Postgraduate Department of Biotechnology and Zoology Saifia College of Science, Bhopal- 462001, India
Corresponding author Email: drshariqueali@yahoo.co.in
Article Publishing History
Received: 10/04/2019
Accepted After Revision: 20/06/2019
Aluminium is a known potent environmental nephrotoxin causing progressive biochemical changes in the kidney. The herb Aloe barbadensis is commonly known as Aloe vera, belongs to the family of Liliacea. It has been widely used in traditional system of medicines and its active compound has many therapeutic potentials. The present study has evaluated the nephroprotective effect of Aloe vera and aloin in aluminium sulphate exposed rats for a period of 45, 90 and 180 days. Aloin from Aloe vera leaf extract was isolated and characterised by HPTLC methods. Serum creatinine, urea and uric acid levels were found to significantly increased (p<0.05) after treatment of Al2(So4)3 in group II compared to control group I animals fed with normal diet. Co treatment with Al2(So4)3 and Aloe vera extract (group III) and Al2(So4)3 and aloin (group IV) showed significant decrease (p<0.05) in creatinine, urea and uric acid. So, our present study has demonstrated that Aloe vera and aloin was effective in reducing Al toxicity in kidney. Hence, Aloe vera and its active compound aloin can be used as adjuvant therapy for the prevention and management of aluminium sulphate induced renal damage.
Aloe Vera, Aluminium, Creatinine, Urea And Uric Acid.
Mahor G, Ali S. A, Parveen N. Aloin from Aloe Vera Leaves: A Potential Natural Aluminium Detoxificant. Biosc.Biotech.Res.Comm. 2019;12(2).
Mahor G, Ali S. A, Parveen N. Aloin from Aloe Vera Leaves: A Potential Natural Aluminium Detoxificant. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2ML2C6L
Copyright © Mahor et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Heavy metals exist in our environment both naturally and from pollution. Some of them are very toxic and ranked as human carcinogens. Accordingly, Aluminium (Al) is a systemic toxic metal known for multiple domestic, industrial, medical and technological applications that contribute to its wide distribution in the environment. Aluminium exposure to human beings occurs through different routes. Common routes of exposure include inhalation, oral, and skin. Exposure is more common in people working in Al industries. The prominent use of Al cookware results in ingestion of some quantity of Al every day. Al is a component of some widely used medications including sucralfate, phosphate binders, and some vaccines. It is also found in preservative, emulsifying agents, colorants, and baking powders. Such widespread use of Al in consumable and non consumable items will eventually lead to entry and deposition in human body, (Gura, 2010; Mitkus et al., 2011; Riihimaki and Antero, 2012; Shaw et al., 2013; Kramer and Heath, 2014; Sjogren et al., 2015; Mahor and Ali, 2015; Exley, 2016; Jakkala et al., 2016; Weidenhamer et al., 2017; Gouda et al., 2018; Mahor and Ali et al., 2018;Yan et al., 2019).
Aluminium does not have any physiological role in the body but upon ingestion it gets stored in human organs such as liver, lungs, kidney and brain. Due to its atomic size and electric charge similar to important elements of our body like calcium, magnesium and iron, it acts as competitive inhibitors of them and causes severe damage. Additionally, it triggers generation of reactive oxygen species (ROS), and depletes the cellular antioxidant capacity. An imbalance of antioxidant pool affects cellular organelles, antioxidant enzymes, and damages membranes, DNA, proteins, and finally destroys the tissues. Therefore, exogenous administration of antioxidant substances would have a beneficial effect on the cells’ antioxidant system to combat aluminium intoxication. In accordance, there are growing interests in using natural compounds to treat aluminium nephrotoxicity, (Garcia et al., 2010; Jing et al., 2011; Abdel Moneim, 2012; Shaw and Tomljenovic, 2013; Mardani et al., 2014; Exley and Mold, 2015; Jakkala and Ali et al., 2015; 2016; Tahir et al., 2016; Stahl et al., 2017; Khan and Strand, 2018; Haese et al., 2019 Mahor and Ali 2019).
Aloe vera L. (Aloe barbendensis Miller) is an important medicinal plant which belongs to the family Liliacea. Aloe vera plant grows readily in hot and dry climate but due to its cosmetic demand, it is cultivated on a large scale irrespective of climatic conditions. It is traded in medicinal drug market for an extensive range of therapeutic applications including wound healing effect, reduction of blood sugar, soothing burns, easing intestinal problems, reducing arthritis swelling. Many studies reports protective effect of Aloe vera and some of its bioactive compounds especially aloin, also called barbaloin is a bitter tasting yellow crystal found in Aloe vera. It is the most important anthraquinone glycoside claimed to be responsible for beneficial effects of Aloe vera, (Herrera et al., 2010; Yebpella et al., 2011; Ali et al., 2012; Lad and Murthy, 2013; Jakkala and Ali et al., 2015; 2016; Vieira et al., 2016; Minjares et al., 2017; Yavari et al., 2018; Mahor and Ali 2018, 2019; Shi et al, 2019).
To our knowledge, this is the first study to evaluate Aloe vera effects against aluminium induced nephrotoxicity in rat (Rattus norvegicus). Therefore, this study aims to investigate the potential protective effects of Aloe vera and its active compound aloin against kidney damage induced by subchronic administration of Aluminium sulphate.
Materials and Methods
Chemicals and Drugs
In this study, Al-sulphate (Al2So4)3 was purchased from Aldrich chemical Company (St. Lousis mo, USA) and Standard Aloin (C21H22O9) was obtained from Sigma. The diagnostic kits required for enzymatic assays were purchased from Span Diagnostics. All other chemicals used in the experiment were of analytical grade. The dose of Al-sulphate (Al2So4)3 was 98.3mg (Al2So4)3 /L (1/25 using Probit analysis based LD50). The dose of A.vera extract and Aloin were 100 mg/kg bw.
Collection and identification of plant material
The fresh leaves of A.vera (Aloe barbadensis) were collected from the Minor Forest Produce Processing and Research Centre (MFP-PARC) Van Parisar, Barkhera Pathani, Bhopal, (M.P.) India. The plant was authenticated by Dr. Zia-Ul-Hassan Head of the Department of Botany at the Saifia College of Science Bhopal, (M.P.) India and the voucher specimen (403/Saifia/Bot/16) has been deposited at the Herbarium of the Saifia Science College, Bhopal, (M.P.) India.
Preparation of extracts
After collection and weighing, fresh leaves of Aloe vera were washed with distilled water to remove dirt and dried under shade separately. The extraction of A. vera leaves was done according to the method (Kumar and Muthuselvam, 2009). Slight modification, Skin of the leaves were pealed and the gel inside was used for extraction. 100 gm of the gel was added to 250ml of ethanol and extracted using the Soxhlet assembly. Later on, the solvent of the extracted material was removed at low temperature in a rotary vacuum evaporator and the resulting dried extract was lyophilized in a freeze dryer.
Quantitative estimation of aloin in Aloe vera extract
Chromatographic separation of extracts of A. Vera was performed on 20 cm x 10 cm aluminium backed HPTLC plates coated with 200 μm layers of silica gel 60F254 (E. Merck, Darmstadt, Germany). Before use, the plates were pre washed with methanol and activated at 110°C for 5 min. Both test and standard samples (5μL each) were applied on to HPTLC plates as 6 mm wide bands and 12 mm apart from middle of bands by spray-on technique along with nitrogen gas supply for simultaneous drying of bands, by means of a Camag Linomat V auto sample applicator fitted with a 100 μL syringe (Hamilton, Bonaduz, Switzerland).
A constant spot application rate of 150 nL was used. Plates were developed to a distance of 165 mm, in the dark, with 30 mL ethyl acetate, methanol and water (10:1:4:1) for aloin, as mobile phase. Before development the chamber was saturated with mobile phase for 15 min at room temperature (25 ± 2°C) and 50% relative humidity. Chromatography was performed in Camag’s twin-trough chamber. Wavelength for detection of aloin was evaluated from complete UV spectrum of aloin. To calculate the concentration of aloin in each sample loaded, following equation was used as developed by Sharma et al., (2012).
Volume made x concentration x total solubility/weight of dried extract x sample loaded x 1000.
Maintenance of animals and approval of protocol
Healthy adult male albino rats (Rattus norvegicus) weighing 120-150g were used for the present investigation. They were housed in a clean polypropylene cage and maintained in an air-conditioned experimental room at 12-hour light: dark cycles. The animals were acclimatized to laboratory condition for one week prior to experiment. Standard pellets were used as a basal diet during the experimental period. The control and experimental animals were provided with purified drinking water ad libitum. The animals were maintained in accordance with the “CPCSEA guidelines for laboratory animal facility” (Committee for the Purpose of Control and Supervision on Experiments on Animals) and the approval number is CPCSEA Registration number SSC/06-06-22/CPCSEA, dated 26/10/2006. Before starting the experiment the animals were carefully marked on different parts of their body, which was later used as identification mark for a particular animal, so that the response of a particular mouse prior to and after the administration could be noted separately.
Acute oral toxicity studies
A.vera extract at the dose range of 100–2500 mg/kg body weight were administered by oral gavage method on different group of mice comprised of 6 rats in each group. Animals were kept under close observation for 4 hours after administering the fraction for behaviour, neurological and autonomic profile and then observed for any change in the general behaviour and physical activities; mortality was recorded within 72 hours. Acute toxicity was determined according to the method of Lorke, (1983).
Animal Grouping /Induction of Toxicity/Experimental design
A total of 24 male (2 months old) Albino rats (Rattus norvegicus) weighing 120-150g were used for the present investigation. The animals were divided into four groups (6 rats/ group): Group I:-was kept as control without giving any treatment. Compared to adult controls, Group II: – animals in this group were given 17±6 ml of water supplemented with Al-sulphate to consume, corresponding to 98 mg of Al per day (Jakkala and Ali et al., 2015; 2016) for 45, 90 and 180 days. Group III: – This group animals were fed with normal diet and received aluminium sulphate (98 mg/ kg body weight) and Aloe vera extract (100mg/kg body weight) for 45, 90 and 180 days. Group IV: – these group animals were fed with normal diet and received aluminium sulphate (98 mg/kg body weight) and Aloin (100mg/kg body weight) for 45, 90 and 180 days.
Collection of Blood Sample
Blood samples were collected by orbital sinus puncture method (Hui et al., 2007). Serum was separated by following procedure. Blood samples were withdrawn from orbital sinus using non heparinised capillary tubes, collected in dried centrifuge tubes and allowed to clot. Serum was separated from the clot by centrifuged at 3000 rpm for 15 min. at room temperature.
Biochemical Assays
Determination of Serum Creatinine, Urea and Uric acid
Serum Creatinine, Urea, Uric acid levels were assayed using reagent kits purchased from Biosystems (Spain), following methods of Young (1995); Kaplan (1984) and Fossati et al., (1980) respectively.
Statistical Analysis of Data
Statistical analysis was performed using Graph Pad Prism 5 software (Graph Pad Software, San Diego, CA). All parameters results were expressed as mean ± standard error (SEM) and all the statistical comparisons were made by means of the one-way ANOVA test, followed by Turkey’s test post hoc analysis. A P value <0.05 was considered significant.
Results
In the chromatogram of Aloe vera extract, many well resolved spots were observed, out of these spots one spot matched with the Rf value shown by standard aloin (0.76). The results of percentage of aloin found in samples are shown in Table 1.
Table 1: Percentage of aloin found in Aloe vera extract
| S.No. | Sample | Rf | Amount of sample applied (ng/spot) | Amount of aloin (%) |
| 1 | Aloe vera extract | 0.76 | 600 | 44.41 |
| 2 | Aloe vera extract | 0.76 | 800 | 59.31 |
| 3 | Aloe vera extract | 0.76 | 1000 | 63.10 |
| 4 | Aloe vera extract | 0.76 | 1200 | 65.56 |
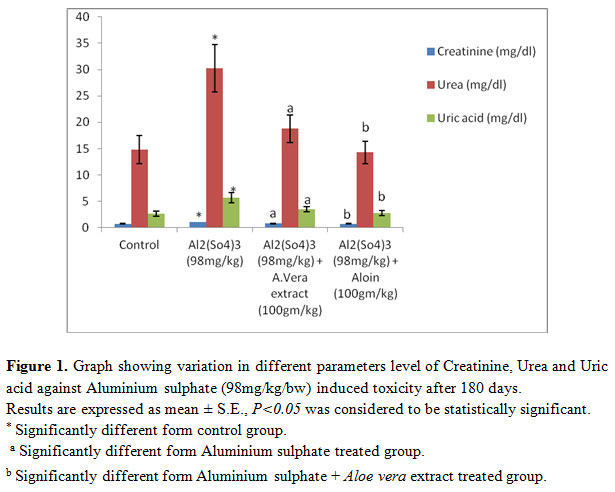
It was observed that all four groups of rats received the following treatment schedule: shows the significant change in all three parameters discussed here. After 45 days (Group II) showed a significant (P<0.05) increase in the level Creatinine, Urea, Uric acid to Al toxicity compared to group I. whereas significant (P<0.05) decrease in Creatinine, Urea and Uric acid level was reported in group III and group IV (Fig: 1).
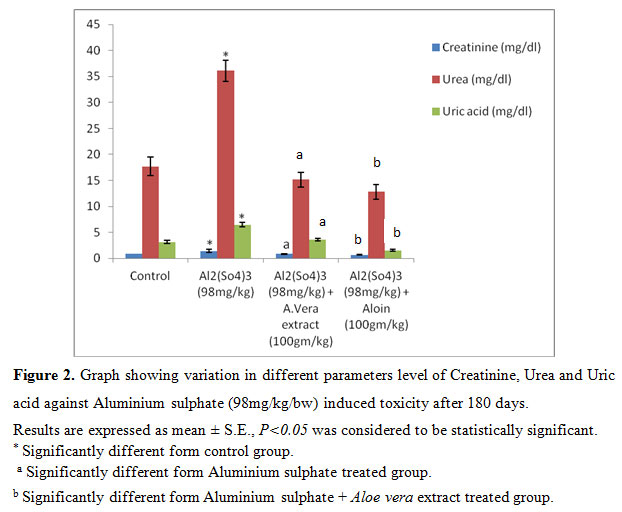
After 90 days (Group II) showed a significant (P<0.05) increase in the level of Creatinine, Urea, Uric acid to Al toxicity compared to group I. whereas significant (P<0.05) decrease in Creatinine, Urea and Uric acid level was reported in group III and group IV, (Fig: 2).
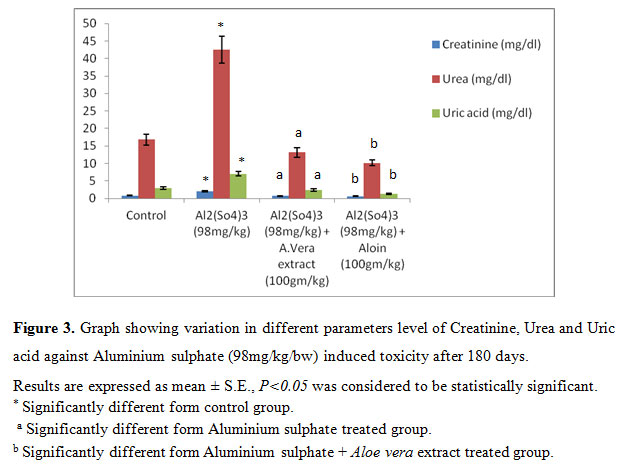
It was observed that Al toxicity enhances compared to 45, 90 days. It means Al on long term exposure induces toxicity in group II whereas A. vera extract and aloin was also effective in reducing Aluminium sulphate toxicity, significant (P<0.05) decrease in kidney function test (Creatinine, Urea and Uric acid) studied after 180 days and last study (180 days) show that in group II Creatinine, Urea and Uric acid level significant (P<0.05) increase compare to normal (control) group I and whereas group III and group IV showed significant (P<0.05) decrease, (Fig.3).
Discussion
Aluminium is one of the trace elements with toxic effect on living organism. However, in recent years, increased attention is being focused on possible adverse effects of aluminium on human health. The present study reveals that the administration of aluminium sulphate significantly (P<0.05) enhanced the levels of creatinine, urea and uric acid. Significantly (P<0.05) elevated creatinine, urea and uric acid were observed in aluminium sulphate fed rats (group II) when compared with control (group I). The rise in creatinine, urea and uric acid may indicate aluminium toxicity in kidney function. This is in consonance with the recent investigation of Ajibade et al., (2019) and Yousef et al., (2019) in which there were biochemical changes observed in the kidney of adult Wistar rats when fed with aluminium chloride. Significant increase (p<0.05) in serum urea and creatinine were observed.
In the present study, significant decreased (p<0.05) level of creatinine, urea and uric acid were observed in aluminium sulphate and Aloe vera extract fed rats (group III) when compared to group II animals. These results are in agreement with the study of Belaid-Nouira et al., (2013; Miraj et al., 2015) using other plant, they have found that fenugreek seeds showed effectiveness in restoring normal plasma values of urea, creatinine in the kidney injured by aluminium chloride. Several other studies using Aloe vera plant, also support our findings concluded that Aloe vera extract showed nephroprotective effect against heavy metal toxicity (Iftikhar et al., 2015; Hussain et al., 2016). In our study, administration of aloin showed significant decreased (p<0.05) level of creatinine, urea and uric acid when compared to group III animals. This is analogous to the study of Al Dera, (2016) who has proposed that standard resveratrol when administered with aluminium chloride showed significant (p<0.05) decreased in serum creatinine and urea. In our previous study it has been showed that aloin also significantly (p<0.05) reduced in Total cholesterol, triglyceride, HDL and LDL (Mahor and Ali, 2018).
Conclusion
In agreement with previous studies, results from this study revealed that aluminium induced nephrotoxicity is indicated by significant (p<0.05) increase in creatinine, urea and uric acid. But, co treatment of aluminium sulphate and Aloe vera extract and Aluminium sulphate and aloin showed significant (p<0.05) reduction in creatinine, urea and uric acid. This suggests that Aloe vera and its active compound aloin is very potent in preventing aluminium toxicity in kidney.
Based on the findings of present work, it can be concluded that the A. vera extract and aloin was effective in reducing Al toxicity in kidney function tests (Creatinine, Urea and Uric acid). Deeper study is needed using histological analysis for gaining better pharmacological information and intervention.
Conflicts of Interest
The authors have no conflict of interest to declare.
Acknowledgement
GM is thankful to UGC, Delhi for awarding RGNF, Vide File No. 2014-15-SC-MAD-67686/2014.
References
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits
Abdel Moneim A.E. (2012) Evaluating the Potential Role of Pomegranate Peel in Aluminum-Induced Oxidative Stress and Histopathological Alteration in Brain of Female Rats. Biological Trace Elements Research, Vol.150, No.1-3, Pages 328-336.
Ajibade A.J., Kehinde B.D., Atanda A.A, Adeleye O.O. (2019) Some morphological and biochemical changes in the kidney of adult Wistar rats following aluminium chloride exposures. Asian Journal of Research in Nephrology. Vol.2, No.1, Pages 1-9.
Ali S. A., Choudhary R.K. and Lakshman J. (2012). Quantitative estimation of Aloin from Aloe vera leaf extract by High Performance Thin Layer Chromatography. Bioscience Biotechnology Research Communications. Vol.5, No.2, Pages 206-209.
Belaid-Nouira Y, Bakhta H, Haouas Z, Flehi-Slim I, Ben Cheikh H. (2013) Fenugreek seeds reduce aluminum toxicity associated with renal failure in rats. Nutrition Research and Practice. Vo.7, No,6, Pages 466-74.
Dera H.S. (2016) Protective effect of resveratrol against aluminium chloride induced nephrotoxicity in rats. Saudi Medical Journal. Vol.37, No.4, Pages 369-378.
Exley C. (2016) The toxicity of aluminium in humans La toxicite de l’aluminium chez l’ home. Morphologic. Vol. 100, No. 329, Pages 51-55.
Exley C. and Mold M.j. (2015) The binding, transport and fate of aluminium in biological cells. Journal of Trace Elements in Medicine and Biology. Vol. 30, Pages 90-95.
Fossati P, Prencipe L, Berti G. (1980) Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urin. Clinical Chemistry. 26:227-31.
Garcia T., Esparza J.L., Montserrat G., Marta R., Domingo J. L., Gomez M. (2010) Protective Role of Melatonin on Oxidative Stress Status and RNA Expression in Cerebral and cerebellum of AβPP Transgenic Mice After Chronic Exposure to Aluminum. Biological Trace Element Research. Vol.135, No.1-3, Pages 220-232.
Gouda AS, El-Nabarawy NA, and Ibrahim S.F. (2018) Moringa oleifera extract (Lam) attenuates Aluminium phosphide-induced acute cardiac toxicity in rats. Toxicology Reports. Vol. 5, Pages 209–212.
Gura K.M. (2010) Aluminium contamination in products use in parenteral nutrition: has anything changed. Nutrition. Vol. 26, No.6, Pages 585-594.
Haese D.P.C., Douglas G., Verhulst A., Neven E., Behets G.J., Vervaet B.A., Finsterle K., Lürling M., Spears B. (2019) Human health risk associated with the management of phosphorus in freshwaters using lanthanum and aluminium. Chemosphere. Vol. 220, Pages 286-299.
Herrera J.D., Delfino I., Salinas C., Silva H. (2010) Irrigation restriction effects on water use efficiency and osmotic adjustment in Aloe vera plants (Aloe barbadensis Miller). Agricultural Water Management. Vol. 97, No.10, Pages1564-1570.
Hui Y.H, Huang N.H., Ebbert L., Bina H., Chiang A., Maples C., Pritt M., Kern T., Patel N. (2007) Pharmacokinetic comparison of tail-bleeding with cannula-or retro-orbital bleeding techniques in rats using six marketed drugs. Journal of Pharmacological and Toxicological Methods. Vol. 56, Pages 256-264.
Hussain N., Chaudhary M.N., Anjum A.A., Abbas N., Khan M.N., Nadeem S.M. (2016) Investigating the Ameliorative Potential of the Aloe barbadesis Aqueous Fraction on Oxidative Stress Markers and Biochemical parameters in Cadmium-Intoxicated Rabbits. Polish Journal of Environmental Studies. Vol. 25, No.6, Pages 2423-2433.
Iftikhar A., Hasan I.J., Sarfraz M., Jafri L., and Ashraf M.A. (2015) Nephroprotective Effect of the Leaves of Aloe barbadensis (Aloe Vera) against Toxicity Induced by Diclofenac Sodium in Albino Rabbits. West Indian Medical Journal. Vol. 64, No.5, Pages 462-467.
Jakkala L.K. and Ali A. (2016) Aloe vera protects the Aluminium induced changes in testicular enzymes activity of Albino rats, Rattus Norvegicus. World Journal of Pharmacy and Pharmaceutical Sciences. Vol. 5, No.5, Pages1091-1104.
Jakkala L.K. and Ali SA. (2015) Aloe vera Protects the Aluminium induced degenerative changes in liver and kidney of Albino Rats, Rattus norvegicus. Journal of Global Biosciences. Vol. 4, No.8 Pages 3158-3164.
Jing H., JiLet W.U., Tiejun L.I., XinMing S., BingZi Z., PinWen Z., Ying Z.X. (2011) Effect of Exposure to Trace Elements in the Soil on the Prevalence of Neural Tube Defects in a High-Risk Area of China. Biomedical and Environmental Sciences. Vol.24, No.2, Pages 94-101.
Kaplan A Urea. In: Kaplan A (Ed). (1984) Clinical Chemistry. St Louis/ Toronto/Princeton: The CV Mosby, Co.St Louis. Toronto. Princeton. Vol.1257, No.60, Pages 418-437.
Khan R.K., Strand M.A. (2018) Road dust and its effect on human health: a literature review. Epidemiology and Health. Vol. 40, Pages 1-11.
Kramer M.F. and Heath M.D. (2014) Aluminium in allergen-specific subcutaneous immunotherapy-A German perspective. Vaccine. Vol. 32, No. 33, Pages 4140-4148.
Kumar A and Muthuselvan S. (2009) Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World Journal of Agricultural Sciences. Vol. 5, No. 5, Pages 572-576.
Lad V.N.and Murthy Z.V.P. (2013) Rheology of Aloe barbadensis Miller: A naturally available material of high therapeutic and nutrient value for food applications. Journal of Food Engineering. Vol. 115, No.3, Pages 279-284.
Lorke D. (1983) A new approach to practical acute toxicity testing. Archives Toxicology. Vol. 54, Pages 275-287.
Mahor G and Ali S.A. (2018) Protective effects of Aloe vera extract on aluminium sulphate induced alterations in serum lipid profile of male albino rats, Rattus norvegicus. Bioscience Biotechnology Research Communications. Vol.1, No.4, Pages727-733.
Mahor G, Ali S.A. (2015). An update on the role of medicinal plants in amelioration of aluminium toxicity. Bioscience Biotechnology Research Communication. Vol. 8, No.2, Pages 175-188.
Mardani S., Nasri H., Hajjan S., Ahmadi A., Kazemi R and Rafieian-Kopaei M. (2014) Impact Momordica Charantia extract on kidney function and structure in mice. Journal of Nephropathology. Vol. 3, No.1, Pages 35-40.
Minjares-Fuentes. R., Rodriguez-Gonzalez V.M., Gonzalez-Laredo R.F., Eim V., Gonzalez-Centeno M.R., Femenia A. (2017) Effect of different drying procedures on the bioactive polysaccharide acemannan from Aloe vera (Aloe barbadensis Miller). Carbohydrate Polymers. Vol.168, Pages 327-336.
Miraj M., Jakkala L., Khan N. and Ali A.S. (2015). On the toxicity of certain metals and its amelioration through herbal extracts. Bioscience Biotechnology Research Communication. Vol.6, No.1, Pages 99-107.
Mitkus R.J., King D.B., Hess D.B., Forshee R.A., Walderhaug M.O. (2011) Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine. Vol.29, No. 51, Pages 9538-9543.
Riihimaki V. and Aitio A. (2012) Occupational exposure to aluminum and its biomonitoring in perspective. Journal of Critical Reviews in Toxicology. Vol. 42, No.10, Pages 827-853.
Sharma V., Shukla V. and Prajapati P.K. (2012) Quantitative estimation of Aloin from Pharmaceutical Dosage By HPTLC. Pharma Science Monitor. Vol. 3, No.1, Pages 104-109.
Shaw C.A. and Tomljienovic L. (2013) Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunologic Research. Vol.56, No. 2-3, Pages 304-316.
Shaw C.A., Li Y., Tomljenovic L. (2013) Administration of aluminium to neonatal mice in vaccine-relevant amounts is associated with adverse long term neurological outcomes. Journal of Inorganic Biochemistry. Vol. 128, Pages 237-244.
Shi X. D., Yin J.Y., Zhang L.J., Li O.Y., Huang X.J., Nie S.P. (2019) Studies on polysaccharides from leaf skin of Aloe barbadensis Miller: Part II. Structural characteristics and molecular properties of two lower molecular weight fractions. Food Hydrocolloids. Vol.86, Pages 50-61.
Sjogren B., Iregren A., Montelius J., Yokel R.A. (2015) Chapter 26 – Aluminum. Hand book on the Toxicology of Metals, Vol.4, No.2, Pages 549-564.
Stahl T, Falk S, Rohrbeck A, Georgii S, Herzog C, Wiegand A, Hotz S, Boschek B, Zorn H, Brunn H. (2017) Migration of aluminum from food contact materials to food-a health risk for consumers? Part I of III: exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environmental Science Europe. Vol. 29, No.1, Pages 1-19.
Tahir I, Khan M.R., Shah N.A. and Aftab M. (2016) Evaluation of phytochemicals, antioxidant activity and amelioration of pulmonary fibrosis with Phyllanthus emblica leaves. BMC Complementary and Alternative Medicine. Vol. 406, No.16, Pages 1-12.
Vieira J.M., Lopez M.L.F, Rodriguez D.J, Sousa M.C.R. (2016) Effect of chitosan-Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biology and Technology. Vol.116, Pages 88-97.
Weidenhamer J.D, Fitzpatrick M.P., Biro A.M., Kobunski P.A., Hudson M.R., Corbin R.W., Gottesfeld P. (2017) Metal exposures from aluminum cookware: An unrecognized public health risk in developing countries. Science of the Total Environment. Vol. 579, Pages 805-813.
Yan L, Riaz M, Du C, Liu Y, Zeng Y, Jiang C. (2019) Ameliorative role of boron to toxicity of aluminum in trifoliate orange roots. Ecotoxicology Environmental Safety. Vol. 179, Pages 212-221.
Yavari Z., Moradi H., Sadeghi H., Golchini B.B. (2018) Evaluation of Aloe vera (Aloe barbadensis Miller) Antioxidant Activity and Some of the Morphological Characteristics in Different Vermicompost Field. Journal of Chemical Health Risks. Vol. 3, No.4, Pages19-28.
Yebpella G.G., Adeyemi Hassan M.M., Hammuel C., Magomya A. M., Aghaji A.S. and Okonkwo E.M. (2011) Phytochemical screening and comparative study of antimicrobial activity of Aloe vera various extracts. African Journal of Microbiology Reseacrh. Vol.5, No.10, Pages 1182-1187.
Young DS. (1997) Effects of Drugs on Clinical Laboratory Tests. Annals of Clinical Biochemistry. Vol. 34, No. 4, Pages 579-581.
Yousef MI, Mutar TF, Kamel MAE (2019) Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicology Reports. Vol.19, No.6, Pages 336-346.