implicated in inflammation and the percentage of this cell type exhibited a dose dependent increase in respect to control.
Monocytes and neutrophils are important white blood cells to protect the body through their elevated phagocytic activity against opportunistic pathogen and parasite infection. The percentage of both monocytes and neutrophils decreased in a dose dependent manner in respect to control. Basophil count also showed an increasing tendency under sublethal concentration of arsenic toxicity. Haematological parameters of fish can be helpful to identify the target organs of toxic effects and also the general health condition of harmful changes in stressed organisms. It is clear from the study that the changes in all the haematological parameters (RBC, WBC, Hb% and Clotting time) for the arsenic + AGE treated group of fishes is less than the arsenic treated group of fishes from its control value (Pragnya et al. 2020).
It signifies a protective role of garlic extract against the toxicological effect of arsenic on the C. Punctatus. Present investigation has also highlighted the use of garlic for detoxification of arsenic which is safe and possibly useful to prevent adverse effects with arsenic exposure. In the present study the accumulated the highest total arsenic content in the liver tissue was 29.25 ± 0.15 ppb and it was found in the group treated with sodium arsenite. The total arsenic concentration in the liver tissue treated concurrently with sodium arsenite and Aqueous Garlic Extract (AGE) were found to be 27.96 ±0.13 ppb which is less than the total arsenic found in the earlier group. In the gill tissue it was observed that arsenic treated group accumulated 36.31 ± 0.21 ppb of total arsenic and arsenic + AGE treated group accumulated 34.94 ± 0.12 ppb of total arsenic.
The results of total arsenic concentration analysis in the kidney tissue showed that 21.8 ± 0.23 ppb total arsenic accumulated for the arsenic treated group and arsenic content reduced to 20.27 ± 0.02 ppb for the group treated simultaneously with arsenic and Aquous Garlic Extract (AGE). The accumulated arsenic was found to be maximum in gill tissue and followed by liver and kidney. Other researchers also reported similar trend of arsenic content in fish and shellfishes ((Pragnya et al. 2020).
The values of arsenic content observed in different tissues from this study were compared with available literature data (Vukadin et al. 1995; Engman and Jorhem 1998; Storelli and Marcotrigiano 2000). High arsenic concentrations in the edible muscle tissue of freshwater fish from arsenic-contaminated and non-contaminated sites in Thailand, was reported by Jankong et al. (2007). Moretto et al. (2004) reported total arsenic in fish muscles. Al Rmalli et al. (2005) observed total arsenic content (0.097–1.32) μg/g; in muscles tissue of the fresh water fishes on the foodstuffs on sale in the United Kingdom and imported from Bangladesh (Al Rmalli et al. 2005; Pragnya et al. 2020).
De Rosemond et al. (2008) investigated five freshwater fish species from Back Bay near Yellowknife, NT, Canada, and reported total arsenic in muscles, intestine and liver. Delgado-Andrade et al. (2003) investigated total arsenic content in muscles tissue of fishes from south-east Spain. Has-Schon et al. (2006) investigated total arsenic content in five fish species from River Neretva, Croatia and observed in muscles, gill and liver tissue. Juresa and Blanusa (2003) observed high concentration of mercury, arsenic, lead and cadmium in fish and shellfish from the Adriatic Sea. Shah et al. (2009) evaluated the total arsenic (As) in five tissues (gills, mouthpiece, intestine, liver and muscles) of 10 different fresh water fish species caught from arsenic contaminated Manchar Lake, Sindh, Pakistan during 2006–2007 (Shah et al. 2009; Pragnya et al. 2020).
Using hydride generation atomic absorption spectrometry (HG-AAS) method they have obtained arsenic concentration to be highest in liver followed by muscles, mouth pieces, intestine and gill (Shah et al. 2009; Pragnya et al. 2020). This survey of literature shows that the total arsenic content observed in the present study in different tissue of Channa punctatus was comparable to the available literature data. In the present study it was observed that arsenic concentration of all the tissues in the group treated with arsenic + garlic was found to be little less than the group treated with only arsenic and the arsenic content were found to be maximum in gill for all groups of fishes except the control group.
A negligible amount of arsenic content is observed in different tissues of control group of fishes. This negligible value of arsenic may be because of some instrumental error, as control group of fished were completely free from any arsenic contamination. The arsenic content in the group treated with arsenic + AGE slightly decreased than the group treated with only arsenic, which signifies that garlic has some restorative properties to minimise the damages caused by arsenic. Analogous results of bioaccumulation of heavy metals (Zn, Pb, Cd, Co, Cu, and Fe) were also observed in fish tissues of various organs of three fish species, i.e., Labeo rohita, Pangasius hypophthalmus and Katsuwonus pelamis (Pragnya et al. 2020).
CONCLUSION
In the present work white blood cell counts and clotting time were found to be increased whereas the RBC count and haemoglobin content were found to be decreased for the arsenic treated group. However the haematological profile of the fishes in the revival group was restored to some extent. From the results of the present investigation it could be concluded that stress due to heavy metals present in the water does create hematological disturbances, erythrocyte destruction (hemolysis), and leukocytosis in fish population which affects the immune system and makes the fish vulnerable to diseases.
The results of the present study also signify that the haematological disturbances are caused by arsenic and its consequences could be reverted to a large extent by using aqueous garlic extract. The observed value of total arsenic accumulation was below the permissible limit set by the Bureau of Indian Standards (50 ppb) but more than the international limit of 10 ppb. The accumulation of arsenic found in liver, gill and kidney in the present study confirms the fact that arsenic toxicity was responsible for the physiological changes in the fishes.
It also indicates the protective role of garlic against arsenic toxicity and how its long term use can be beneficial to eliminate arsenic from blood and soft tissues. There is a scope of further investigation in to the mechanism of reduced arsenic accumulation by prolonged AGE treatment. These results may be used to guide ecological monitoring programs that measure the bioavailability of arsenic in freshwater fishes.
ACKNOWLEDGEMENTS
The authors are thankful to Department of Zoology, Gauhati University, Guwahati, Assam for providing necessary infrastructure and facilities to conduct this research work.
Conflict of Interests:The authors declare no conflict of interests with respect to the research, authorship, and / or publication of this article.
REFERENCES
Alam, M. G. M., Tanaka, A., Allinson, G., Laurenson, L. J. B., Stagnitti, F., and Snow, E. T. (2002). A comparison of trace element concentrations in cultured and wild carp (Cyprinus carpio) of lake Kasumigaura, Japan. Ecotoxicol Environ Saf, 53, 348–354.
Al Rmalli, S. W., Haris, P. I., Harrington, C. F., and Ayub, M. (2005). A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Sci Total Environ, 337, 23–30.
Amsath, A., Sugumaran, J., and Vanitha, S. (2017). Effect of arsenic (As2O3) on haematological parameters of freshwater air breathing fish, Channa punctatus (Bloch). Curr Trend Biomed Eng Biosci, 7, 555702.
Angerer, J. and Schaller, K.H., (1997). Analyses of hazardous substances in biological materials. Vol. 5.
Arora, A., Seth, K., and Shukla, Y. (2004). Reversal of p-glycoprotein- mediated multi drug resistance by diallyl sulfide in K562 leukemic cells and in mouse liver. Carcinogen, 25, 941–949.
Bala, S., Sinha, M. P., and Mehrutra, P. N. (1994). Toxicity of sublethal concentration of some heavy metal salts on haematology of Channa punctatus Erythrocyte counts. J Freshwat Biol, 6, 187-190.
Chowdhury, R., Dutta, A., Ray Chaudhuri, S., Sharma, N., Giri, A. K., and Chaudhuri, K. (2008). In vitro and in vivo reduction of sodium arsenite induced toxicity by aqueous garlic extract. Food Chem Toxicol, 46, 740–751.
Christensen, G. M., McKim, J. M., Brungs, W. A., and Hunt, E. P. (1972). Changes in the blood of the brown bullhead (Ictalurus nebulosus (Lesueur)) following short- and long-term exposure to copper (II). Toxicol Appl Pharmacol, 23, 417-427.
Das, T. and Goswami, M. (2020). Acute toxicity impact of sodium arsenite on behavioural changes and histopathology of kidney and intestine of the freshwater fish, Channa punctatus and its revival with aqueous garlic extract. Biosc Biotech Res Comm, 13, 913-922.
De Rosemond, S., Xie, Q. and Liber, K. (2008). Arsenic concentration and speciation in five freshwater fish species from Back Bay near Yellowknife, NT, Canada. Environ. Monit Assess, 147, 199-210.
Delgado-Andrade C., Navarro M., Lopez H. and Lopez M. C. (2003). Determination of total arsenic levels by hydride generation atomic absorption spectrometry in foods from south-east Spain: Estimation of daily dietary intake. Food Addit Contamin, 20, 923–932.
Dheer, J. M. S. (1988). Haematological, haematopietric and biochemical responses to thermal stress in an air breathing freshwater fish Channa punctatus (Bloch.). J Fish Biol, 32, 197-206.
Elnabris, K. J., Muzyed, S. K., and El-Ashgar, N. M. (2013). Heavy metal concentrations in some commercially important fishes and their contribution to heavy metals exposure in Palestinian people of Gaza Strip (Palestine). J Assoc Arab Univ Basic Appl Sci, 13, 44-51.
Engman, J. and Jorhem, L. (1998). Toxic and essential elements in fish from Nordic waters, with the result seen from the perspective of analytical quality assurance. Food Addit Contamin, 15, 884–892.
Goel, K. A., Gupta, K. and Sharma, M. L. (1985). Haematological characteristic of Heteropneustes fossilis under the stress of zinc. Ind. J. Fish. 36, 186-188.
Goel, K. A. and Sharma, S. D. (1987). Haematological characteristics of Clarias batrachus under metallic stress of arsenic. Comp Physiol Ecol, 12, 63-66.
Gong, Z., Xiufen, L., Mingsheng, M., Corinna, W., and Le Chris, X. (2002). Arsenic speciation analysis. Arch. Environ. Contamin Toxicol, 58, 77–96.
Has-Schon, E., Bogut, I. and Strelec, I. (2006). Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch Environ Contamin Toxicol. 50, 545–551.
Humtsoe, N., Davoodi, R., Kulkarni, B. G. and Chavan, B. (2007). Effect of Arsenic on the Enzymes of the Rohu Carp, Labeo rohita (Hamilton, 1822). The Raffles Bull Zoo, 14, 17-19.
Idrees, N., Sarah, R., Tabassum, B. and Abd_Allah, E. F. (2020). Evaluation of some heavy metals toxicity in Channa punctatus and riverine water of Kosi in Rampur, Uttar Pradesh, India. Saudi J Bio Sci, 27, 1191–1194.
Iqbal, M. J., Ali, S. S. and Shakoon, A. R. (1997). Toxicity of lead in freshwater fish Cirrhinus mrigala Haematological changes. J Ecotoxi Environ Moni. 7, 139-143.
Jalbani, N., Kazi, T. G., Jamali, M. K., Arain, M. B., Afridi, H. I. and Baloch, A. (2007). Evaluation of aluminum contents in different bakery foods by electrothermal atomic absorption spectrometer. J Food Compos Anal. 20, 226–231.
Jankong, P., Chalhoub, C., Kienzl, N. B., Goessler, W., Francescon, K. A., and Visoottiviseth, P. (2007). Arsenic accumulation and speciation in freshwater fish living in arsenic-contaminated waters. Environ Chem 4, 11–17.
Jha, D. K., Mishra, B. B., Thakur, K. R., Pranay, K., Sayrav, K., Vikash, K., Verma, P. and Khan, P. K. (2017). Toxicological Effects of Arsenic Exposure on Haematology of Fresh Water Fish Channa punctatus. Der Pharma Chemica. 9, 1-5.
Joshi, P. K., Bose, M., and Harish D. (2002). Haematological changes in the blood of Clarias battrachus exposed to mercuric chloride. Ecotoxicol Environ Monit, 12, 119-122.
Juresa, D. and Blanusa, M. (2003). Mercury, arsenic, lead and cadmium in fish and shellfish from the Adriatic Sea. Food Add Conta, 20, 241–246.
Mazon, A. F., Monteiro, E. A., Pinheiro, G. H. and Fernandes, M. N. (2002). Hematological and physiological changes induced by short-term exposure to copper in the freshwater fish, Prochilodus scrofa. Braz J Biol, 62, 621-631.
McKim, J. M., Christensen, G. M., and Hunt, E. P. (1970). Changes in the blood of the brook trout Salvelinus fontinalis after short-term and log-term exposure to copper. J Fish Res Board Can, 27, 1883-1889.
Mehra S. and Chadha P. (2020). Bioaccumulation and toxicity of 2-naphthalene sulfonate: an intermediate compound used in textile industry. Tox Res, 9, 127–136.
Moretto, A. L., and Cadore, S. (2004). Determination of arsenic in food samples by hydride generation–atomic absorption spectrometry. Microchim Acta, 146, 239–244.
Mishra, A., and Niyogi, P. A. (2011). Haematological changes in the Indian Murrel (Channa punctatus, Bloch) in response to phenolic industrial wastes of the Bhilai steel plant (Chhaittisgarh, India). Int J Res Chem Environ, 1, 83-91.
Mukherjee S., Ray D., Adhikari D. and Ghosh T. (2015). Impairment of haematological profile of Channa punctatus exposed to sodium arsenite. Inter J App Bio Pharmaceu Tech, 6, 223-229.
Nath, R., and Banerjee, V. (1995). Effects of various concentrations of lead nitrate on haematological parameters of an air breathing fish, Clarias batrachus. J Freshwater Biol. 7, 267-268.
Navarro, M., Lopez, H., Lopez, M. C. and Sanchez, M. (1992). Determination of Arsenic in Fish by Hydride Generation Atomic Absorption Spectrometry. J Anal Toxicol 16, 169-171.
Olaganathan, R. and Patterson, J. (2013). Effect of anthraquinone dyes on the carbohydrate, protein and lipid content in the muscle of Channa punctatus and Cyprinus carpio Int J Pharma App, 4, 11-19.
Pamila, D., Subbaiyan, P. A. and Ramaswamy, M. (1991). Toxic effect of chromium and cobalt on Sartherodon mossambicus (peters). Ind J Environ Hlth, 33, 218-224.
Pandey, B. N. (1977). Haematological studies in relation to environmental temperature and different periods of breeding cycle in Heteropneustes fossils in relation to body weight. Folia Haematol, 104, 69–74.
Pandey, K. and Shukla, J. P. (2005). A textbook of Fish and Fisheries. Rastogi Publications, Meerut, India.
Patriche, T., Patriche, N., Bocioc, E., and Coada, M. T. (2011) Serum biochemical parameters of farmed carp (Cyprinus carpio), Aquaculture, aquarium, conservation and legislation. Int J Bioflux Soc, 4, 137–140.
Podeti, R. K. and Benarjee, G. (2017). Haematological changes in South Indian fresh water murrel, Channa punctatus have both EUS and A. hydrophila infection. J Parasit Dis, 41, 329–335.
Pragnya, M., Kumar, S. D., Raju, A. J. S. and Murthy, L. N. (2020). Bioaccumulation of heavy metals in different organs of Labeo rohita, Pangasius hypophthalmus, and Katsuwonus pelamis from Visakhapatnam, India. Mar Pollut Bull, 157, 111326
Prakash, S. and Verma, A. K. (2020). Effect of arsenic on serum biochemical parameters of a fresh water cat fish, Mystus vittatus. Int J Bio Inno, 2, 11-19.
Radu, D., Oprea, L., Bucur, C., Costache, M. and Oprea, D. (2009) Characteristics of haematological parameters for carp culture and Koi (Cyprinus carpio Linneaus, 1758) reared in an intensive system. Bull. UASVM J. Anim Sci Biotechnol, 66, 336-342.
Sampath, K., James, R. and Akbar Ali, K. M. (1998). Effects of copper and zinc on blood parameters and prediction of their recovery in Oreochromis mossambicus. Ind J Fish, 45, 129-139.
Saravanam, M., Ramesh, M., Malarvizhi, A. and Petkam, R., (2011). Toxicity of neem leaf extracts (Azadirachta indica A. Juss) on some haematological, ionoregulatory, biochemical and enzymological parameters of Indian major carp, Cirrhinus mrigala. J Trop Environ, 1, 14-26.
Satheeshkumar, P., Ananthan, G., Senthilkumar, D., Khan, A. B. and Jeevanantham, K. (2012). Comparative investigation on haematological and biochemical studies on wild marine teleost fishes from Vellar estuary, southeast Coast of India. Comp. Clin. Pathol. 21, 275–281.
Senapati, S. K., Dey, S. and Dwivedi S. K. (2001). Effect of garlic (Allium sativum L) extract on tissue lead level in rats. J Ethnopharma. 76, 229–232.
Shah, A. Q., Kazi, T. G., Arain, M. B., Jamali, M. K., Afridi, H. I., Jalbani, N., Baig J. A., and Kandhro G. A. (2009). Accumulation of arsenic in different fresh water fish species – potential contribution to high arsenic intakes. Food Chem. 112, 520–524.
Singh, M., (1995). Haematological responses in a fresh water teleost, Channa punctatus to experimental copper and Cr poisoning. J Environ Biol 16, 339-341.
Singh, D., Nath, K., Trivedi, S. P., and Sharma, Y. K. (2008). Impact of copper on haematological profile of freshwater fish, Channa punctatus. J Env Bio, 29, 253-257.
Sinha, A. K., Sinha, M. K. and Adhikari, S. (2000). Effect of the copper toxicity on haematological profile of Indian major corp, Lobeo rohita. Hand book Industry Environment and Pollution. 172p.
Smedley, P. L. and Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem, 17, 517–568.
Soiveo, A. and Nikinmaa, A. (1981). The swelling of erythrocytes in relation to the oxygen affinity of the blood of the rainbow trout, Salmo gairdneri Richardson. In: Stress and fish (Ed.: A.D. Pickering). Academic Press, London
Soylak, M., Tuzen, M., Narin, I., and Sari, H. (2004). Comparison of microwave, Dry 371y and wet digestion procedures for the determination of trace metal contents in spice samples produced in turkey. J Food Drug Ana, 12, 254–258.
Storelli, M. M., and Marcotrigiano, G. O. (2000). Organic and inorganic arsenic and lead in fish from the South Adriatic Sea, Italy. Food Addit Contam, 17, 763–768.
Tariq, J., Jaffor, M., and Ashraf, M. (1991). Levels of selected heavy metals in commercial fish from five fresh water lake Pakistan. Toxicology and Enviro Chem, 33, 133–140.
ThangaMalathi, S. and Anuradha V. (2020). Lithium Induced Toxicity Profile of Oxygen Consumption, Haematological Parameters and Biochemical Profiles of Channa punctatus and
Oreochromis niloticus. Nat Enviro Poll Tech, 19, 677-685
Vanitha, S., Amsath, A., Muthukumaravel, K., and Sugumaran, J. (2017). Effect of Arsenic on Haemetological Parameters of Fresh Water Fish, Channa punctatus (bloch). Inter J Zoo App Biosci. 3, 117-121.
Va´zquez, G. R., and Guerrero G. A. (2007). Characterization of blood cells and hematological parameters in Cichlasoma dimerus (Teleostei, Perciformes). Tissue Cell, 39, 151–160.
Vincent, S., Ambrose, T., Cyril, L., Kumar, A. and Selvanayagan, M. (1996). Heavy metal cadmium influenced anaemia in the riverine major carp, Catla (Ham). J Environ Biol, 17, 81-84.
Voegborlo, R. B., Atta, A., and Agorku, E. S. (2012). Total mercury distribution in different tissues of six species of freshwater fish from the Kpong hydroelectric reservoir in Ghana. Environ Mod Assess, 184, 3259–3265.
Vukadin, I., Zvonaric, T., and OdZak, N. (1995). Fate and distribution of toxic heavy metals in some marine organisms from the eastern Adriatic coast. Helgolander Meeresuntersuchungen, 49, 679–688.
Ybanez, N., Cervera, M. L., and Montoro, R. (1992). Determination of arsenic in dry ashed seafood products by hydride generation atomic absorption spectrometry and a critical comparative study with platform furnace Zeeman-effect atomic absorption spectrometry and inductively coupled plasma atomic emission spectrometry, Analytica Chimica Acta, 258, 61-71.
Yilmaz, F., Özdemir, N., Demirak, A., and Tuna, A. L. (2007). Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chem, 100, 830–835.
Zhao, S., Feng, C., Quan, W., Chen, X., Niu, J., and Shen, Z. (2012). Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Marine Poll Bull, 64, 1163-1171.
Zwart, D., and Trivedi, R. C. (1995) Manual on Integrated Water Quality Evaluation. RIVM, Bilthoven. RIVM Report 208023003, Appendix 6, 1-101.


